Abstract
Aims
Hydrogen sulfide (H2S) at low concentrations serves as a physiological endogenous vasodilator molecule, while at higher concentrations it can trigger cytotoxic effects. The aim of our study was to elucidate the potential mechanisms responsible for the effects of H2S on vascular tone.
Main methods
We measured the vascular tone in vitro in precontracted rat thoracic aortic rings and we have tested the effect of different oxygen levels and a variety of inhibitors affecting known vasodilatory pathways. We have also compared the vascular effect of high concentrations of H2S to those of pharmacological inhibitors of oxidative phosphorylation. Furthermore, we measured adenosine triphosphate (ATP)-levels in the same vascular tissues.
Key findings
We have found that in rat aortic rings: (1) H2S decreases ATP levels; (2) relaxations to H2S depend on the ambient oxygen concentration; (3) prostaglandins do not take part in the H2S induced relaxations; (4) the 3':5'-cyclic guanosine monophosphate (cGMP) – nitric oxide (NO) pathway does not have a role in the relaxations (5) the role of KATP channels is limited, while Cl−/HCO3− channels have a role in the relaxations. (6): We have observed that high concentrations of H2S relax the aortic rings in a fashion similar to sodium cyanide, and both agents reduce cellular ATP levels to a comparable degree.
Significance
H2S, a new gasotransmitter of emerging importance, leads to relaxation via Cl−/HCO3− channels and metabolic inhibition and the interactions of these two factors depend on the oxygen levels of the tissue.
Keywords: hydrogen sulfide, vasorelaxation, oxidative phosphorylation
INTRODUCTION
Hydrogen sulfide (H2S) has been best known for decades as a pungent toxic gas in the contaminated environmental atmosphere (Smith and Gosselin 1979). However, H2S has been recognized recently as a novel gasotransmitter in the central nervous and in the circulatory systems, similar to the other gasotransmitters nitric oxide (NO) and carbon monoxide (CO). H2S is formed endogenously by pyridoxal-5’-phosphate-dependent enzymes such as cystathionine-γ-lyase (CSE; E.C. 4.4.1.1) and cystathionine-β-synthetase (CBS; E.C. 4.2.1.22). CSE is expressed in various vessels of the vascular system, while CBS is highly expressed in the brain, but not detectable in the blood vessels (Hosoki et al. 1997; Kimura 2000 Zhao et al. 2001). The physiological concentration of sulfide in blood and in tissues is in a broad range (1-160 μM) and appears to depend on the used method (Bhatia et al. 2005a; Li et al. 2005; Whitfield et al. 2008; Zhao et al. 2001).
H2S has been implicated in many inflammatory, neural and cardiovascular diseases such as acute pancreatitis (Bhatia et al. 2005a), carrageenan-induced hindpaw edema (Bhatia et al. 2005b), Down-syndrome (Kamoun 2001), septic shock(Li et al. 2005), haemorrhagic shock (Mok et al. 2004), hypertension (Yan et al. 2004) and myocardial ischemia-reperfusion injury (Elrod et al. 2007). For a comprehensive current review on hydrogen sulfide and its therapeutic potential see: (Szabo 2007).
In the cardiovascular system, H2S was shown to affect vascular tone by complex mechanisms, exerting contraction or relaxation (or both) of blood vessels (Ali et al. 2006; Dombkowski et al. 2005). Despite the early finding that H2S induced vasodilation of rat aortic rings in a concentration-dependent manner starting from 60 μM via activating ATP-sensitive K+ channels (KATP channels) (Zhao et al. 2001), the precise signaling mechanism of H2S on vascular tone is far from understood. Recently published work found that the KATP channel inhibitor glibenclamide failed to affect the contractile and relaxant activity of NaHS in mouse aorta and had only partial effect on rat aorta (Kubo et al. 2007). In other experiments using nonvascular smooth muscle glibenclamide also failed to block the effect of NaHS (Teague et al. 2002). These results suggest that KATP channels may not be the only signaling mechanism responsible for the vasorelaxation of H2S. In fact, H2S was shown recently to have a role in the regulation of intracellular pH, which would lead to relaxation (Lee et al. 2007). It was also demonstrated that H2S interferes with cytochrome c oxidase and has a potency to induce metabolic inhibition (Hill et al. 1984), and H2S administration led to reduction of tissue oxygen uptake and modified the neurotransmitter concentrations in the brain (Nicholson et al. 1998). Recently, the effect of H2S on vascular tone was found to be highly oxygen-dependent also (Koenitzer et al. 2007). Therefore the aim of our study was to investigate the mechanism of sulfide-induced vascular relaxations, with focus on the possibility of release of secondary vasodilatory pathways (e.g. nitric oxide, prostaglandins etc.), as well as KATP channels, Cl−/HCO3− channels and metabolic inhibition.
MATERIALS AND METHODS
Animals
Male Wistar rats (Charles Rivers) weighing 300-325 g were used. Animals were maintained on lab chow and tap water ad lib with a 12 h day-night cycle in the conventional animal facility of University of Medicine and Dentistry of New Jersey.
Measurement of isometric force in aortic rings
Animals were anaesthetized with pentobarbital (50 mg/kg, Nembutal, Ovation, Deerfield, IL, USA). The thorax was opened and the circulation was flushed with ice-cold heparinized saline, then the thoracic aorta was excised and immediately immersed in ice-cold Krebs’ solution composed of CaCl2 1.5mM, MgSO4 1.2mM, NaCl 118mM, NaHCO3 14.8mM, KCl 4.6mM, NaH2PO4 1.2mM, glucose 11.1mM. The aorta was cleaned of all fat and adherent connective tissue in a Petri dish containing ice-cold Krebs’ solution and cut to six ring segments (4mm in length). Two stainless-steel triangles were inserted through each vessel ring with care to preserve the endothelial layer. Each aortic ring was suspended in a water-jacketed organ bath (6ml) maintained at 37°C and aerated with a gas mixture of 95% O2 and 5% CO2. One triangle was anchored to a stationary support and the other connected to an isometric force transducer (Kent Scientific, Torrington, CT, USA). The rings were stretched passively by imposing a resting tension of 1.0g, which was maintained throughout the experiment. Each ring was equilibrated in the organ bath solution for 60 min before the experiment and fresh Krebs was provided at 20 min intervals. Isometric contractions were recorded using a computerized data acquisition system (PowerLab/8SP, ADInstruments, Castle Hill, NSW, Australia) and recorded on a PC using Chart 5.4.2 software.
We investigated the relaxations to our H2S formulation (20-320μM) in 4 separate sets of experiments. First we studied the relaxations after different kinds of contraction. We used either 120mM KCl containing Krebs’ solution or 1μM epinephrine solution. In all of the following sets 1μM epinephrine was used. Second we checked the effect of various well-known inhibitors on H2S induced vasorelaxations after 1μM epinephrine. The following inhibitors were used (incubation times): 1μM atropine (10 min); 30μM lidocaine (10 min); 10μM indomethacin (10 min); 100μM glibenclamide (30 min); 100μM NG-Methyl-L-arginine (L-NMMA,10 min); 0.3μM HOE140 (selective bradykinin-2-receptor antagonist, 20 min); 1mM 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt hydrate (DIDS, Cl−/HCO3− channel inhibitor, 10 min). In the third set of experiments we tested the effect of different oxygen tensions on H2S induced relaxation after the rings were contracted with 1μM epinephrine. We have investigated the vascular responses to sulfide with and without oxygenation. The approximate O2 concentrations in these conditions are, based on literature, ~200μM for air-saturated condition and ~20μM at the non-oxygenated condition (Koenitzer et al. 2007). Finally, we compared the effects of H2S to the complex IV inhibitor hydrogen cyanide (HCN) and to the mitochondrial uncoupler 2,4-dinitrophenol (2,4-DNP) at 320μM.
Measurement of vascular ATP levels
We compared the effect of sulfide on vascular tone with the effect of cyanide (HCN) and dinitrophenol (2,4-DNP), with the hypothesis being that if sulfide relaxes the blood vessels via inhibition of cellular ATP generation, then the pattern of vasorelaxation after sulfide and after HCN and 2,4-DNP would be similar. Vascular ring samples from the last set of experiments were snap frozen at three different time points: before, at 10 sec and at 3 min after administration of H2S, HCN or 2,4-DNP. The samples were homogenized in 500μL 2% trichloroacetic acid using Dounce homogenizator (Wheaton) and then centrifuged at 13 000 g at 4°C for 25 min. The supernatant was used to measure ATP using a commercially available kit (Promega) following the manufacturer’s instructions.
Chemicals
The hydrogen sulfide formulation (pharmaceutical name: IK-1001) was a pharmaceutical-grade aqueous solution of H2S produced and formulated to pH neutrality and isoosmolarity by Ikaria Inc. (Seattle, WA) using H2S gas (Matheson, Newark, CA) as a starting material. The pharmaceutical effects of this formulation have previously been characterized in multiple published studies (Bengtsson et al. 2008; Elrod et al. 2007; Esechie et al. 2008; Insko et al. 2008; Leviten et al. 2008; Simon et al. 2008; VandenEckart et al. 2008.). As H2S is chemically present in different forms under physiological pH in vivo (H2S and HS−) we used the term ‘sulfide’ to collectively define all these species. The sealed bottles containing the H2S formulation were freshly opened prior to each experiment and diluted in Krebs’ solution to the desired concentration and used immediately. All other chemicals were purchased from Sigma. Atropin, lidocaine, indomethacin, L-NMMA and HOE140 were diluted to working concentration in Krebs’ solution, while glibenclamide and DIDS were diluted in dimethyl sulfoxide (DMSO). DMSO didn’t reach higher concentration than 0.5% in the baths and at this concentration it had no effect on relaxation (data not shown).
Statistical analysis
All values are reported as mean±sem with n representing the number of experimental animals per group. Statistical analysis was performed using one-way ANOVA and Tukey post-hoc tests using Graphpad Prism 4.03 statistical software. Values greater than two standard deviations outside the mean were considered statistical outliers.
RESULTS
Characterization of the relaxations of the rat thoracic aortic rings to H2S
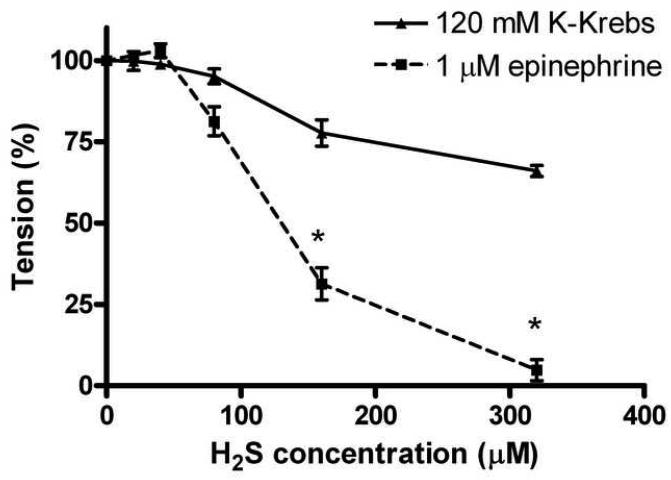
In the first set of our experiments relaxations to increasing concentrations of H2S showed marked differences, depending on the precontracting agent used (depolarizing potassium vs. epinephrine). The relaxant effect of sulfide was concentration-dependent and pronounced when 1 μM epinephrine was used, while the relaxations were weak in the case of K-Krebs precontraction. At the highest H2S concentration used (320μM) the maximal relaxation was 34±2 % for the K-Krebs contracted rings whereas for the epinephrine-contracted rings it was 95±3% (Fig. 1). It is noteworthy that at low H2S concentrations (20-40μM) a small, non-significant tendency to contraction was observed, when epinephrine (but not when potassium-depolarization) was used for precontraction.
Fig 1.
Concentration-dependent, H2S induced relaxations in rat thoracic aortic rings precontracted with either 1uM epinephrine or 120mM K-Krebs buffer. Relaxations were more pronounced after 1μM epinephrine (n=26) and reached near total relaxation at 320μM, albeit small contractions were observed at 20-40μM. H2S caused only partial relaxation after K-Krebs (n=6). Data expressed as mean ± SEM, *P <0.05 compared to K-Krebs induced relaxation.
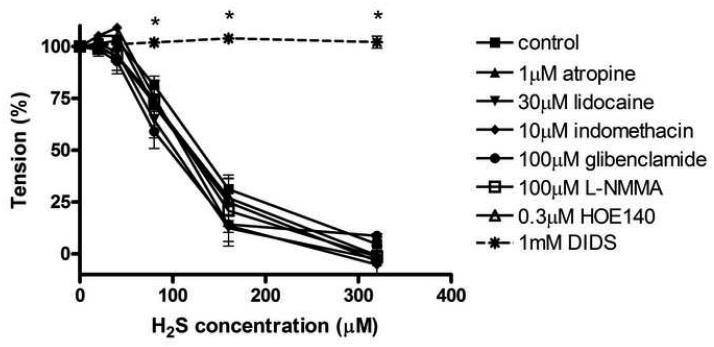
In the experiments where several inhibitors of various potential vasorelaxant pathways were tested on the sulfide-induced relaxations, none of the inhibitors tested had significant effect on the H2S concentration-response curve, At the highest sulfide concentration used (320μM), sulfide caused a relaxation of 95±3% in the presence of no pharmacological agents, whereas in the presence of atropine (1μM), lidocaine (30μM), indomethacin (10μM), L-NMMA (100μM) or HOE140 (0.3μM) pretreatment, the relaxations amounted to were, respectively:, 97±7%, 98±2%, 95±10%, 99±3% and 99±11%. When the KATP channel inhibitor glibenclamide (100μM) was tested, the relaxations also remained unaffected 91±2%. However, when DIDS (1mM), the inhibitor of Cl−/HCO3− channels was used, relaxations were abolished (2±3%).
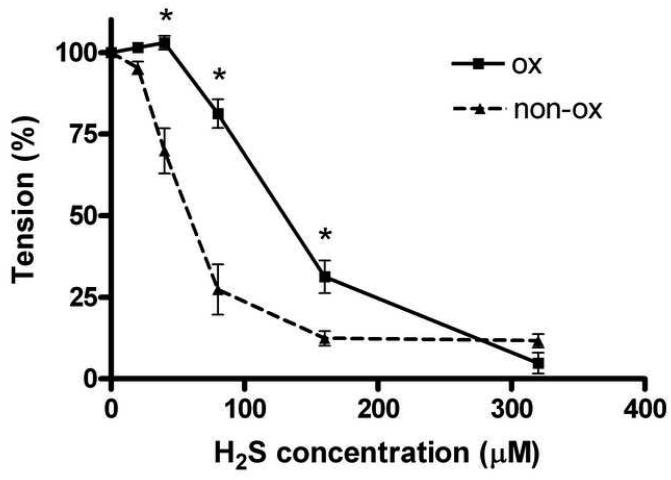
Experiments conducted in the presence of different oxygen tensions demonstrated that sulfide’s vasorelaxant effect is dependent on the ambient oxygen concentration. The concentration-response curve was shifted to the left when the organ bath did not receive oxygen. However, the maximal relaxations in the presence vs. the absence of oxygen remained the same: 95±3%, 88±2%, respectively (Fig. 3).
Fig 3.
Relaxations to H2S in rat thoracic aortic rings precontracted with 1uM epinephrine in the presence of 95%O2/5%CO2 oxygenation (ox) or in its absence (non-ox). The H2S concentration-response curve shifted to the left when the organ baths did not receive oxygen supply. The maximal relaxations for oxygenated (n=26) and non-oxygenated (n=11) groups remained the same. Data expressed as mean ± SEM, *P <0.05 vs. non-ox.
Potential metabolic inhibitory mechanisms of sulfide-induced relaxations
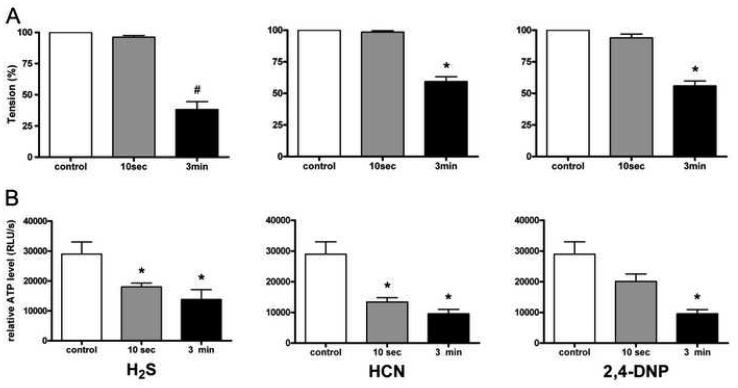
The time-course of the relaxations to sulfide, HCN and 2,4-DNP was comparable: at 10 sec after the administration of the respective agents, vascular tone was reduced by 4±1%, 2±1% and 6±3%, respectively, and reached significant (P<0.001) degrees of relaxation by 3 minutes after their administration, amounting to 62±6%, 41±4% and 44±4%, respectively (Fig. 4A). At this time point H2S caused a significantly more pronounced degree of vasorelaxation than HCN or 2,4-DNP (p<0.01). The levels of ATP measured in the rings after 320 μM H2S, HCN and 2,4-DNP showed similar patterns: a strong tendency for a decrease at 10 seconds and a pronounced decrease in ATP levels by 3 minutes (Fig. 4B). Therefore sulfide and the agents affecting mitochondria exerted similar effects on the vascular tone, as well as on the ATP content of the vascular rings.
Fig 4.
Vascular tension (A) and ATP content (B) in rat thoracic aortic rings precontracted with 1 uM epinephrine in control conditions and various times after treatment with H2S (left panels), HCN (middle panels) or 2,4-DNP (right panels). (A) H2S, HCN, 2,4-DNP caused distinct relaxations H2S causing the more pronounced one at 3 minutes #p<0.001 vs. control and <0.01 vs. HCN at 3min and 2,4-DNP at 3min, *P <0.001 vs. control. (B) ATP levels decreased as the relaxations developed after administration of H2S, HCN and 2,4 DNP (all 320 μM). Data expressed as mean ± SEM, *P <0.001 vs. control, n=6 in all groups.
DISCUSSION
In the present study we examined the effect of H2S on the tone of precontracted rat aortic vascular rings and found that sulfide induces concentration-dependent relaxations except for at low concentrations in the presence of oxygen, in which case a slight vasoconstriction was observed, which converted into vasorelaxation at higher sulfide concentrations. We have noted that the relaxant effect of sulfide was dependent on the precontractile agent used with the relaxations being less pronounced when depolarizing potassium solution was applied, and more pronounced in the case of epinephrine. We have also observed that the relaxations are dependent on the oxygen tension, with the relaxations being more distinct at lower ambient oxygen tension. We have been unable to inhibit the relaxant effect of sulfide by inhibiting vascular prostaglandin synthesis, nitric oxide synthesis, bradykinin receptors or ATP-dependent potassium channels. However, a pharmacological inhibitor of the vascular Cl−/HCO3− channels completely blocked the sulfide-induced relaxations. We have also noted striking similarities between the effects of sulfide and two agents affecting mitochondrial oxidative phosphorylation, both in terms of vascular tone, as well as in terms of vascular ATP content.
After many years of investigation, the mechanisms of vascular actions of H2S are still unclear. Initially H2S was shown to induce relaxation responses in rat aorta and mesentery artery in vitro (Cheng et al. 2004; Hosoki et al. 1997; Zhao et al. 2001). In contrast, other experiments (Dombkowski et al. 2005; Olson 2005) have shown that H2S has a potency to contract the rat aorta, while it exhibited both contractile and relaxant activities in rat pulmonary artery. The method of precontraction seems to be important, as the abovementioned studies used different agents at different concentrations. Indeed, our own results also confirmed the importance of the way of precontraction. The vasorelaxations appear to involve endothelium-dependent and independent mechanisms, and a number of publications attribute the relaxant effect, at least in part, to the activation of ATP-sensitive potassium (KATP) channels. However, the relaxations to H2S are only partially inhibited by the KATP channel inhibitor glibenclamide, and in some cases no inhibition was achieved at all (Cheng et al. 2004; Zhao et al. 2001).
In our study we investigated a number of possibilities to characterize the sulfide-induced relaxations. The used concentrations of sulfide were in line with the previous studies and has been shown to be relevant in physiological and pathophysiological situations (Ali et al. 2006; Cheng et al. 2004; Li et al. 2005; Zhao et al. 2001). Our first results showed that relaxations to 20-320μM H2S after 120mM K-Krebs were not as effective as in the case of 1μM epinephrine. These findings support the possibility that mechanism of relaxation by H2S may be connected to K+-channels. However, the relaxations were not completely blocked, which implicates other possible mechanisms. Our findings confirm the earlier report (Zhao et al. 2001) showing that precontraction using 100mM KCl containing Krebs blocks the relaxations to H2S – even though in our case only partial inhibition occurred.
Next, we investigated several inhibitors affecting the vascular L-arginine-nitric oxide pathway, because some of the effects of H2S have been ascribed to nitric oxide (Lefler et al. 2006; Zhao et al. 2001). H2S was found to potentiate the expression of inducible NO synthase (iNOS) following stimulation with interleukin-1β in cultured rat vascular smooth muscle cells (Jeong et al. 2006), while H2S was found to inhibit the expression of iNOS in RAW264.7 macrophages stimulated with lipopolysaccharide (Oh et al. 2006). Sulfide was also reported to directly inhibit endothelial NOS (Kubo et al. 2007). Recently published work also showed that H2S and NO forms a nitrosothiol compound that may modulate the biological activity of both sulfide and NO (Whiteman et al. 2006). We used the isoform-nonselective NO synthase inhibitor NG-methyl-L-arginine (L-NMMA), and found no effects of this inhibitor on the relaxations in the current experimental system. Atropine was used to test the potential of an intra-vascular cholinergic vasorelaxant pathway, whereas the bradykinin receptor antagonist HOE140 (Gorlach and Wahl 1996) was used to test the possibility of an autocrine bradykinin-mediated relaxant pathway. None of these had an effect on concentration-effect curve of H2S.
We have also investigated the possibility of prostaglandins in the vasodilatatory effect of H2S. Relaxations in the presence of the cyclooxygenase inhibitor, 10μM indomethacin did not show any difference compared to control. Although it was shown recently that indomethacin could inhibit H2S induced vasoconstriction at lower O2 levels (Koenitzer et al. 2007) we did not find significant effect on vasoconstriction either, the reason for which could be the pronounced O2 sensitivity of this inhibiting effect demonstrated by the same authors.
The KATP channel inhibitor glibenclamide failed to inhibit relaxations to H2S in our experiments. We used high concentration (100μM) of this inhibitor, because in our earlier pilot studies we have tested the effects of glibenclamide at smaller (10, 50 μM), more KATP channel specific concentrations, but we did not find any inhibition at any of the concentrations tested (data not shown). The use of glibenclamide at concentrations higher than 10 μM, causes non-specific actions, such as inhibition of Na+-K+ pumps, L-type Ca2+ channels and CFTR (cystic fibrosis transmembrane regulator) Cl− channels (Sheppard and Robinson 1997), therefore the failure of glibenclamide at this concentration also means that these pumps and channels do not take part in the vasorelaxant effect of H2S. The reasons for the failure of glibenclamide to affect the vasorelaxant effect of H2S are not clear. Some earlier reports also found that glibenclamide failed to affect the contractile and relaxant activity of NaHS (Kubo et al. 2007; Teague et al. 2002). These results open the possibility that in certain conditions other mechanisms than KATP channels play important role in the vasorelaxation of H2S.
1mM DIDS completely inhibited relaxations to H2S in our experiments. DIDS is an inhibitor of the Cl−/HCO3− exchanger, which has a role in the intracellular pH regulation in vascular smooth muscle cells and DIDS was shown to partially block H2S mediated pH decrease (Lee et al. 2007). It is possible that in certain metabolic situations this decrease in pH increases KATP currents which leads to relaxation (Ishizaka and Kuo 1996). The decrease in pH was only partially blocked by DIDS and the remaining amount cannot be accounted for the weak acidic property of H2S, because the concentration of H2S used would exhibit only very small pH change in vascular smooth muscle cells (Lee et al. 2007). DIDS affects the mitochondrial inner membrane anion channel as well (Beavis and Davatol-Hag 1996), but this channel is implicated in mitochondrial related ROS production. Therefore, we feel that it is unlikely that this mechanism can significantly contribute to the relaxations. Inhibition of ROS production could lead to increased NO-level in the tissue, but NO was shown using L-NMMA not to take part in the vasorelaxant effects of H2S.
Lowered oxygen levels shifted to the left the concentration–response curve for H2S related vasorelaxations which is in line with earlier observations (Koenitzer et al. 2007). In addition to these results, we have shown that the maximal relaxation remained the same in both situations. One explanation for this phenomenon could be that sulfide competes with oxygen at the level of cytochrome c, therefore the vasorelaxant effect of sulfide is less pronounced in the presence of higher ambient oxygen concentration. This oxygen dependency would suggest that H2S acts differently on the pulmonary vascular tone and it might have a role in pulmonary hypertension (Zhang et al. 2003). Clearly, ambient oxygen level modulates the effect of H2S. Further studies on the underlying mechanism are warranted.
The current results demonstrate for the first time that H2S decreases ATP levels in vascular tissues. The patterns and time courses of sulfide, HCN and 2,4-DNP were comparable both in terms of vascular tone and vascular ATP concentrations. The rapid fall of the ATP level shows that ATP utilization maintaining contraction is very high in the vessels and production is very low. Based on the earlier results that H2S blocks cytochrome c oxidase (Hill et al. 1984; Khan et al. 1990) we hypothesize that H2S changes the metabolic state of the cell to switch to anaerobic glycolysis which leads to energy deficit and intracellular pH decrease. In fact, it was shown recently that above 80μM H2S concentration O2 consumption decreases in rat aorta (Koenitzer et al. 2007). In other studies metabolic inhibition has been shown to reduce ATP levels by about a third in rabbit mesenteric artery and by 59% in uterine smooth muscle (Post and Jones 1991; Wray 1990). The possibility for H2S to take part in metabolic regulation makes sense from an evolutionary standpoint, as it once played oxygen's molecular part in metabolism when oxygen was scarce. In recent studies it was shown that H2S inhibited cardiac mitochondrial respiration and had cardioprotective effects, at least in part via metabolic inhibition (Elrod et al. 2007; Pan et al. 2006). It is possible that effects of H2S differ in ischaemic tissue, where metabolism is compromised and this possibility needs further investigation.
CONCLUSION
Based on the current and recent published results, we presume that H2S leads to vascular relaxation via mechanisms involving metabolic inhibition, intracellular pH and the Cl−/HCO3− channels. We hypothesize that the inhibition of cytochrome c oxidase leads to loss of mitochondrial ATP generation, thus energy deficit and intracellular acidosis ensue, which in turn activates Cl−/HCO3− channels and the combination of these effects leads to relaxation. The interactions of these mechanisms depend on the oxygen levels of the tissue. Clearly, the vascular effects reported here may underlie the toxic pathological vasodilatory effects of sulfide, which may be relevant, for instance for the pathophysiology of hydrogen sulfide inhalation toxicology. In addition, as hydrogen sulfide is viewed as a physiological vasorelaxant, the current results open the intriguing possibility that a cytochrome c oxidase/cellular energetics/cellular pH-related mechanism may serve as a physiological mechanism for the regulation of vascular tone.
Fig 2.
Effect of 1μM atropine, 30μM lidocaine, 10μM indomethacin, 100μM glibenclamide, 100μM L-NMMA, 0.3μM HOE140 and 1mM DIDS on H2S induced relaxations in rat thoracic aortic rings precontracted with 1uM epinephrine. DIDS achieved a complete inhibition on relaxations after 1μM epinephrine. None of the other inhibitors tested, including glibenclamide had any significant effects on the relaxations. Data expressed as mean ± SEM, n=6-20/group *P <0.05 vs. control.
ACKNOWLEDGEMENTS
This study was supported by a grant from Ikaria Inc. The authors thank Mr. Paul Hill (Ikaria, Seattle, WA) for preparing and supplying IK-1001, the H2S formulation used in this study.
Footnotes
Conflict of interest statement: C.S. is an officer and shareholder of IKARIA, a for-profit organization involved in the commercial development of hydrogen sulfide.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ali MY, Ping CY, Mok YY, Ling L, Whiteman M, Bhatia M, Moore PK. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? British Journal of Pharmacology. 2006;149:625–634. doi: 10.1038/sj.bjp.0706906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavis AD, Davatol-Hag H. The mitochondrial inner membrane anion channel is inhibited by DIDS. Journal of Bioenergetics and Biomembranes. 1996;28:207–214. doi: 10.1007/BF02110652. [DOI] [PubMed] [Google Scholar]
- Bengtsson A, Johnson J, Hill P, Mulligan J, Leviten D, Insko M, Mebel E, Vertz P, VandenEkart E, Szabo C, Deckwerth T, Wintner EA. Use of mono-bromo-bimane to derivatize sulfide in whole blood: comparison of blood sulfide levels during atmospheric hydrogen sulfide exposure and intravenous sulfide infusion. Experimental Biology meeting abstracts. 2008 Abstract #749.15. [Google Scholar]
- Bhatia M, Sidhapuriwala J, Moochhala SM, Moore PK. Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. British Journal of Pharmacology. 2005;145:141–144. doi: 10.1038/sj.bjp.0706186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. The FASEB Journal. 2005;19:623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. American Journal of Physiology-Heart and Circulatory Physiology. 2004;287:H2316–2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- Dombkowski RA, Russell MJ, Schulman AA, Doellman MM, Olson KR. Vertebrate phylogeny of hydrogen sulfide vasoactivity. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2005;288:R243–252. doi: 10.1152/ajpregu.00324.2004. [DOI] [PubMed] [Google Scholar]
- Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proceedings of the National Academy of Sciences U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esechie A, Kiss L, Olah G, Horvath EM, Hawkins H, Szabo C, Traber DL. Protective effect of hydrogen sulfide in a murine model of acute lung injury induced by combined burn and smoke inhalation. Clinical Science. 2008;115:91–97. doi: 10.1042/CS20080021. [DOI] [PubMed] [Google Scholar]
- Gorlach C, Wahl M. Bradykinin dilates rat middle cerebral artery and its large branches via endothelial B2 receptors and release of nitric oxide. Peptides. 1996;17:1373–1378. doi: 10.1016/s0196-9781(96)00223-9. [DOI] [PubMed] [Google Scholar]
- Hill BC, Woon TC, Nicholls P, Peterson J, Greenwood C, Thomson AJ. Interactions of sulphide and other ligands with cytochrome c oxidase. An electron-paramagnetic-resonance study. Biochemical Journal. 1984;224:591–600. doi: 10.1042/bj2240591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochemical and Biophysical Research Communications. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- Insko MA, Deckwerth T, Szabo C, Toombs C. Detection of exhaled sulfide gas: a potential in vivo, continuous monitoring technique for therapeutic sulfide donor compounds. Experimental Biology meeting abstracts. 2008 Abstract #749.8. [Google Scholar]
- Ishizaka H, Kuo L. Acidosis-induced coronary arteriolar dilation is mediated by ATP-sensitive potassium channels in vascular smooth muscle. Circulation Research. 1996;78:50–57. doi: 10.1161/01.res.78.1.50. [DOI] [PubMed] [Google Scholar]
- Jeong SO, Pae HO, Oh GS, Jeong GS, Lee BS, Lee S, Kim du Y, Rhew HY, Lee KM, Chung HT. Hydrogen sulfide potentiates interleukin-1beta-induced nitric oxide production via enhancement of extracellular signal-regulated kinase activation in rat vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 2006;345:938–944. doi: 10.1016/j.bbrc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Kamoun P. Mental retardation in Down syndrome: a hydrogen sulfide hpothesis. Medical Hypotheses. 2001;57:389–392. doi: 10.1054/mehy.2001.1377. [DOI] [PubMed] [Google Scholar]
- Khan AA, Schuler MM, Prior MG, Yong S, Coppock RW, Florence LZ, Lillie LE. Effects of hydrogen sulfide exposure on lung mitochondrial respiratory chain enzymes in rats. Toxicology and Applied Pharmacology. 1990;103:482–490. doi: 10.1016/0041-008x(90)90321-k. [DOI] [PubMed] [Google Scholar]
- Koenitzer JR, Isbell TS, Patel HD, Benavides GA, Dickinson DA, Patel RP, Darley-Usmar VM, Lancaster JR, Jr., Doeller JE, Kraus DW. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. American Journal of Physiology-Heart and Circulatory Physiology. 2007;292:H1953–1960. doi: 10.1152/ajpheart.01193.2006. [DOI] [PubMed] [Google Scholar]
- Kubo S, Kurokawa Y, Doe I, Masuko T, Sekiguchi F, Kawabata A. Hydrogen sulfide inhibits activity of three isoforms of recombinant nitric oxide synthase. Toxicology. 2007;241:92–97. doi: 10.1016/j.tox.2007.08.087. [DOI] [PubMed] [Google Scholar]
- Lee SW, Cheng Y, Moore PK, Bian JS. Hydrogen sulphide regulates intracellular pH in vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 2007;358:1142–1147. doi: 10.1016/j.bbrc.2007.05.063. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Parfenova H, Jaggar JH, Wang R. Carbon monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation. Journal of Applied Physiology. 2006;100:1065–1076. doi: 10.1152/japplphysiol.00793.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviten D, Mulligan J, Bengtsson A, Insko M, Mebel E, VandenEckart E, Vertz P, Toombs C, Wintner EA, Szabo C, Deckwerth TL. Distinguishing endogenous and exogenous sulfide by mass spectroscopy. Experimental Biology meeting abstracts. 2008 Abstract #1154.2. [Google Scholar]
- Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. The FASEB Journal. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- Mok YY, Atan MS, Yoke Ping C, Zhong Jing W, Bhatia M, Moochhala S, Moore PK. Role of hydrogen sulphide in haemorrhagic shock in the rat: protective effect of inhibitors of hydrogen sulphide biosynthesis. British Journal of Pharmacology. 2004;143:881–889. doi: 10.1038/sj.bjp.0706014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson RA, Roth SH, Zhang A, Zheng J, Brookes J, Skrajny B, Bennington R. Inhibition of respiratory and bioenergetic mechanisms by hydrogen sulfide in mammalian brain. Journal of Toxicology and Environmental Health. 1998;54:491–507. doi: 10.1080/009841098158773. Part A. [DOI] [PubMed] [Google Scholar]
- Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim HR, Jeon SB, Jeon WK, Chae HJ, Chung HT. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radical Biology & Medicine. 2006;41:106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Olson KR. Vascular actions of hydrogen sulfide in nonmammalian vertebrates. Antioxidants & Redox Signaling. 2005;7:804–812. doi: 10.1089/ars.2005.7.804. [DOI] [PubMed] [Google Scholar]
- Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. Journal of Molecular and Cellular Cardiology. 2006;40:119–130. doi: 10.1016/j.yjmcc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Post JM, Jones AW. Stimulation of arterial 42K efflux by ATP depletion and cromakalim is antagonized by glyburide. American Journal of Physiology. 1991;260:H848–854. doi: 10.1152/ajpheart.1991.260.3.H848. [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Robinson KA. Mechanism of glibenclamide inhibition of cystic fibrosis transmembrane conductance regulator Cl-channels expressed in a murine cell line. The Journal of Physiology. 1997;503(Pt 2):333–346. doi: 10.1111/j.1469-7793.1997.333bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon F, Giudici R, Duy CN, Schelzig H, Oter S, Groger M, Wachter U, Vogt J, Speit G, Szabo C, Radermacher P, Calzia E. Hemodynamic and metabolic effects of hydrogen sulfide during porcine ischemia/reperfusion injury. Shock. 2008 Feb 21; doi: 10.1097/SHK.0b013e3181674185. in press. [DOI] [PubMed] [Google Scholar]
- Smith RP, Gosselin RE. Hydrogen sulfide poisoning. Journal of Occupational Medicine. 1979;21:93–97. doi: 10.1097/00043764-197902000-00008. [DOI] [PubMed] [Google Scholar]
- Szabo C. Hydrogen sulphide and its therapeutic potential. Nature Reviews. Drug Discovery. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- Teague B, Asiedu S, Moore PK. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. British Journal of Pharmacology. 2002;137:139–145. doi: 10.1038/sj.bjp.0704858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandenEckart E, Bengtsson A, Johnson J, Mulligan J, Leviten D, Insko M, Mebel E, Vertz P, Toombs C, Wintner EA, Szabo C, Deckwerth TL. Bioequivalence between inhaled hydrogen sulfide and intravenously administered sodium sulfide. Experimental Biology meeting abstracts. 2008 Abstract #749.16. [Google Scholar]
- Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochemical and Biophysical Research Communications. 2006;343:303–310. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2008;294:R1930–1937. doi: 10.1152/ajpregu.00025.2008. [DOI] [PubMed] [Google Scholar]
- Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313:22–27. doi: 10.1016/j.bbrc.2003.11.081. [DOI] [PubMed] [Google Scholar]
- Wray S. The effects of metabolic inhibition on uterine metabolism and intracellular pH in the rat. The Journal of Physiology. 1990;423:411–423. doi: 10.1113/jphysiol.1990.sp018030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Du J, Bu D, Yan H, Tang X, Si Q, Tang C. [The regulatory effect of endogenous hydrogen sulfide on hypoxic pulmonary hypertension] Beijing Da Xue Xue Bao. 2003;35:488–493. [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. The EMBO Journal. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]