Abstract
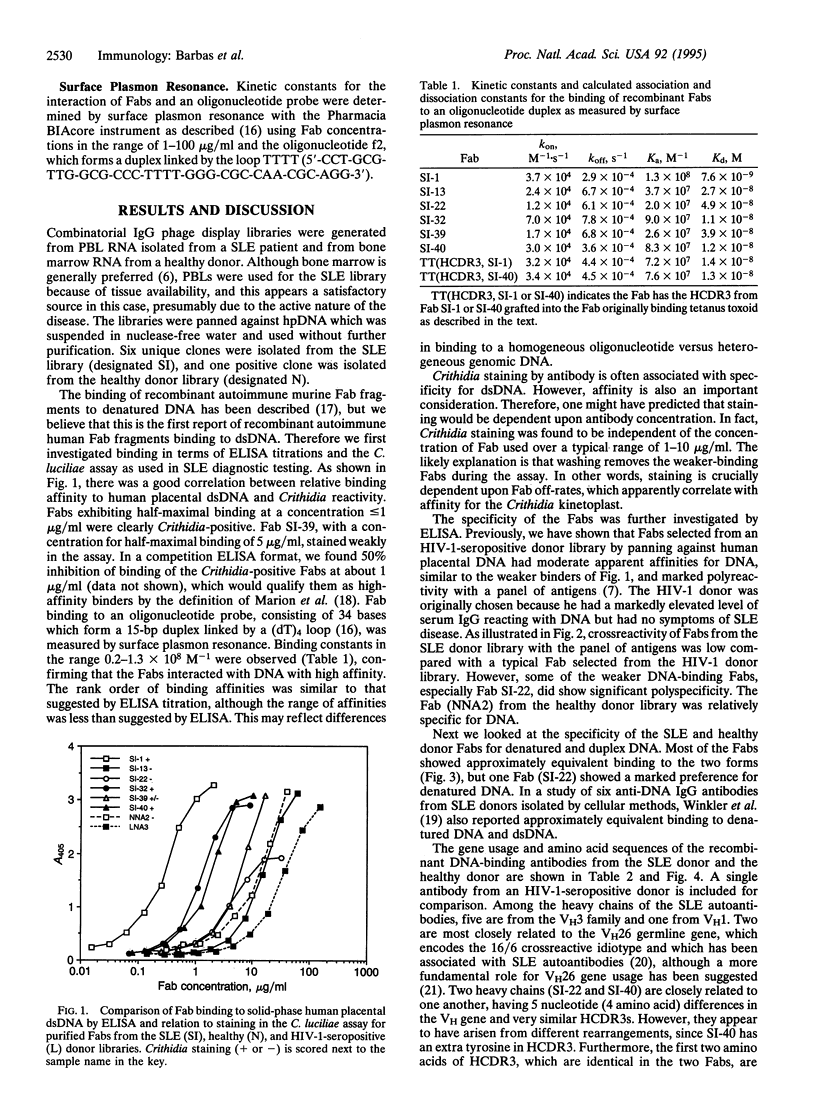
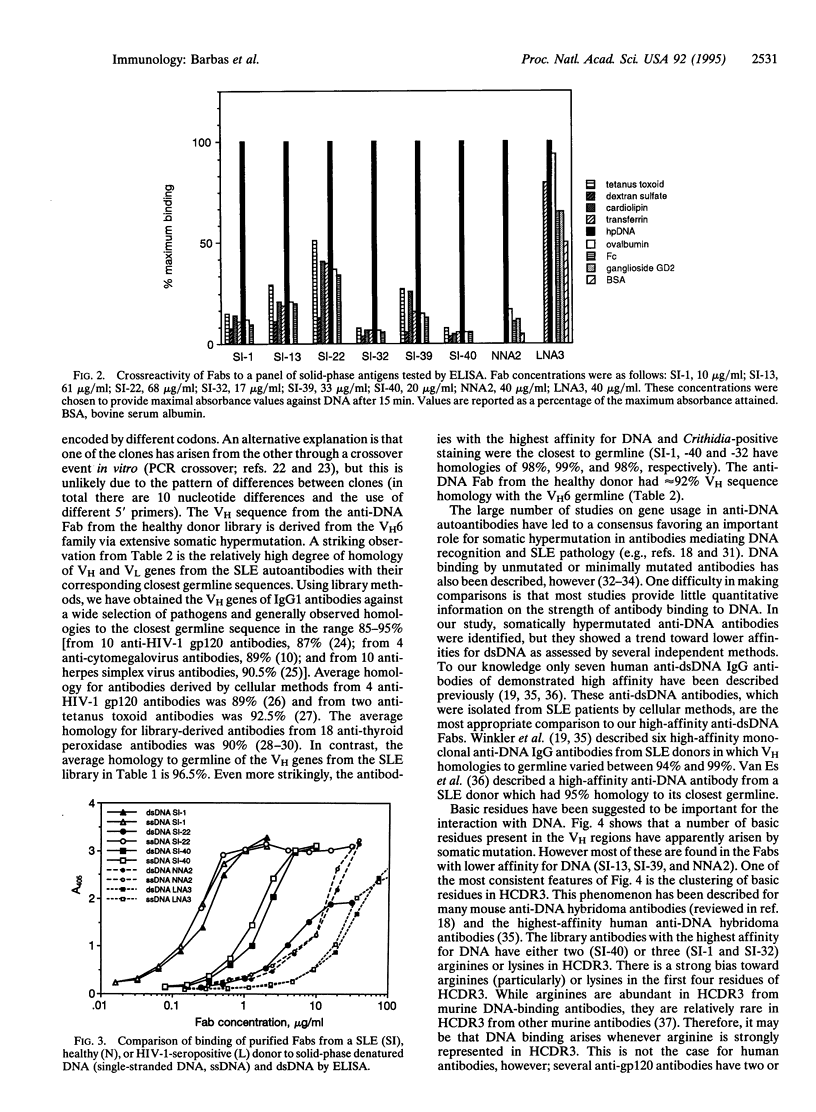
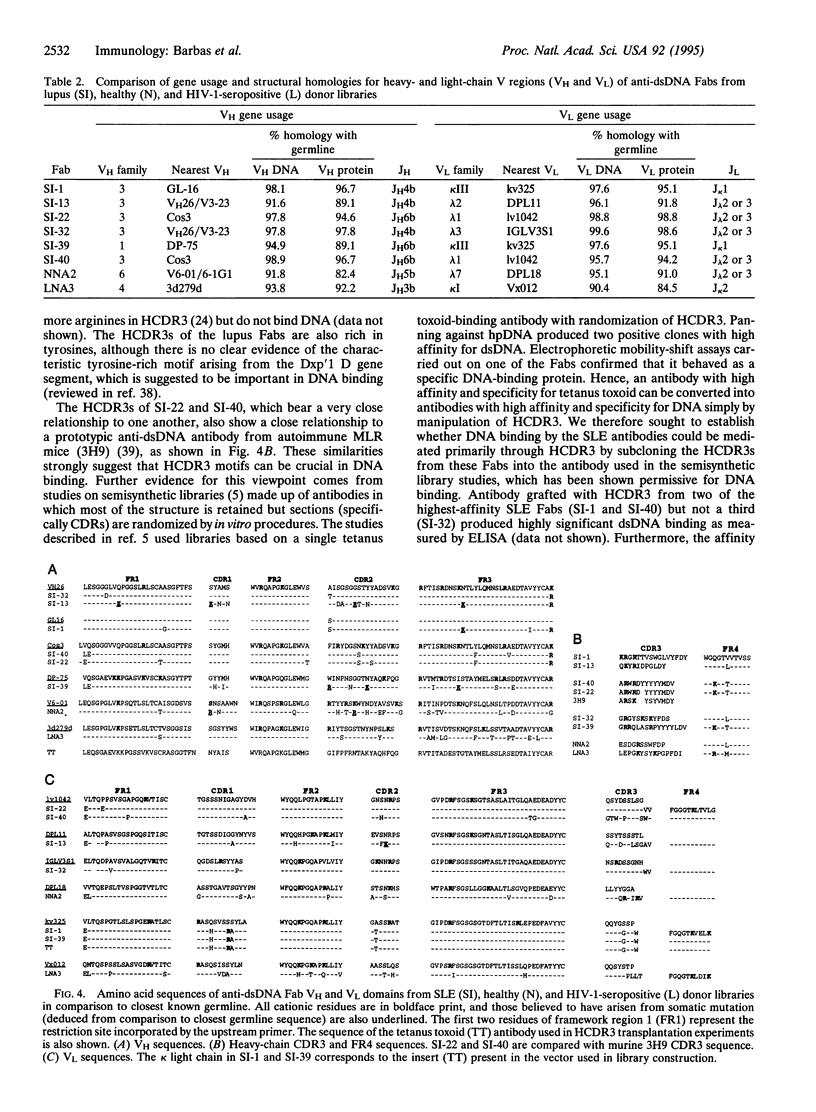
Combinatorial IgG Fab phage display libraries prepared from a systemic lupus erythematosus (SLE) donor and a healthy donor were affinity selected against human placental DNA. Human monoclonal antibody Fab fragments specific for DNA were isolated from both libraries, although Fabs of the highest affinity were isolated only from the lupus library. Generally, apparent affinities of the Fabs for human placental DNA, purified double-stranded DNA, and denatured DNA were approximately equivalent. Surface plasmon resonance indicated Fab binding constants for a double-stranded oligodeoxynucleotide of 0.2-1.3 x 10(8) M-1. The higher-affinity Fabs, as ranked by binding to human placental DNA or to the oligonucleotide probe, tested positive in the Crithidia luciliae assay commonly used in the diagnosis of SLE, and interestingly the genes encoding the heavy-chain variable regions of these antibodies displayed evidence of only minimal somatic hypermutation. The heavy chains of the SLE Fabs were characterized by a predominance of basic residues toward the N terminus of complementarity-determining region 3 (CDR3). The crucial role of heavy-chain CDR3 (HCDR3) in high-affinity DNA recognition was suggested by the creation of DNA binding in an unrelated antibody by HCDR3 transplantation from SLE antibodies. We propose that high-affinity DNA-binding antibodies can arise in SLE without extensive somatic hypermutation in the variable-region genes because of the expression of inappropriate HCDR3s.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andris J. S., Johnson S., Zolla-Pazner S., Capra J. D. Molecular characterization of five human anti-human immunodeficiency virus type 1 antibody heavy chains reveals extensive somatic mutation typical of an antigen-driven immune response. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7783–7787. doi: 10.1073/pnas.88.17.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Bain J. D., Hoekstra D. M., Lerner R. A. Semisynthetic combinatorial antibody libraries: a chemical solution to the diversity problem. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4457–4461. doi: 10.1073/pnas.89.10.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Collet T. A., Amberg W., Roben P., Binley J. M., Hoekstra D., Cababa D., Jones T. M., Williamson R. A., Pilkington G. R. Molecular profile of an antibody response to HIV-1 as probed by combinatorial libraries. J Mol Biol. 1993 Apr 5;230(3):812–823. doi: 10.1006/jmbi.1993.1203. [DOI] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Crowe J. E., Jr, Cababa D., Jones T. M., Zebedee S. L., Murphy B. R., Chanock R. M., Burton D. R. Human monoclonal Fab fragments derived from a combinatorial library bind to respiratory syncytial virus F glycoprotein and neutralize infectivity. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10164–10168. doi: 10.1073/pnas.89.21.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Kang A. S., Lerner R. A., Benkovic S. J. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Rosenblum J. S., Lerner R. A. Direct selection of antibodies that coordinate metals from semisynthetic combinatorial libraries. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6385–6389. doi: 10.1073/pnas.90.14.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender E., Pilkington G. R., Burton D. R. Human monoclonal Fab fragments from a combinatorial library prepared from an individual with a low serum titer to a virus. Hum Antibodies Hybridomas. 1994;5(1-2):3–8. [PubMed] [Google Scholar]
- Burioni R., Williamson R. A., Sanna P. P., Bloom F. E., Burton D. R. Recombinant human Fab to glycoprotein D neutralizes infectivity and prevents cell-to-cell transmission of herpes simplex viruses 1 and 2 in vitro. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):355–359. doi: 10.1073/pnas.91.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D. R., Barbas C. F., 3rd Human antibodies from combinatorial libraries. Adv Immunol. 1994;57:191–280. doi: 10.1016/s0065-2776(08)60674-4. [DOI] [PubMed] [Google Scholar]
- Burton D. R., Barbas C. F., 3rd, Persson M. A., Koenig S., Chanock R. M., Lerner R. A. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcutt M. J., Kremer M. T., Giblin M. F., Quinn T. P., Deutscher S. L. Isolation and characterization of nucleic acid-binding antibody fragments from autoimmune mice-derived bacteriophage display libraries. Gene. 1993 Dec 27;137(1):77–83. doi: 10.1016/0378-1119(93)90254-z. [DOI] [PubMed] [Google Scholar]
- Dersimonian H., Schwartz R. S., Barrett K. J., Stollar B. D. Relationship of human variable region heavy chain germ-line genes to genes encoding anti-DNA autoantibodies. J Immunol. 1987 Oct 1;139(7):2496–2501. [PubMed] [Google Scholar]
- Diamond B., Katz J. B., Paul E., Aranow C., Lustgarten D., Scharff M. D. The role of somatic mutation in the pathogenic anti-DNA response. Annu Rev Immunol. 1992;10:731–757. doi: 10.1146/annurev.iy.10.040192.003503. [DOI] [PubMed] [Google Scholar]
- Ditzel H. J., Barbas S. M., Barbas C. F., 3rd, Burton D. R. The nature of the autoimmune antibody repertoire in human immunodeficiency virus type 1 infection. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3710–3714. doi: 10.1073/pnas.91.9.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster M. H., Cizman B., Madaio M. P. Nephritogenic autoantibodies in systemic lupus erythematosus: immunochemical properties, mechanisms of immune deposition, and genetic origins. Lab Invest. 1993 Nov;69(5):494–507. [PubMed] [Google Scholar]
- Griffiths A. D., Williams S. C., Hartley O., Tomlinson I. M., Waterhouse P., Crosby W. L., Kontermann R. E., Jones P. T., Low N. M., Allison T. J. Isolation of high affinity human antibodies directly from large synthetic repertoires. EMBO J. 1994 Jul 15;13(14):3245–3260. doi: 10.1002/j.1460-2075.1994.tb06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hexham J. M., Partridge L. J., Furmaniak J., Petersen V. B., Colls J. C., Pegg C., Rees Smith B., Burton D. R. Cloning and characterisation of TPO autoantibodies using combinatorial phage display libraries. Autoimmunity. 1994;17(3):167–179. doi: 10.3109/08916939409010651. [DOI] [PubMed] [Google Scholar]
- Hexham M., Pegg C., Burton D., Petersen V. B., Horimoto M., Furmaniak J., Rees Smith B. Variable region sequence of a human monoclonal thyroid peroxidase autoantibody. Autoimmunity. 1992;14(2):169–172. doi: 10.3109/08916939209083138. [DOI] [PubMed] [Google Scholar]
- Katz M. S., Foster M. H., Madaio M. P. Independently derived murine glomerular immune deposit-forming anti-DNA antibodies are encoded by near-identical VH gene sequences. J Clin Invest. 1993 Feb;91(2):402–408. doi: 10.1172/JCI116214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. H., Dixon F. J. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968 Mar 1;127(3):507–522. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrick J. W., Wallace E. F., Coloma M. J., Bruderer U., Lang A. B., Fry K. E. Therapeutic human antibodies derived from PCR amplification of B-cell variable regions. Immunol Rev. 1992 Dec;130:69–85. doi: 10.1111/j.1600-065x.1992.tb01521.x. [DOI] [PubMed] [Google Scholar]
- Marion T. N., Tillman D. M., Jou N. T., Hill R. J. Selection of immunoglobulin variable regions in autoimmunity to DNA. Immunol Rev. 1992 Aug;128:123–149. doi: 10.1111/j.1600-065x.1992.tb00835.x. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., André-Schwartz J., Manser T., Wysocki L. J., Breitman L., Stollar B. D., Gefter M., Schwartz R. S. A single germline VH gene segment of normal A/J mice encodes autoantibodies characteristic of systemic lupus erythematosus. J Exp Med. 1986 Aug 1;164(2):614–626. doi: 10.1084/jem.164.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portolano S., McLachlan S. M., Rapoport B. High affinity, thyroid-specific human autoantibodies displayed on the surface of filamentous phage use V genes similar to other autoantibodies. J Immunol. 1993 Sep 1;151(5):2839–2851. [PubMed] [Google Scholar]
- Radic M. Z., Mackle J., Erikson J., Mol C., Anderson W. F., Weigert M. Residues that mediate DNA binding of autoimmune antibodies. J Immunol. 1993 Jun 1;150(11):4966–4977. [PubMed] [Google Scholar]
- Rubin R. L., Joslin F. G., Tan E. M. An improved ELISA for anti-native DNA by elimination of interference by anti-histone antibodies. J Immunol Methods. 1983 Oct 28;63(3):359–366. doi: 10.1016/s0022-1759(83)80009-x. [DOI] [PubMed] [Google Scholar]
- Shefner R., Kleiner G., Turken A., Papazian L., Diamond B. A novel class of anti-DNA antibodies identified in BALB/c mice. J Exp Med. 1991 Feb 1;173(2):287–296. doi: 10.1084/jem.173.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M. J., Aucoin A. H., Pisetsky D. S., Weigert M. G. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M., Mascelli M., Shan H., Radic M. Z., Pisetsky D., Marshak-Rothstein A., Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990 Jan 1;171(1):265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A. K., Huang C., Long A. A., Stollar B. D., Schwartz R. S. VH-gene representation in autoantibodies reflects the normal human B-cell repertoire. Immunol Rev. 1992 Aug;128:101–122. doi: 10.1111/j.1600-065x.1992.tb00834.x. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tomlinson I. M., Walter G., Marks J. D., Llewelyn M. B., Winter G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J Mol Biol. 1992 Oct 5;227(3):776–798. doi: 10.1016/0022-2836(92)90223-7. [DOI] [PubMed] [Google Scholar]
- Williamson R. A., Burioni R., Sanna P. P., Partridge L. J., Barbas C. F., 3rd, Burton D. R. Human monoclonal antibodies against a plethora of viral pathogens from single combinatorial libraries. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4141–4145. doi: 10.1073/pnas.90.9.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler T. H., Fehr H., Kalden J. R. Analysis of immunoglobulin variable region genes from human IgG anti-DNA hybridomas. Eur J Immunol. 1992 Jul;22(7):1719–1728. doi: 10.1002/eji.1830220709. [DOI] [PubMed] [Google Scholar]
- Winkler T. H., Jahn S., Kalden J. R. IgG human monoclonal anti-DNA autoantibodies from patients with systemic lupus erythematosus. Clin Exp Immunol. 1991 Sep;85(3):379–385. doi: 10.1111/j.1365-2249.1991.tb05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Yang W. P., Barbas C. F., 3rd Building zinc fingers by selection: toward a therapeutic application. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):344–348. doi: 10.1073/pnas.92.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es J. H., Gmelig Meyling F. H., van de Akker W. R., Aanstoot H., Derksen R. H., Logtenberg T. Somatic mutations in the variable regions of a human IgG anti-double-stranded DNA autoantibody suggest a role for antigen in the induction of systemic lupus erythematosus. J Exp Med. 1991 Feb 1;173(2):461–470. doi: 10.1084/jem.173.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]



