Abstract
Social isolation in the pre-stroke environment leads to poorer outcomes after an ischemic injury in both animal and human studies. However, the impact of social isolation following stroke, which may be more clinically relevant as a target for therapeutic intervention, has yet to be examined. In this study, we investigated both the sub-acute (2 weeks) and chronic (7 weeks) effects of social isolation on post-stroke functional and histological outcome. Worsened histological damage from ischemic injury and an increase in depressive-like behavior was observed in isolated mice as compared to pair-housed mice. Mice isolated immediately after stroke showed a decrease in the levels of brain-derived neurotrophic factor (BDNF). These changes, both histological and behavioral, suggest an overall negative effect of social isolation on stroke outcome, potentially contributing to post-stroke depression and anxiety. Therefore, it is important to identify patients who have perceived isolation post-stroke to hopefully prevent this exacerbation of histological damage and subsequent depression.
Keywords: Social isolation, middle cerebral artery occlusion, Brain Derived Neurotrophic Factor, forced swim test
1. Introduction
Stroke is the fourth leading cause of death and the primary cause of long-term adult disability in the United States (American Stroke Association, 2012). Stroke affects physical, cognitive, and social functioning. Perceived social isolation contributes to mortality and morbidity in patients with cerebrovascular disease, but the underlying mechanisms for this are unknown [1-3]. Pre-stroke social isolation leads to poor functional and cognitive recovery in humans, and has been linked to an increased risk of post-stroke depression (PSD) and post-stroke anxiety (PSA) [4-7]. PSA occurs with PSD in approximately 25% of isolated stroke patients [8, 9]. Stroke patients report higher perceived social isolation after stroke than age-matched healthy individuals [10], which is thought to contribute to the higher recurrent stroke rates seen in isolated individuals [4].
Importantly, the detrimental effects of social isolation can be modeled in animals. Isolation prior to an induced stroke exacerbates histological ischemic damage in rodents [11-13], an effect mediated in part by an increased pro-inflammatory signaling via NF-ĸB [13]. However, since many “isolated” patients are not identified until they come to medical attention after a stroke has occurred, assessing the effects of social isolation after stroke on outcome is critical to translational efforts targeting social factors in stroke recovery.
Across 51 clinical studies, approximately one-third of stroke survivors are diagnosed with post-stroke depression (PSD) [14]. Depression is associated with higher morbidity and mortality [15], greater disability, and poorer recovery after stroke [16-18]. Depression correlates with markers of inflammation such as interleukin-6 (IL-6) and C reactive protein (CRP) in humans [19]. The detrimental effects of pre-stroke isolation in animal models has been linked to an enhanced neuroinflammatory response to injury and elevations in IL-6 but the link between social behavior, inflammation, and depression remains unclear [13, 20, 21]. Much less is known regarding the contribution and involvement of these pro-inflammatory signaling pathways in isolation that occurs after stroke.
Neurotrophins play an important role in depression and are emerging as a possible role depression following stroke [22-25]. Hippocampal levels of brain-derived neurotrophic factor (BDNF) clinically correlated with mood elevation after pharmacological treatment for depression in humans [26] and in rodents [27, 28]. BDNF is a known regulator of neuronal plasticity and neurogenesis [29] in addition to regulating behavioral and cellular metabolic responses to environmental stimuli [30] and environmental enrichment [31], and is higher in pair housed than in isolated rodents [21, 32]. BDNF is also known to be neuroprotective after experimental brain injury [30] and enhances synaptic plasticity and functional outcome after MCAO [33]. Therefore BDNF may play a role in both PSD and repair after ischemic injury. While the effects of pair-housing mice prior to an induced stroke leads to a positive impact on stroke recovery and infarct size in the stroke animal, the nature of the behavioral interactions induced by the partner have not yet been explored [13]. Living with an unwell individual as opposed to a healthy caretaker may affect stroke recovery and mood in both the patient and the caretaker. We investigated whether there was a benefit to housing with a healthy partner in our post-stroke isolation design.
This study investigates the interaction of post-stroke social isolation on histological outcomes, functional recovery, and depressive phenotypes. BDNF levels in brain, serum inflammatory markers, motor function, and depression-like and anxiety-like behavior in mice isolated after experimental stroke were examined.
2. Methods
2.1. Experimental Animals
Eight-week-old male C57BL/6 mice (23-27 g) were obtained from Harlan Laboratories, Inc (IN). Mice were housed in a temperature controlled room (74 ± 2 °F) with ad-libitium access to food and water and maintained under a 12-hour light/dark cycle (lights on at 7:00 AM). Experimental procedures were conducted during the light phase. After arrival and acclimation for one week, mice were group-housed for one week and then pair-housed. Depending on the experimental group, mice were randomly assigned to remain pair-housed (PH) or were housed individually (socially isolated: “ISO”) after surgery (Figure 1). All experimental procedures were conducted in accordance with protocols approved by the Animal Care and Use Committee of the University of Connecticut Health Center. All behavioral testing was performed by an investigator blinded to housing condition.
Figure 1. Experimental timeline.
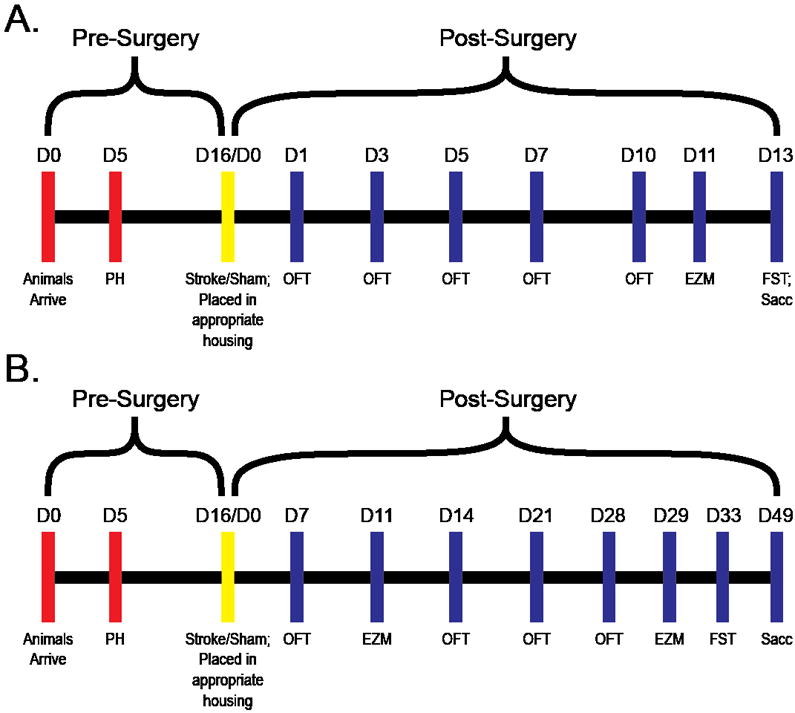
A) In experiment 1animals were pair-housed (PH) (D5), underwent surgery (D16/D0), were placed into experimental housing conditions (D16/D0), and underwent behavioral testing depicted by the blue hash marks. At the end of experiment 1 animals were sacrificed (D13). B) In experiment 2 animals underwent a similar housing and surgery procedure; however, animals underwent weeks of behavioral testing and were sacrificed on D49. OF = Open Field Test, EZM = Elevated Zero Maze, FST = Forced Swim Test, Sacc = animals were sacrificed.
2.2. Middle Cerebral Artery Occlusion Model
Focal transient cerebral ischemia was induced by 60 minutes of reversible right middle cerebral artery occlusion (MCAO) under Isofluorane anesthesia followed by reperfusion as described previously [34, 35]. Sham animals underwent the same procedure but the suture was not advanced into the MCA. During surgery and ischemia, rectal temperature was monitored with a Monotherm system (V WR LabShop, Batavia, IL, USA) and maintained at approximately 37 °C with an automated temperature control feedback system. Cerebral blood flow reduction of >80% of baseline after suture insertion was confirmed in all stroke animals by Laser Doppler Flowmetry (Moor Instruments). All animals, both stroke and sham, were administered 0.2 mL saline subcutaneously day 0-5 post-stroke to ensure survival and had free access to wet mashed food.
2.3. Experiment 1
In order to assess the impact of stoke and social isolation on anxiety- and depression-like behaviors, mice were randomized and subjected to a transient stroke (ST) or a sham surgery (SH). Immediately after surgery, mice were assigned one of six groups using 2 × 3 experimental design with stroke condition, sham (SH) or stroke (ST) as the first between-subjects factor and post-stroke housing condition, housed with sham (SH), housed with stroked (ST) for the healthy partner studies, or housed in isolation (ISO) as the second between-subjects factor. Thus, the six groups were: SH/SH (n= 10), SH/ST (n= 10), SH/ISO (n= 8), ST/SH (n= 10), ST/ST (n= 10), ST/ISO (n= 10). Mice remained in these housing conditions throughout the experiment.
The mice were then subject to behavioral testing by a blinded investigator. The open field test (OFT) was administered on days 1, 3, 5, 7, and 10 post-stroke. On day 11, the elevated zero maze (EZM) test was conducted, followed by the forced swim test (FST) on day 13. Mice were sacrificed on day 13, two hours after the FST (Figure 1A).
2.4. Experiment 2
Experiment 2 was designed to assess long-term depression- and anxiety-like behavior after stroke. The same experimental procedures used in Experiment 1 were used. Behavior on the EZM was assessed on day 11 post-stroke and the FST was conducted on day 33 post-stroke. Animals were sacrificed on day 49. The experimental design was identical to that in experiment 1, and the experimental groups were as follows: SH/SH (n= 8), SH/ST (n= 9), SH/ISO (n= 10), ST/ST (n= 9), ST/SH (n= 8), ST/ISO (n= 8) (Figure 1B). Mice remained in these housing conditions throughout the experiment.
2.5. Neurological Scoring
Neurological deficit scores (NDS) were obtained on days 0-5 post MCAO surgery and after behavioral tests using a five point scoring scale. The scoring used was as follows: 0, no deficit; 1, forelimb weakness and torso turning to the ipsilateral side when held by tail; 2, circling to affected side; 3, unable to bear weight on affected side; and 4, no spontaneous locomotor activity or barrel rolling as described previously [36].
2.6. Behavioral Testing
Mice were acclimated to the testing room in their home cages 1 hour before behavioral testing. All apparatuses were wiped with 70% ethanol between animals. All testing was performed at the same time of day to avoid circadian variations in activity.
2.6.1. Open Field Test
General locomotor activity and anxiety-like behavior was assessed with the OFT Mice were placed in the front right corner of a clear acrylic box (16” × 16”) and allowed to explore the box for 20 minutes. Locomotor activity was quantified as the total number of beam breaks by a computer operated PAS Open Field system (San Diego Instruments, San Diego, CA). The percentage of beam breaks in the center zone (13” × 13”) was used as a measure of anxiety-like behavior [37]. Tests were administered at several timepoints to view trends without hindrance by habituation [38].
2.6.2. Elevated Zero Maze
The EZM was also used to assess anxiety-like behavior. The maze consisted of a circular track divided into four equal sections with two open quadrants and two closed quadrants enclosed by 6”-tall walls (San Diego Instruments, Inc, San Diego, CA). Mice were placed at the entry way of a closed arm and allowed to explore the maze for 5 minutes. Time spent in the open arms and number of entries into the open arms were recorded by an observer and a video camera. Anxiety-like behavior was determined by the percent of time spent in the open arms [39]. The EZM is a variant of the elevated plus maze. However it eliminates the ambiguity of the open/closed area at the center of the elevated plus maze [39].
2.6.3. Forced Swim Test
To assess depression-like behavior, mice were placed in an open glass cylinder (diameter: 10 cm, height: 25 cm), which was filled to a height of 15 cm with water (25 ± 1 °C) for 6 minutes. Sessions were recorded by a video camera. Time spent immobile was later scored by a blinded observer. Immobility was defined as floating passively in the water, performing only movements necessary to remain afloat. After the 6 minute session the mice were removed from the water, dried off with a paper towel, and returned to their home cages [40]. Immobility was assessed during last four minutes of the six-minute trial as previously described [41].
2.7. Histological Assessment
Mice were anesthetized with a 0.1mL/10g body weight dose of avertin (Sigma) dissolved in 2-Methyl-2-Butanol. Animals were perfused transcardially with cold phosphate-buffered saline followed by 4% paraformaldehyde. The brain was then removed from the skull, post-fixed for 24h, and subsequently placed in cyroprotectant (30% sucrose) for a minimum of three days prior to sectioning. The brain tissue was cut into 30-μm free-floating coronal sections on a freezing microtome. Every eighth slice was stained using cresyl violet (CV) for evaluation of ischemic cell damage [42]. Images were acquired by a charge-coupled device camera (QImaging, Surrey, BC, Canada) and analyzed using Sigmascan Pro5 (Systat Software Inc., Chicago, IL, USA) as previously described [43]. Infarct volumes were expressed as a percentage of the contralateral hemisphere to correct for edema (Swanson’s method) [44, 45].
2.8. Immunofluorescence
Alternating (every other) thirty micron sections (between 1.70mm and -0.82mm from Bregma) were slide mounted and incubated in blocking solution as previously described [43]. Briefly, sections were incubated overnight with rabbit anti-BDNF (1:1000; Millipore) and subsequently incubated for 60 min with a fluorescein-conjugated anti-rabbit secondary antibody. The slides were then dipped in DAPI nuclear stain solution (1:1000; Invitrogen) for 5 min. Images were then visualized using an inverted light Zeiss axiovert fluorescence microscope. BDNF positive cells were counted in a pre-specified region of the ipsilateral striatum (approximately 2.5mm laterally and 2.5mm ventrally from the midsagittal line) and cortical region (approximately 3mm laterally and 2 mm ventrally from midsagittal line) by a blinded investigator in four sections per mouse in 6 mice per group.
2.9. ELISA
Serum was collected at harvest, and interleukin-6 (IL-6) (eBiosciences, San Diego, CA) and C-reactive protein (CRP) levels (Life Diagnostics, Inc., West Chester, PA) were assessed by ELISA per the manufacturer’s instructions.
2.10. Statistical Analysis
All data, except FST, are presented as means ± SEM. FST is presented as mean with maximum and minimum. Effects were considered statistically significant at p ≤ .05. EZM, and FST data were analyzed using a 2 × 3 analysis of variance [19] with surgery and housing condition as between subject factors. A 2 × 3 × 5 repeated-measures ANOVA was used for the OFT with day post-stroke as a repeated measure. Neurological deficit scores were analyzed using the Freidman test, which is a non-parametric alternative to a repeated-measures ANOVA. All statistical analyses were conducted using SPSS Statistical Software 16.0 (SPSS, 2011).
When there was no statistical difference between groups, data was collapsed for convenience to the reader (i.e. all sham (SH/SH, SH/ST, SH/ISO) groups were collapsed into a single “Sham” group). Often, the ST/ST group was an intermediate between the ST/SH and ST/ISO group, so the ST/ST and ST/SH groups were combined as a single “Pair-Housed” group to show the effect of isolation. There are no error bars in the OFT for convenience of visualization of trends within and between groups.
3. Results
3.1. Open Field
In experiment 1, there was no significant effect of stroke or housing, and no significant interaction between stroke and housing condition on overall locomotor activity in the OFT [F (1, 49) = .45, p=.50, F (2, 49) = .41, p=.66, and F (2, 49) = .22, p=.80, respectively] (Figure 2A). There was no significant main effect of stroke or housing, and no significant interaction between stroke and housing on anxiety-like behavior [F (1, 49) = .54, p=.47; F (2, 49) = .14, p=.87, and F (2, 49) = 1.46, p=.24, respectively] (Figure 2C).
Figure 2. Open Field Testing.
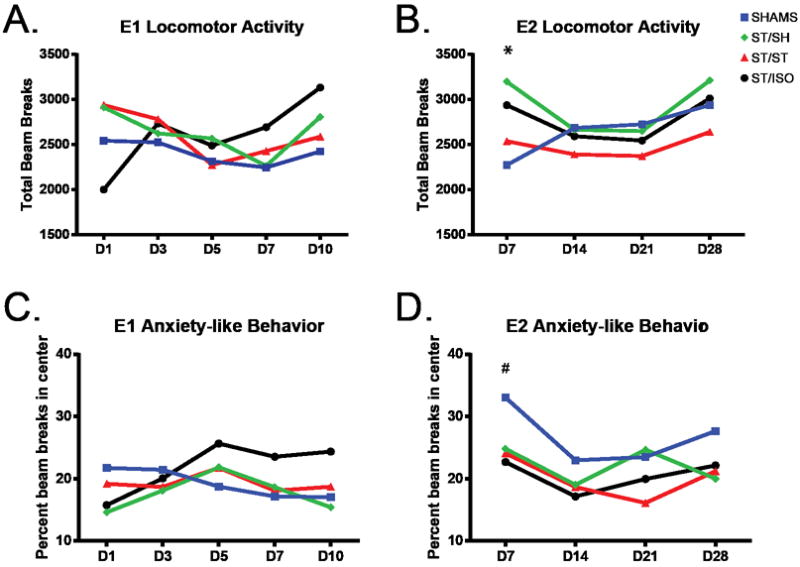
Locomotor activity, measured by the total number of beam breaks the animal makes in the enclosed area, in experiment 1 (A) showed that all groups have similar activity by Day 3 of testing. Anxiety-like behavior in experiment 1 (C), measured by the percent of beam breaks in the center, the lower the percentage the more anxiety-like the behavior, was similar among all groups. Locomotion in experiment 2 (B) was consistent among all groups, except for day 7 post-stroke, where all stroke mice showed an increase in locomotor activity compared to shams (* p<.001). Anxiety-like behavior in experiment 2 (D) was increased in all stroke groups compared to shams on day 7, (# p=.002). There are no error bars in the OFT for convenience of visualization of trends within and between groups.
On chronic assessment in experiment 2, we found no significant main effect of stroke, and no significant interaction between stroke and housing on overall locomotor activity [F (1,44)=.11, p=.75; F (2,44)=.59, p=.56, respectively]. However there was a significant effect of housing [F (2,44)=.3.72, p=.032] (Figure 2B). A Tukey post-hoc test for housing showed a significant decrease in locomotor activity of mice that lived with a stroke animal versus a sham animal (p=.024). Within-subject analysis showed a significant increase in locomotor activity of stroke mice on day 7 compared to shams [F (3,132)=6.91, p<.001]. This reflects post-stroke hyperactivity which has been seen previously in stroke mice [46], which then normalizes to the sham mice by day 14. Assessment of anxiety-like behavior showed a significant main effect of stroke [F (1,44)=10.61, p=.002], where stroke mice exhibited increased anxiety-like behavior compared to shams. There was no significant effect of housing or the interaction between stroke and housing on anxiety-like behavior [F (2,44)=1.51, p=.23; F (2,44)=.37, p=.69, respectively] (Figure 2D).
3.2. Elevated Zero Maze
In experiment 1, EZM testing showed no significant main effect of stroke and no significant interaction between stroke and housing condition on locomotor activity [F (2, 52) = .04, p=.85 and F (2, 52) = .32, p=.73 respectively]. However, there was a significant effect of housing [F (2, 52) = 5.17, p=.001]. A pairwise comparison showed that the mice that were pair-housed with a sham made significantly fewer entries into the open arms than mice that were pair-housed with a stroke, (p= .01), and with mice living in isolation (p <.01) (Figure 3A). Overall, locomotor activity assessed by the EZM was lower in mice housed with a sham, yet we observed that locomotor activity was not affected by stroke and is consistent with the locomotor activity seen in the OFT.
Figure 3. Elevated Zero Maze.
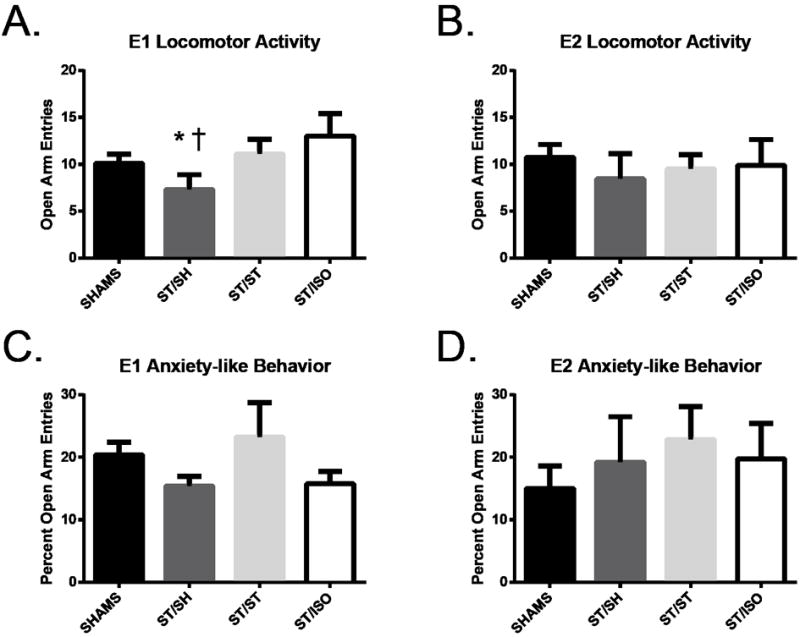
On Day 11, mice housed with a sham showed less locomotor activity on the EZM when compared to mice who lived with a stroke († p=.01) and mice who were socially isolated (* p<.001) in experiment 1 (A). In experiment 2, all mice showed similar locomotor activity (C). Anxiety-like behavior expressed as percentage of time spent in the open arms, the lower the percentage the more anxiety-like the behavior, was similar among all stroke groups in experiment 1 (B) and experiment 2 (D).
In experiment 2, there was no significant effect of stroke or housing, and no significant interaction between stroke and housing on percent of time spent in the open arms, [F (1, 52) = .61, p=.45, F (2, 52) = 1.44, p=.44, F (2, 52) = .39, p=.67 respectively] (Figure 3C). Thus, there was no difference in anxiety-like behavior between groups.
Trends seen in locomotor activity as assessed by EZM in experiment 1 were also seen in experiment 2, however there was no significant effect of stroke, housing and the interaction between stroke and housing [F (1,46)=.64, p=.429; F(2,46)=.51, p=.603; F(2,46)=.69, p=.51, respectively] (Figure 3B). There was also no statistical significance of stroke, housing and the interaction between housing and surgery when comparing the percent time spent in the open arms [F (1,46)=.04, p=.843; F (2,46)=3.42, p=.041; F (2,46)=2.58, p=.087, respectively] (Figure 3D). Tukey’s post-hoc analysis on housing showed no statistical significance between groups.
3.3. Forced Swim Test
In experiment 1, there was a main effect of stroke on mobility in the FST, [F (1, 49) = 8.83, p < .01]. There was significantly less immobility in stroke mice than in sham mice (data not shown), consistent with the hyperactivity seen in previous tests [43]. There was no main effect of housing, [F (2, 49) = .91, p = .41], but a significant interaction between stroke and housing was observed in mobility in the FST [F (2, 49) = 3.70, p < .05]. Using a two-tailed independent variable t-test, there was a significant difference between stroke animals that were PH (ST/SH and ST/ST groups) and isolated stroke animals (ST/ISO), p =.02 (Figure 4A).
Figure 4. Forced Swim Test.
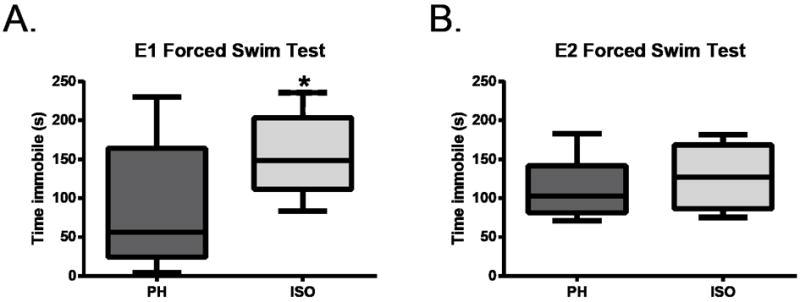
Plots are means with the whiskers being minimum and maximum. On day 13 in experiment 1, isolated stroke mice (ISO) spent more time immobile than stroke mice that were pair-housed (PH), which includes both ST/ST and ST/SH groups, p=.02 (A). However, on day 33 in experiment 2, all stroke mice, isolated and pair-housed, spent a similar amount of time immobile (B).
Assessment of immobility in experiment 2 (Figure 4B) showed no main effect of housing or interaction between surgery and housing on immobility [F (2, 44) = 1.03, p = .37and F (2, 44) = 1.60, p = .21 respectively]. Yet, there was an observed significant effect of stroke on FST (data not shown), with immobility being greater in the stroke groups compared to the sham groups [F (1, 44) = 4.17, p = .05], suggesting deficits were mediated by stroke rather than housing manipulations.
3.4. Histology
At 13 days post stroke, the ischemic damage had evolved into either stable infarcts, glial scarring, or in some cases the tissue had begun to atrophy (Figure 5). Due to these multi-faceted outcomes, the volume of infarct or damage is difficult to analyze quantitatively. However, qualitative analysis shows that ST/SH and ST/ST infarcts are stable, whereas ST/ISO brains have more atrophy and enhancement of glial scarring leading to the formation of necrotic cysts in 60% of the brains. ST/SH had no necrotic cysts and ST/ST brains only had cysts 25% of the time.
Figure 5. Cresyl violet staining of stroke brains at 13 days post-stroke (top panel) and 49 days post-stroke (bottom panel).
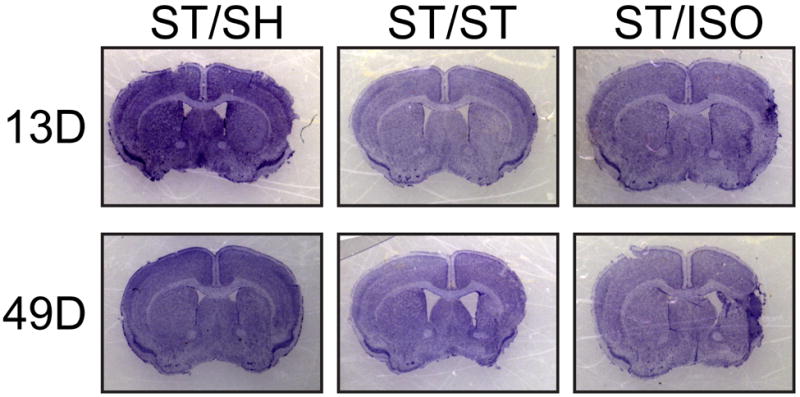
ST/ISO brains at 13 days post-stroke developed necrotic cysts, whereas pair-housed animals still have infarct regions. At 49 days post-stroke, ST/SH brains have mild tissue and ventricle loss whereas ST/ST brains have moderate tissue loss and ventricular hypertrophy. ST/ISO brains have ventricular hypertrophy and necrotic cysts.
At 49 days post-stroke, ST/SH brains show less infarct and atrophy than ST/ST brains, which had moderate atrophy, while ST/ISO brains demonstrated both enhanced atrophy as the glial scar walled off necrotic cysts (Figure 5).
Immunofluorescence for BDNF showed no difference between groups at day 13 post-stroke (data not shown). However, at day 49 post-stroke, BDNF immunofluorescence showed that ST/SH and ST/ST brains have significantly higher levels of BDNF in the ipsilateral cortical tissue when compared to ST/ISO cohorts [F (2, 20) =16 p<.01]. ST/SH and ST/ST also have significantly higher levels of BDNF in the ipsilateral striatum when compared to the ST/ISO striatum [F (2,19)=3.91 p<.05] (Figure 6).
Figure 6. Brain-derived neurotrophic factor (BDNF) Immunohistochemistry.
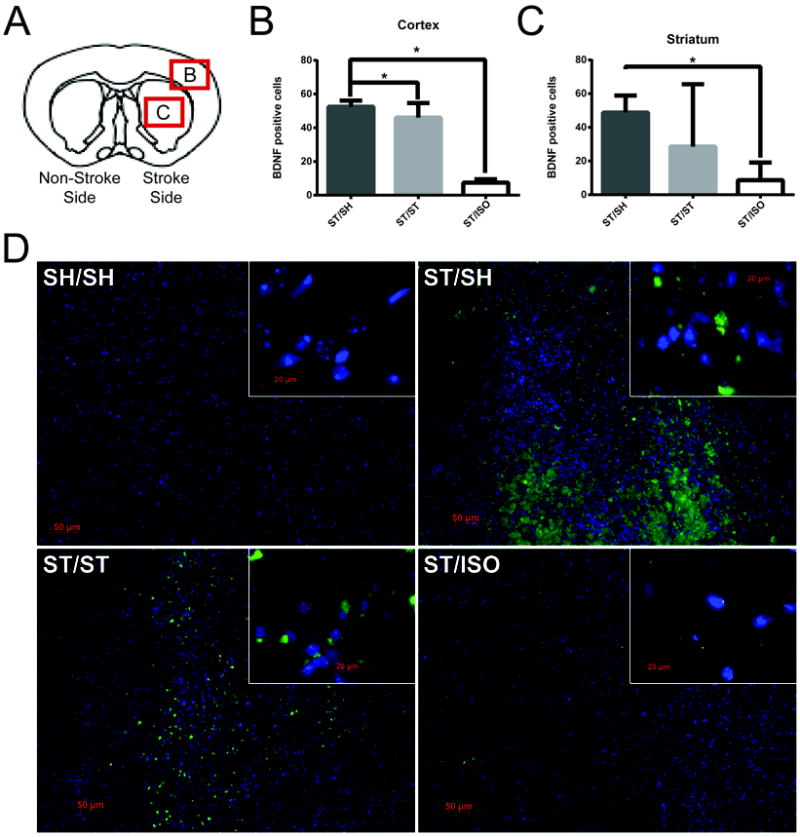
Four slices from each brain were stained and quantified in the region of 1.70mm to -0.82mm from Bregma. BDNF positive cells were counted in the infarcted cortical region (box labeled B, approximately 3mm laterally and 2 mm ventrally from midsagittal line), and the infarcted striatum (box labeled C, approximately 2.5mm laterally and 2.5mm ventrally from the midsagittal line), in all stroke animals (ST/SH, ST/ST, ST/ISO) at 20x (n=6 per group) (A). Quantification shows that there are significantly less BDNF positive cells in the cortex of ST/ISO mice compared to ST/SH and ST/ST (** p <0.001) showing the detrimental effects of isolation on BDNF expression (B). In the striatum there are significantly less BDNF positive cells in ST/ISO than ST/SH (* p<0.05) (C). The differences between groups can be visualized in these micrographs of the striatum (D), where BDNF is represented by green, and DAPI marks the nucleus in blue.
3.5. ELISA
At 13 days post-stroke, there was no effect of surgery or housing in serum IL-6 [F (1,12=1.3, p=.28; F(2,12)=1.5, p=2.6, respectively] or C-reactive protein (CRP) [F (1,21)=3.18, p=.09; F(2,21)=.019, p=.981, respectively] (data not shown).
3.6. Neurological Deficit Scores and Mortalities
For experiment 1, ST/SH (χ2(2)=11.3, p=.004) and ST/ST (χ2(2)=8.2, p=.017) groups recovered faster than the ST/ISO group (χ2(4)=10.0, p=.04) in terms of their neurological deficit score. Similarly, in experiment 2, ST/SH (χ2(1)=6.0, p=.014) and ST/ST (χ2(6)=13.3, p=.038) recovered more quickly than the ST/ISO group (χ2(9)=18.5, p=.03).
Mortality was highest in the ST/ISO groups in both experiments (30% in experiment 1; 27% in experiment 2) compared with ST/ST (17% in experiment 1; 11% in experiment 2) and ST/SH (0% in both experiments) supporting the results seen in previous studies [47].
4. Discussion
Pre-stroke isolation is detrimental to stroke outcome in both clinical [1] and experimental rodent models [12, 13, 48]. PSD and PSA have been linked to pre-stroke isolation in stroke patients when compared to age matched controls [5, 6]. In this work, consistent with reports in the literature we found that experimental ischemia can induce hyperactivity and anxiety [46]. We found significant effects of stroke on depressive-like phenotypes in the FST, and anxiety like behavior in the OFT. However, few studies have investigated the influence of social isolation after a stroke on depressive-like behavior in experimental models which was the focus of this work. In the present study, we examined the individual and combined effects of stroke and post-stroke social environment on depression-like and anxiety-like behavior in mice. Different stroke social environment paradigms were created by housing mice with a healthy sham partner, an unhealthy stroke partner, or alone in social isolation.
After the acute treatment period, post-stroke care can be given by either a healthy caretaker at home, or the patient can go to a rehabilitation center where he/she often rooms with another unwell patient. It is unknown if the interaction with the caretaker/partner is responsible for the behavioral benefits seen in stroke animals when they are pair housed, and a more detailed assessment of the type of interaction between the pair housed mice is necessary. Therefore, we designed this experiment to separate pair-housed mice into two categories: living with a healthy partner, or living with an unhealthy (stroke) partner to better understand the nature of the potential “social support” given by the partner.
Because of the high prevalence of post-stroke depression in post-stroke patients, it is important to delineate which patients are most susceptible to depression so providers can prevent its occurrence [5]. While there has been a correlation between social isolation and depression, this experiment aimed to elucidate the importance of “perceived” isolation. While a stroke victim may at a nursing home receiving multiple interactions, the quality of those interactions may be lacking, which can lead to perceived isolation. Analysis which demonstrated no effect between stroke mice housed with a healthy partner and stroke mice housed with an unhealthy partner were collapsed into one group, termed “pair-housed”.
By testing locomotor activity in the OFT, we were able to ascertain that stroke mice in all cohorts were able to regain overall mobility by day 3. This finding allows us to be comfortable in assuming that mobility did not hinder our other behavioral assays. In the present study, anxiety-like behavior was increased in all stroke mice regardless of housing in experiment 2 in the OFT. However, neither stroke nor housing condition after stroke affected anxiety-like behavior in the EZM, which is a much more sensitive test for assessing anxiety-like behavior than the OFT [49]. Therefore, we conclude that neither isolation nor partner’s heath affected measures of PSA, although OFT does show an effect of the stroke itself. Because PSA is seen more often in posterior circulation strokes in clinical populations, the MCAO model may not be ideal to induce these types of deficits [50].
At day 13 post-stroke, socially isolated stroke mice showed increased depression-like behavior by FST assessment when compared to stroke mice housed with a partner, regardless if the partner is healthy or unwell. This suggests that isolation induces depressive like phenotypes. As there was no “partner effect”, we collapsed these two groups into one “pair-housed” group for further analysis. Although all stroke mice exhibit greater mobility than their sham controls, we believe this is non-specific stroke – induced hyperactivity as has been previously reported in mice [46]. The more striking finding was the effect of social isolation in the FST, which suggests that isolation induces depressive-like behavior after stroke. This effect between housing conditions was not seen in isolated mice at day 33 post-stroke, perhaps due to more chronic recovery. However changes in BDNF were seen at day 33. It is possible that if animals were challenged the depressive phenotype would be unmasked, which will be evaluated in future studies. Importantly, mice from experiment 2 were not tested on day 13, so a direct comparison cannot be made between the two groups. This potentially “recovered” phenotype may be rodent specific, as mice, unlike humans, tend to recover from even large ischemic injuries very rapidly, a recognized limitation of rodent models. Further, other studies have shown an increase in immobility in socially isolated mice when compared to pair-housed mice that was only seen with repeated challenge, which could explain the lack of a difference in the FST on day 33 post-stroke [51].
While quantitative analysis of infarct and tissue damage was difficult to assess since ischemic damage progressed through different stages at different rates and was thus highly variable, qualitative analysis exhibited discrepancies in infarct stability and necrotic cyst development between groups. Socially isolated stroke mice formed necrotic cysts as early at 13 days, whereas stroke mice that lived with other mice still showed stable infarcts. At 49 days, necrotic cyst formation was seen in the stroke mice that lived with a cage-mate as well as socially isolated stroke mice. Further, when looking at the neurological deficit scores in both experiments, socially isolated stroke mice show a much slower recovery as compared with stroke mice housed with a partner, which could be attributed to their exacerbated histological damage. Additionally, socially isolated stroke mice were noted to have a high percentage of penile prolapses, suggesting a lack of self-grooming. These may be key attributes leading to increased mortality seen in the socially isolated group. It has been shown that physical contact appears to be necessary to see the complete beneficial effects of affiliative housing [52].
Clinical findings have shown that inflammatory markers such as IL-6 and CRP play an important role in depression [20]. While early post-stroke histological damage has been demonstrated to be inflammatory-mediated [11], we found no effect of surgery or housing on key inflammatory markers such as IL-6 and CRP at day 13 post-stroke. Therefore, for our chronic cohort we turned our attention from inflammation to BDNF as a potential mechanism for the improved behavioral recovery due to its involvement in depression, neurogenesis, and environmental enrichment (for a review, see [22, 23]. Because ST/SH have higher BDNF levels in the stroke hemisphere, this could be a potential target for preventing PSD and poor recovery. Studies in rats have shown that chronic antidepressant treatment with monoamine oxidase inhibitors, tricyclics and selective serotonin reuptake inhibitors (SSRIs), enhance neurogenesis [53]. Therefore, administration of an anti-depressant post-stroke may prevent PSD and enhance neurotrophic-mediated recovery in patients, especially in patients that are socially isolated after stroke.
Studies have emphasized the importance for individuals to seek out social support to decrease both the risk of stroke, and enhance recovery after a stroke has occurred [13, 54]. While previous studies have demonstrated the detrimental effects of isolation prior to an induced ischemic event, this is the first study to demonstrate that social support after a stroke also enhances recovery, improves histological outcomes, and increases neurotrophin levels. This suggests that the window for intervention may be wider for “social interventions” than for many other current acute stroke therapies. A longitudinal study of BDNF promoter methylation status showed that patients with higher methylation, which leads to lower production of BDNF, was independently associated with PSD [55]. Therefore, treating patients with an antidepressant post-stroke, especially if they are susceptible to perceived isolation, may influence stroke recovery. The translational relevance is high, and if the mechanisms underlying the behavioral benefits of social interaction can be determined, new strategies can be developed to enhance these pharmacologically.
5. Conclusion
The results of this study suggest that the timing of intervention post-stroke might be crucial to post-stroke recovery. While acute changes may be inflammatory-mediated, chronic recovery may be regulated through a different mechanism. Therefore, inflammatory effects should be targeted for development of acute treatments, whereas chronic treatments should involve the enhancement of BDNF. Whether treatment with anti-depressants can enhance BDNF when administered after injury, and potentially mitigate detrimental effects of isolation (or perceived isolation) will be the subject of future studies.
Highlights.
Isolated mice have exacerbated stroke-induced histological injury compared to pair housed animals
Locomotion across all stroke mice is equivalent by day 3 in the Open Field Test (OFT)
Isolation leads to increased immobility in the Forced Swim Test (FST) at 13 days post-stroke
Pair-housed animals have increased BDNF levels in stroke tissue at 49 days post-stroke compared to isolated cohorts
Acknowledgments
This work was supported by the NIH (NINDS RO1 NSO77769 to LDM) and the American Heart Association (Post-Doctoral Fellowship to VV).
Abbreviations
- MCAO
Middle cerebral artery occlusion
- ST
Stroke surgery
- SH
Sham surgery
- BDNF
Brain Derived Neurotrophic Factor
- SI or ISO
Social Isolation
- PH
Pair-Housed
- PSD
Post-stroke depression
- PSA
Post-stroke anxiety
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241(4865):540–5. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 2.Glass TA, et al. Impact of social support on outcome in first stroke. Stroke. 1993;24(1):64–70. doi: 10.1161/01.str.24.1.64. [DOI] [PubMed] [Google Scholar]
- 3.Venna VR, McCullough LD. “Won’t you be my neighbor?”: deciphering the mechanisms of neuroprotection induced by social interaction. Stroke. 2011;42(12):3329–30. doi: 10.1161/STROKEAHA.111.632570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boden-Albala B, et al. Social isolation and outcomes post stroke. Neurology. 2005;64(11):1888–92. doi: 10.1212/01.WNL.0000163510.79351.AF. [DOI] [PubMed] [Google Scholar]
- 5.Whyte EM, et al. Depression after stroke: a prospective epidemiological study. J Am Geriatr Soc. 2004;52(5):774–8. doi: 10.1111/j.1532-5415.2004.52217.x. [DOI] [PubMed] [Google Scholar]
- 6.Ouimet MA, Primeau F, Cole MG. Psychosocial risk factors in poststroke depression: a systematic review. Can J Psychiatry. 2001;46(9):819–28. doi: 10.1177/070674370104600905. [DOI] [PubMed] [Google Scholar]
- 7.Astrom M, Adolfsson R, Asplund K. Major depression in stroke patients. A 3-year longitudinal study. Stroke. 1993;24(7):976–82. doi: 10.1161/01.str.24.7.976. [DOI] [PubMed] [Google Scholar]
- 8.Starkstein SE, et al. Relationship between anxiety disorders and depressive disorders in patients with cerebrovascular injury. Arch Gen Psychiatry. 1990;47(3):246–51. doi: 10.1001/archpsyc.1990.01810150046008. [DOI] [PubMed] [Google Scholar]
- 9.Barker-Collo SL. Depression and anxiety 3 months post stroke: prevalence and correlates. Arch Clin Neuropsychol. 2007;22(4):519–31. doi: 10.1016/j.acn.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Ebrahim S, Barer D, Nouri F. Use of the Nottingham Health Profile with patients after a stroke. J Epidemiol Community Health. 1986;40(2):166–9. doi: 10.1136/jech.40.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craft TK, et al. Social interaction improves experimental stroke outcome. Stroke. 2005;36(9):2006–11. doi: 10.1161/01.STR.0000177538.17687.54. [DOI] [PubMed] [Google Scholar]
- 12.Karelina K, et al. Social contact influences histological and behavioral outcomes following cerebral ischemia. Exp Neurol. 2009;220(2):276–82. doi: 10.1016/j.expneurol.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Venna VR, et al. NF-kappaB contributes to the detrimental effects of social isolation after experimental stroke. Acta Neuropathol. 2012;124(3):425–38. doi: 10.1007/s00401-012-0990-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hackett ML, et al. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36(6):1330–40. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- 15.Noel PH, et al. Depression and comorbid illness in elderly primary care patients: impact on multiple domains of health status and well-being. Ann Fam Med. 2004;2(6):555–62. doi: 10.1370/afm.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chemerinski E, Robinson RG, Kosier JT. Improved recovery in activities of daily living associated with remission of poststroke depression. Stroke. 2001;32(1):113–7. doi: 10.1161/01.str.32.1.113. [DOI] [PubMed] [Google Scholar]
- 17.Bogousslavsky J. William Feinberg lecture 2002: emotions, mood, and behavior after stroke. Stroke. 2003;34(4):1046–50. doi: 10.1161/01.STR.0000061887.33505.B9. [DOI] [PubMed] [Google Scholar]
- 18.Ayerbe L, et al. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry. 2013;202(1):14–21. doi: 10.1192/bjp.bp.111.107664. [DOI] [PubMed] [Google Scholar]
- 19.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150(3):736–44. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Su S, et al. Common genetic contributions to depressive symptoms and inflammatory markers in middle-aged men: the Twins Heart Study. Psychosom Med. 2009;71(2):152–8. doi: 10.1097/PSY.0b013e31819082ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry A, et al. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology. 2012;37(6):762–72. doi: 10.1016/j.psyneuen.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Nestler EJ, et al. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 23.Duric V, Duman RS. Depression and treatment response: dynamic interplay of signaling pathways and altered neural processes. Cell Mol Life Sci. 2013;70(1):39–53. doi: 10.1007/s00018-012-1020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thakker-Varia S, Alder J. Neuropeptides in depression: role of VGF. Behav Brain Res. 2009;197(2):262–78. doi: 10.1016/j.bbr.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stuller KA, Jarrett B, DeVries AC. Stress and social isolation increase vulnerability to stroke. Exp Neurol. 2012;233(1):33–9. doi: 10.1016/j.expneurol.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Chen B, et al. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50(4):260–5. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 27.Altar CA, et al. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry. 2003;54(7):703–9. doi: 10.1016/s0006-3223(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Steven Richardson J, Li XM. Dose-related effects of chronic antidepressants on neuroprotective proteins BDNF, Bcl-2 and Cu/Zn-SOD in rat hippocampus. Neuropsychopharmacology. 2003;28(1):53–62. doi: 10.1038/sj.npp.1300009. [DOI] [PubMed] [Google Scholar]
- 29.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothman SM, et al. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann N Y Acad Sci. 2012;1264(1):49–63. doi: 10.1111/j.1749-6632.2012.06525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chourbaji S, et al. The impact of environmental enrichment on sex-specific neurochemical circuitries - effects on brain-derived neurotrophic factor and the serotonergic system. Neuroscience. 2012;220:267–76. doi: 10.1016/j.neuroscience.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Scaccianoce S, et al. Social isolation selectively reduces hippocampal brain-derived neurotrophic factor without altering plasma corticosterone. Behav Brain Res. 2006;168(2):323–5. doi: 10.1016/j.bbr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Cui X, et al. Therapeutic benefit of treatment of stroke with simvastatin and human umbilical cord blood cells: neurogenesis, synaptic plasticity, and axon growth. Cell Transplant. 2012;21(5):845–56. doi: 10.3727/096368911X627417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, et al. Effects of metformin in experimental stroke. Stroke. 2010;41(11):2645–52. doi: 10.1161/STROKEAHA.110.589697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longa EZ, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 36.Liu F, et al. Sex differences in caspase activation after stroke. Stroke. 2009;40(5):1842–8. doi: 10.1161/STROKEAHA.108.538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463(1-3):3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 38.Moy SS, et al. Preweaning sensorimotor deficits and adolescent hypersociability in Grin1 knockdown mice. Dev Neurosci. 2012;34(2-3):159–73. doi: 10.1159/000337984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulkarni SK, Singh K, Bishnoi M. Elevated zero maze: a paradigm to evaluate antianxiety effects of drugs. Methods Find Exp Clin Pharmacol. 2007;29(5):343–8. doi: 10.1358/mf.2007.29.5.1117557. [DOI] [PubMed] [Google Scholar]
- 40.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327–36. [PubMed] [Google Scholar]
- 41.Venna VR, et al. PUFA induce antidepressant-like effects in parallel to structural and molecular changes in the hippocampus. Psychoneuroendocrinology. 2009;34(2):199–211. doi: 10.1016/j.psyneuen.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 42.Li X, et al. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187(1):94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Manwani B, et al. Functional recovery in aging mice after experimental stroke. Brain Behav Immun. 2011;25(8):1689–700. doi: 10.1016/j.bbi.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu F, Schafer DP, McCullough LD. TTC, fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179(1):1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swanson RA, et al. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10(2):290–3. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 46.Winter B, et al. Anxious and hyperactive phenotype following brief ischemic episodes in mice. Biol Psychiatry. 2005;57(10):1166–75. doi: 10.1016/j.biopsych.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Karelina K, et al. Social isolation alters neuroinflammatory response to stroke. Proc Natl Acad Sci U S A. 2009;106(14):5895–900. doi: 10.1073/pnas.0810737106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winocur G, et al. The effects of high- and low-risk environments on cognitive function in rats following 2-vessel occlusion of the carotid arteries: A behavioral study. Behav Brain Res. 2013;252C:144–156. doi: 10.1016/j.bbr.2013.05.052. [DOI] [PubMed] [Google Scholar]
- 49.Chirumbolo S. Plant-derived extracts in the neuroscience of anxiety on animal models: biases and comments. Int J Neurosci. 2012;122(4):177–88. doi: 10.3109/00207454.2011.635829. [DOI] [PubMed] [Google Scholar]
- 50.Castillo CS, et al. Generalized anxiety disorder after stroke. J Nerv Ment Dis. 1993;181(2):100–6. doi: 10.1097/00005053-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Koike H, et al. Behavioral abnormality and pharmacologic response in social isolation-reared mice. Behav Brain Res. 2009;202(1):114–21. doi: 10.1016/j.bbr.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 52.Fuss J, et al. Are you real? Visual simulation of social housing by mirror image stimulation in single housed mice. Behav Brain Res. 2013;243:191–8. doi: 10.1016/j.bbr.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 53.Malberg JE, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark CJ, et al. Neighborhood cohesion is associated with reduced risk of stroke mortality. Stroke. 2011;42(5):1212–7. doi: 10.1161/STROKEAHA.110.609164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim JM, et al. A longitudinal study of BDNF promoter methylation and genotype with poststroke depression. J Affect Disord. 2013 doi: 10.1016/j.jad.2013.01.008. [DOI] [PubMed] [Google Scholar]


