Abstract
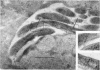
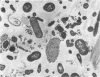
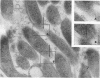
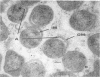
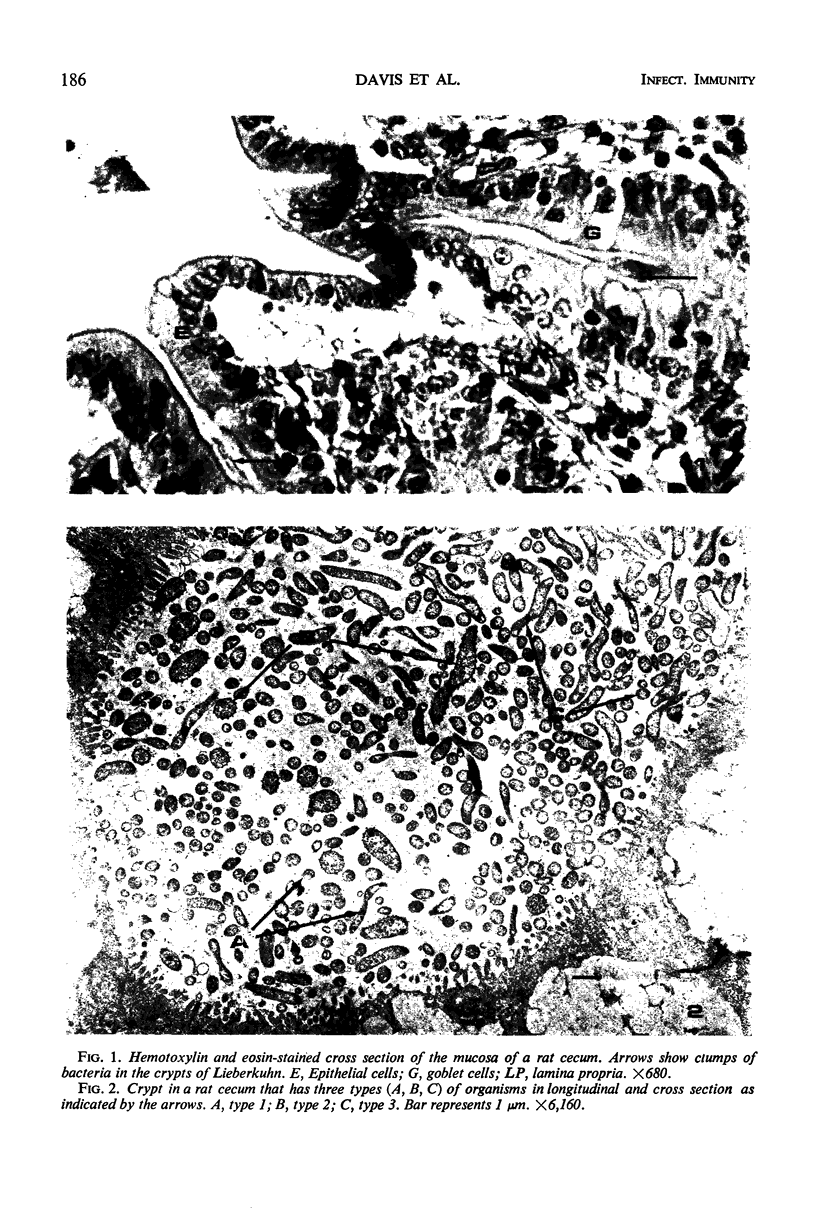
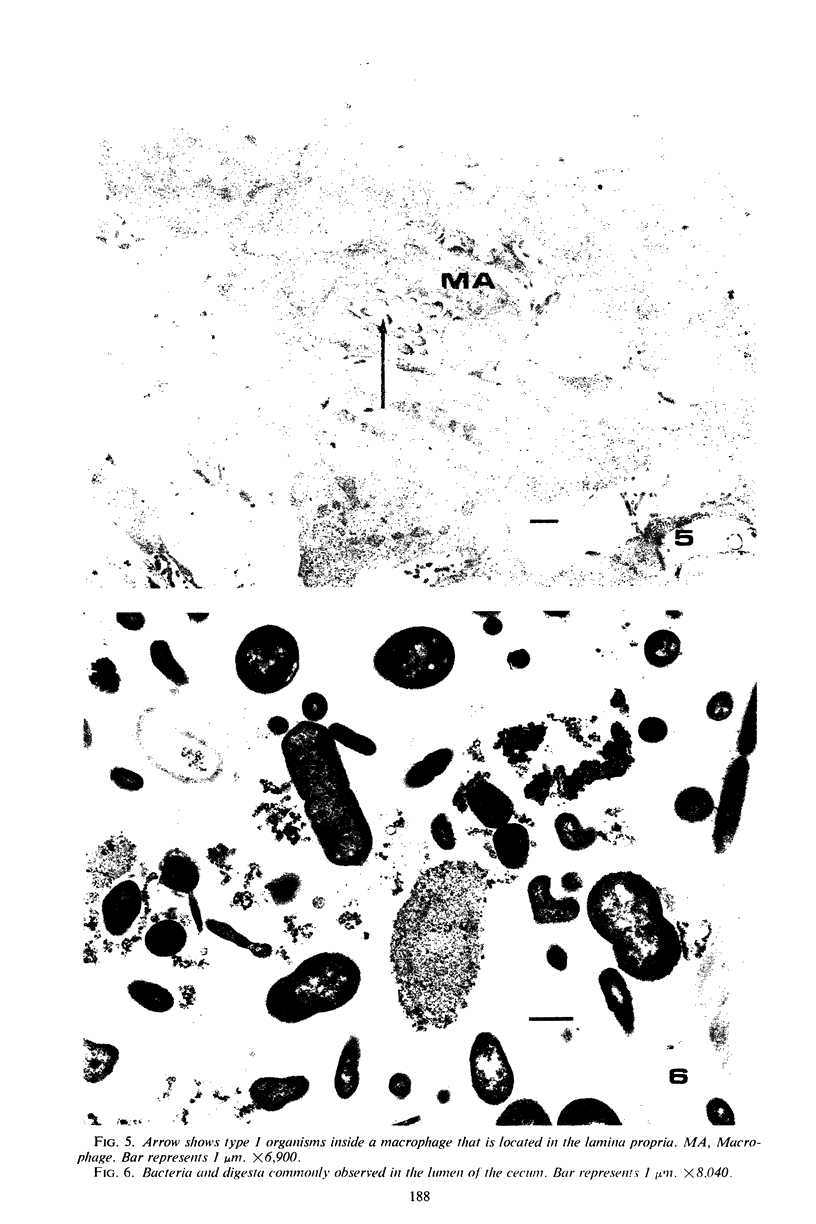
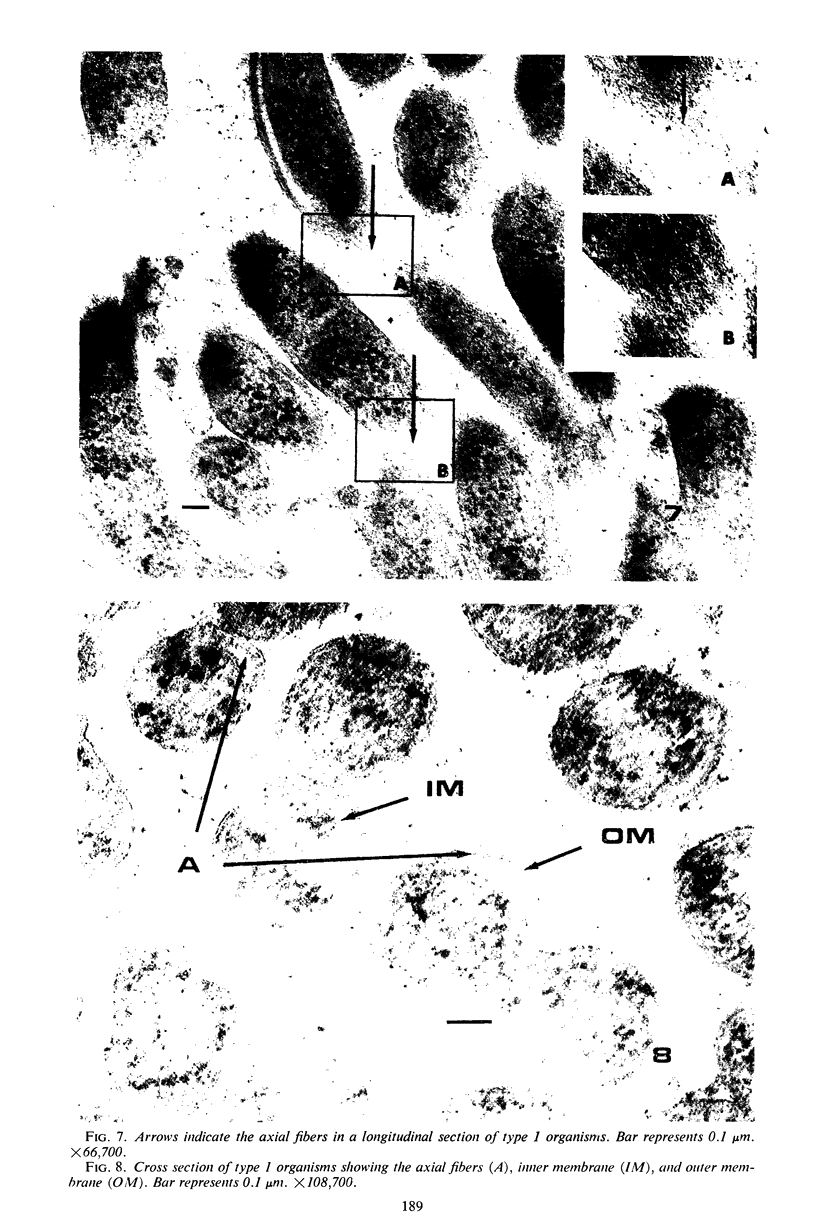
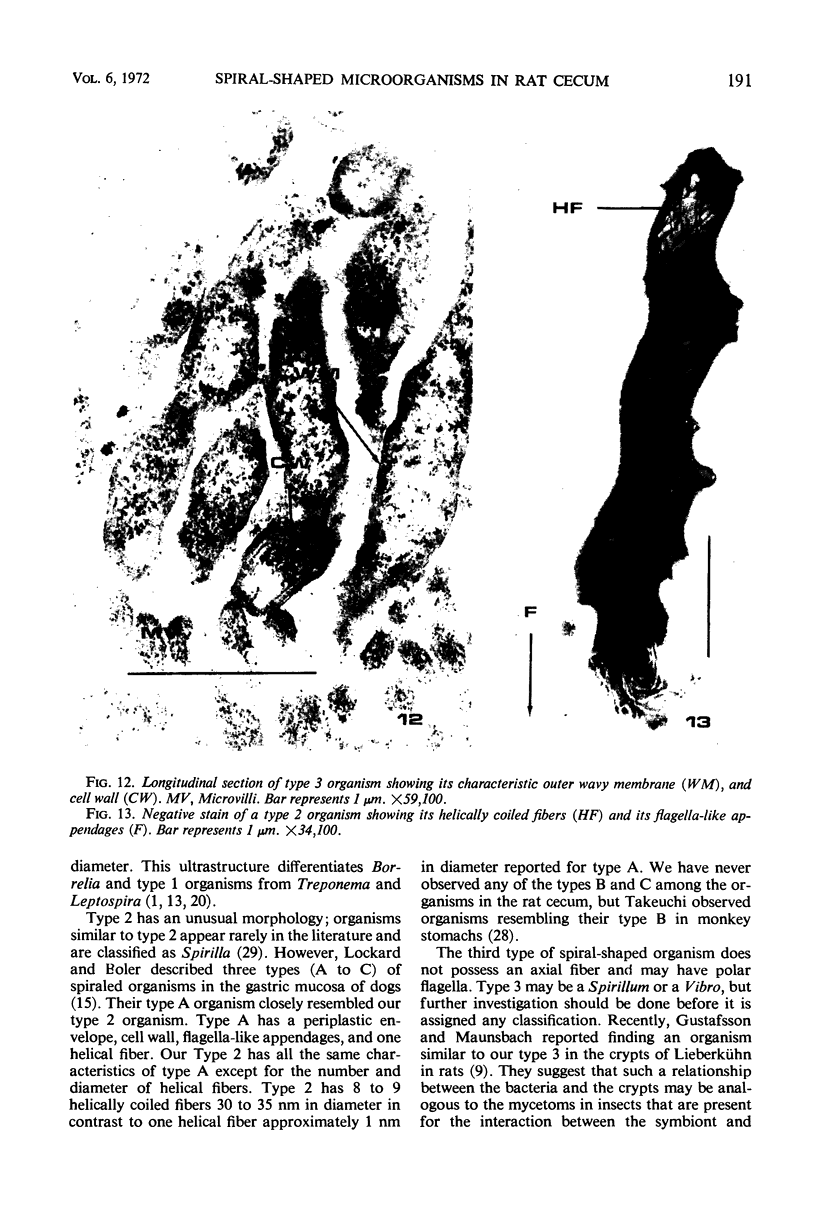
Some indigenous microorganisms have been shown to localize in certain anatomical sites of the digestive tract of mammals. We studied the ceca of normal adult rats by light and electron microscopy to determine whether any specific bacterial population localizes in this area. All rats studied showed that the crypt was packed with organisms whose morphological character differs from those of the cecal lumen. Organisms localized in the crypt were often identified topographically close to the microvilli of the epithelial cells. These organisms could be differentiated into three types according to their characteristic ultrastructure. Type 1 was a thin spiral-shaped microbe that resembled a Borrelia. Type 2 possessed helically coiled fibers and flagella-like appendages. Type 3 was spiral-shaped but lacked axial fibers. Types 1 and 2 were both capable of penetrating through the crypt epithelium into the lamina propria where they were found in either phagocytes or extracellular locations. These observations are discussed in relation to other host-microflora localization patterns.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUBOS R., SCHAEDLER R. W., COSTELLO R., HOET P. INDIGENOUS, NORMAL, AND AUTOCHTHONOUS FLORA OF THE GASTROINTESTINAL TRACT. J Exp Med. 1965 Jul 1;122:67–76. doi: 10.1084/jem.122.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Gordon H. A., Pesti L. The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol Rev. 1971 Dec;35(4):390–429. doi: 10.1128/br.35.4.390-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. H., Dubos R. The anaerobic bacterial flora of the mouse cecum. J Exp Med. 1970 Aug 1;132(2):251–260. doi: 10.1084/jem.132.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B. E., Maunsbach A. B. Ultrastructure of the enlargec cecum in germfree rats. Z Zellforsch Mikrosk Anat. 1971;120(4):555–578. doi: 10.1007/BF00340589. [DOI] [PubMed] [Google Scholar]
- LISTGARTEN M. A., SOCRANSKY S. S. ELECTRON MICROSCOPY AS AN AID IN THE TAXONOMIC DIFFERENTIATION OF ORAL SPIROCHETES. Arch Oral Biol. 1965 Jan-Feb;10:127–138. doi: 10.1016/0003-9969(65)90064-6. [DOI] [PubMed] [Google Scholar]
- LISTGARTEN M. A., SOCRANSKY S. S. ELECTRON MICROSCOPY OF AXIAL FIBRILS, OUTER ENVELOPE, AND CELL DIVISION OF CERTAIN ORAL SPIROCHETES. J Bacteriol. 1964 Oct;88:1087–1103. doi: 10.1128/jb.88.4.1087-1103.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Gordon J., Dubos R. Enumeration of the oxygen sensitive bacteria usually present in the intestine of healthy mice. Nature. 1968 Dec 14;220(5172):1137–1139. doi: 10.1038/2201137a0. [DOI] [PubMed] [Google Scholar]
- Lee A., Gordon J., Lee C. J., Dubos R. The mouse intestinal microflora with emphasis on the strict anaerobes. J Exp Med. 1971 Feb 1;133(2):339–352. doi: 10.1084/jem.133.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockard V. G., Boler R. K. Ultrastructure of a spiraled microorganism in the gastric mucosa of dogs. Am J Vet Res. 1970 Aug;31(8):1453–1462. [PubMed] [Google Scholar]
- Loeschke K., Gordon H. A. Water movement across the cecal wall of the germfree rat. Proc Soc Exp Biol Med. 1970 Apr;133(4):1217–1222. doi: 10.3181/00379727-133-34657. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWAIN R. H. Electron microscopic studies of the morphology of pathogenic spirochaetes. J Pathol Bacteriol. 1955 Jan-Apr;69(1-2):117–128. doi: 10.1002/path.1700690117. [DOI] [PubMed] [Google Scholar]
- Savage D. C., Dubos R., Schaedler R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968 Jan 1;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., McAllister J. S. Cecal enlargement and microbial flora in suckling mice given antibacterial drugs. Infect Immun. 1971 Feb;3(2):342–349. doi: 10.1128/iai.3.2.342-349.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., McAllister J. S., Davis C. P. Anaerobic bacteria on the mucosal epithelium of the murine large bowel. Infect Immun. 1971 Oct;4(4):492–502. doi: 10.1128/iai.4.4.492-502.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER A. F., SCHMITTDIEL E. F. Electron microscopic and bacteriologic studies of spirilla isolated from the fundic stomachs of cats and dogs. Am J Vet Res. 1962 May;23:422–427. [PubMed] [Google Scholar]