Abstract
Purpose
Dp71 is the main product of the Duchenne muscular dystrophy (DMD) gene in the central nervous system. While studying the impact of its absence on retinal functions, we discovered that mice lacking Dp71 also developed a progressive opacification of the crystalline lens. The purpose of this study was to perform a detailed characterization of the cataract formation in Dp71 knockout (KO-Dp71) mice.
Methods
Cataract formations in KO-Dp71 mice and wild-type (wt) littermates were assessed in vivo by slit-lamp examination and ex vivo by histological analysis as a function of aging. The expression and cellular localization of the DMD gene products were monitored by western blot and immunohistochemical analysis. Fiber cell integrity was assessed by analyzing the actin cytoskeleton as well as the expression of aquaporin-0 (AQP0).
Results
As expected, a slit-lamp examination revealed that only one of the 20 tested wt animals presented with a mild opacification of the lens and only at the most advanced age. However, a lack of Dp71 was associated with a 40% incidence of cataracts as early as 2 months of age, which progressively increased to full penetrance by 7 months. A subsequent histological analysis revealed an alteration in the structures of the lenses of KO-Dp71 mice that correlated with the severity of the lens opacity. An analysis of the expression of the different dystrophin gene products revealed that Dp71 was the major DMD gene product expressed in the lens, especially in fiber cells. The role of Dp71 in fiber cells was also suggested by the progressive disorganization of the lens fibers, which was observed in the absence of Dp71 and demonstrated by irregular staining of the actin network and the aqueous channel AQP0.
Conclusions
While its role in the retina has been well characterized, this study demonstrates for the first time the role played by Dp71 in a different ocular tissue: the crystalline lens. It primarily demonstrates the role that Dp71 plays in the maintenance of the integrity of the secondary lens fibers.
Introduction
The crystalline lens is an ocular component of high transparency, with a refractive index that is responsible for focusing light rays onto the photosensitive retina. The mammalian lens consists of two populations of specialized epithelial cells organized into distinct spatial patterns. Differentiated cells called fiber cells, due to their morphology and composition, make up the bulk of the lens, while a monolayer of epithelial cells covers the anterior surface of the fibers. The development and growth of the lens, which take place throughout life, depend on the proliferation of the epithelial cells and their differentiation into lens fiber cells at the equator. During these processes, major structural changes as well as extensive modifications in the gene expression occur. These include alterations in tissue hydration, phase separations in molecular components, and the reorganization of the cellular architecture, including a breakdown of cellular organelles [1]. Alterations in lens epithelial cell function or maturation, as well as disruptions to fiber lens organization, result in clouding of the lens and cataract formation. While age and associated stress accumulation are the main risk factors leading to age-related cataracts, losses in the functions of specific proteins due to mutations or targeted silencing in genetically engineered mice has led to the identification of genetic factors involved in the formation of juvenile or congenital cataracts.
Duchenne muscular dystrophy (DMD) is an X-linked genetic disorder causing progressive muscle degeneration and death. The main product of the DMD gene in muscles is dystrophin, a large protein of 427 kDa with an amino acid sequence homology related to the spectrin family of membrane-associated cytoskeletal proteins [2]. Dystrophin is normally present under the sarcolemma of the skeletal muscle as part of a large protein complex that forms a linkage between the cytoskeleton, the sarcolemma, and the extracellular matrix [3,4]. It is also involved in the proper localization of transmembrane proteins, such as voltage-gated sodium [3], potassium and water channels [5], or membrane-associated proteins including NO synthase [6]. The mdx mouse mutant, which lacks an expression of the 427-kDa dystrophin, has been shown to develop subcapsular cataracts over time [7].
The DMD gene also encodes several smaller products controlled by internal promoters [8]. Dp71, which consists of a unique seven-residue N-terminus fused to the cysteine-rich and C-terminal domains of dystrophin, is the most abundant product of the gene in non-muscular tissues [9]. Among numerous other tissues, it is expressed in the central nervous system, including in the retina [10]. Similar to dystrophin at the neuromuscular junction, Dp71 is involved in large multi-protein complexes, including β-dystroglycan, α-dystrobrevin, and α1-syntrophin [11]. We have shown that this complex, which binds to the actin cytoskeleton and extracellular matrix protein laminin, is required for the proper localization of Kir4.1 and aquaporin-4 (AQP4) channels at discreet locations in the retinal Müller glial cells [12]. Outside the ocular system, it is also responsible for the proper localization of the voltage-dependent Na+ (μI) and K+ (Kv1.1) channels, as well as the nNOS enzyme in mouse spermatozoa [13]. To further study the specific role of Dp71 in those various tissues, Dp71 has been specifically inactivated by replacing its first and unique exons and part of the concomitant intron with a β-galactosidase reporter gene [14]. The original analysis of the expression of this reporter demonstrated the activation of the Dp71 promoter in the developing lens, especially in the developing lens fiber cells, which was not activated in the adjacent anterior capsular epithelial cells at the lens margin [14].
While these data suggested the potential role of dystrophin proteins and, more specifically, Dp71 in the crystalline lens, their specific expressions and localizations have never been studied. Here, we show that while several products are detected, Dp71 is the predominantly expressed DMD gene product in the mouse lens. Using immunohistochemistry, we substantiated the localization of Dp71 at the cell surface of secondary lens fibers. Consistent with these findings, we demonstrated that a lack of the Dp71 expression in the lens was associated with progressive cataract formation and fiber cell disorganization. These findings provide direct evidence that the loss of Dp71 leads to a time-dependent alteration in the integrity of the crystalline lenses in mice.
Methods
Animals
Dp71 knockout (KO-Dp71) mice and their littermates were bred in our laboratory. Mice were originally generated following the method described [14] and then bred in our laboratory. Briefly, the first and unique exons of Dp71 and a small part of the Dp71 first intron were replaced by a sequence encoding a β-gal-neomycin-resistance chimeric protein (b-geo), only abolishing the expression of Dp71.
Mice were identified through an analysis of PCR products using the following oligonucleotide primers: Dp71F, 5’-ATG GAA CAG CTC AAA GG-3’ and Dp71R, 5’-TGC AGC TGA CAG GCT CAA GA-3’. Control immunoblot experiments were also performed on brain extracts to verify the Dp71-null phenotype. Because of the previously demonstrated influence of genetic background on cataract formation, and particularly due to beaded filament mutations and differential expressions, the cataract phenotype reported in this study was evaluated in the S129 original background as well as in mice backcrossed on either C57Bl/6 (pigmented) or Balb/c (albino) genetic backgrounds. All experiments were conducted in compliance with the European Communities Council Directives (86/609/EEC) for animal care and experimentation (HMG). Mice were handled in accordance with the ARVO statement for the use of animals in ophthalmic and vision research.
Clinical evaluation of mice eyes
Mice eyes were examined for lens opacity using a slit lamp and an ophthalmoscope. Mice were physically restrained during the analysis and their eyes were dilated with 1% tropicamide for at least 15 min before evaluation. Both wild-type (wt) (n = 20) and KO-Dp71 littermate mice (n = 31) were analyzed and mixed in a randomized order, without the evaluating investigators’ prior knowledge of the genotypes.
Electrophoresis, western blotting, and immunodetection
Retinas and lenses from 2-month-old mice were homogenized at 4 °C in 10 vol (wt/vol) of extraction buffer (50 mM Tris-HCl pH 7.2, 150 mM NaCl, 1% Triton X-100, 0.01% sodium deoxycholate, 0.1% SDS) containing a mixture of protease inhibitors from Sigma (Saint-Quentin Fallavier, F), and it was centrifuged at 1,000 ×g for 5 min. Protein concentrations were determined by Bradford assays (Bradford, 1970). The proteins were separated using either NuPAGE Tris-Acetate 3%–8% or Tris-Glycine 4%–12% gradient gels (Invitrogen, Cergy Pontoise, F), and they were electrotransferred to either polyvinylidene difluoride membranes (Millipore, Saint-Quentin-en-Yvelines, F) or nitrocellulose (Schleicher & Schuell, Dassel, G), according to the manufacturer instructions. Blots were blocked with 5% dry milk (Bio-Rad, Hertfordshire, UK) in PBS for 1 h, and they were then incubated for 2 h with a primary antibody at room temperature. After washing, they were probed with an HRP-labeled goat anti-mouse secondary antibody (Interchim, France). Chemiluminescence was performed using the ECL+ plus western blotting detection system (Amersham Biosciences, UK), and it was documented on film (Kodak).
Lens histology, immunohistochemistry, and phalloidin staining
For a gross morphology analysis of the lenses, the dissected lenses were placed in a warm tissue culture Medium 199 containing Earle’s salts and l-glutamine (Cellgro, Mediatech, Inc., Manassas, VA). Transparency was then assessed using a dissecting microscope with photographing lenses placed on a 200-mesh electron microscopy (EM) grid using bright field conditions to optimize the focus on the grid, as previously described [15].
For histology, after enucleation, the eyes of 2-month-old mice were fixed for 1 h in 4% paraformaldehyde, transferred to PBS (pH 7.4), dehydrated in increasing concentrations of ethanol, and embedded in paraffin. Sections (8 µm) were stained with hematoxylin and eosin (H&E), and they were viewed by light microscopy or prepared for immunohistochemical analysis. Four to six individual images of the H&E-stained sections were combined for a full representation of the whole lens.
Immunohistochemical labeling was performed using the indirect immunofluorescence method on previously deparaffinized and rehydrated sections. After blocking in PBS (pH 7.4) containing 10% normal goat serum (NGS), 1% bovine serum albumin (BSA), and 0.05% Triton X-100, sections were incubated with the primary antibody in PBS (pH 7.4) supplemented with 3% NGS, 1% BSA, and 0.1% Tween-20. Primary antibodies were detected using a secondary goat anti-mouse IgG antibody coupled to Alexa fluorophore (Molecular Probes, Eugene, OR). Cell nuclei were counterstained using DAPI, 1:500 in PBS (pH 7.4; Molecular Probes, Eugene, OR). Controls were prepared by omitting the primary antibody during incubation; in these controls, no specific staining could be detected.
Phalloidin staining was performed on isolated lenses from wt and KO-Dp71 mice. Freshly isolated lenses were fixed for an hour in 4% paraformaldehyde before being washed and permeabilized in a PBS (pH 7.4) solution containing 0.1% Triton X-100 for 15 min. Lenses were then incubated for 2 h in a solution of 1% BSA, 0.25% Triton X-100 containing Alexa488-labeled phalloidin, and Draq5 for nuclei visualization. Lenses were finally washed in 0.1% Triton X-100 and stored at 4 °C until image acquisition by confocal microscopy.
Antibodies
The mouse monoclonal Dys2 antibody used to detect dystrophins was from Tebu (Novocastra, Newcastle-on-Tyne, UK). The AQP0 and actin are mouse monoclonal antibodies from Abcys (Paris, F) and Millipore (Saint-Quentin-en-Yvelines, F), respectively.
Results
Incidence of cataract formation in KO-Dp71 mice
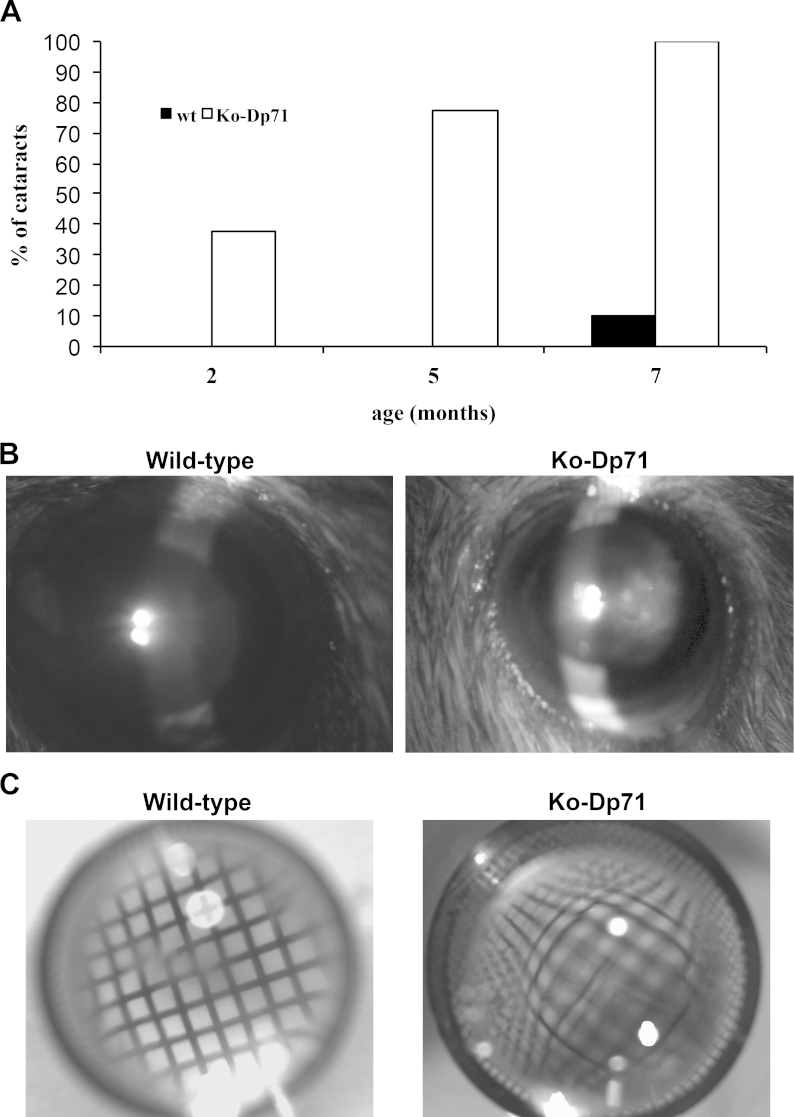
Following the expression of the inserted reporter gene, the original characterization of the KO-Dp71 mice suggested that Dp71 was expressed in the crystalline lens [14]. To determine whether Dp71 plays a critical role in the crystalline lens, we assessed the lens transparency in wt and KO-Dp71 littermate mice strains at different ages post-development, including 2, 5, and 7 months of age. Although the initial characterization of KO-Dp71 mice shows that the gross anatomy of the eye appears normal (this study and 15), cataracts were observed in 37.5% of the 2-month-old KO-Dp71 mice. This proportion strongly increased in older mice to reach 100% of the 7-month-old mice tested (Figure 1A). In contrast and as expected, spontaneous cataracts were observed in only one of the 7-month-old wt littermates, thus indicating that the absence of Dp71 induces the development of juvenile congenital cataracts (Figure 1B). This result is consistent with the similar, but slower developing, congenital cataracts observed in the dystrophin mutant mdx3cv mice, which suffer a drastic reduction in—but not a disappearance of—all the DMD gene products (data not shown).
Figure 1.
A dramatic increase of the incidence of cataracts in the absence of Dp71. A: Time-course analysis of cataract formation in Dp71 knockout (KO-Dp71) mice. Observations were performed at 2, 5, and 7 months in wild-type (wt; black; n = 20) and KO-Dp71 mice (white; n = 31). While virtually no cataracts were observed in wt mice at any of the ages tested, the incidence of cataracts increased dramatically with age in KO-Dp71 mice until affecting all the KO animals tested at 7 months. B: Representative photographs of the eyes of sedated wt and KO-Dp71 mice at 7 months of age using a slit lamp. Whereas the lenses of the wt mice are entirely transparent, a strong opacification is observed in the lenses of the 7-month-old KO-Dp71 mice. C: Representative photographs of isolated crystalline lenses of wt and KO-Dp71 mice at 7 months of age on top of an EM microscopy grid showing the undistorted image obtained through a wt lens; the image is strongly distorted in the KO-Dp71 mice, indicating a refractive defect.
Histological analysis of lenses from KO-Dp71 mice
Gross morphological analyses of the isolated lenses of wt and KO-Dp71 mice showed that while lenses from the wt mice showed no morphological defects at the light level, an absence of Dp71 was clearly associated with refractive defects when placed on a 200-mesh EM grid (Figure 1). To better characterize the impact of the lack of Dp71 on the development and maintenance of a functional translucent lens, a histological analysis was performed on lenses from wt and KO-Dp71 mice at different ages. First, we observed that the size of the lens was consistently reduced in KO-Dp71 mice when compared with their wt littermates. This finding was maintained throughout life and represented here at 2 (65.2 mm3 in wt versus 36.8 mm3 in KO-Dp71) and 7 months (91.5 mm3 in wt versus 57.5 mm3 in KO-Dp71) to match the in vivo data (Figure 2). Careful observations of the wt lenses at these ages revealed normal lens development without cellular damage or disorganization in epithelial or fiber cells. While the overall structure and organization of the lenses were normal in the KO-D71 mice, consistent with the in vivo observations, subtle alterations were already detectable in lenses from 2-month-old KO-Dp71 mice, which was exacerbated at 7 months. At 2 months of age, a few instances of structural defects, such as the formation of vacuoles and a loss in the juxtaposition of fiber cells, were observed (Figure 2D, white arrows). Consistent with the slit-lamp observations, those defects seem to accumulate with age (Figure 2H, white arrows). Additionally, the distinct and typical bow-like organization of the cell nuclei observed in the wt mice began to be disrupted in 2-month-old KO-Dp71 mice. In KO-Dp71 animals, cell nuclei could be found much more centrally and deeply inside the fiber cell layers, well beyond the bow region of the equator. This was a phenotypic observation that was exacerbated with age, as demonstrated by images of lenses from 7-month-old KO-Dp71 mice (Figure 2H, arrowhead).
Figure 2.
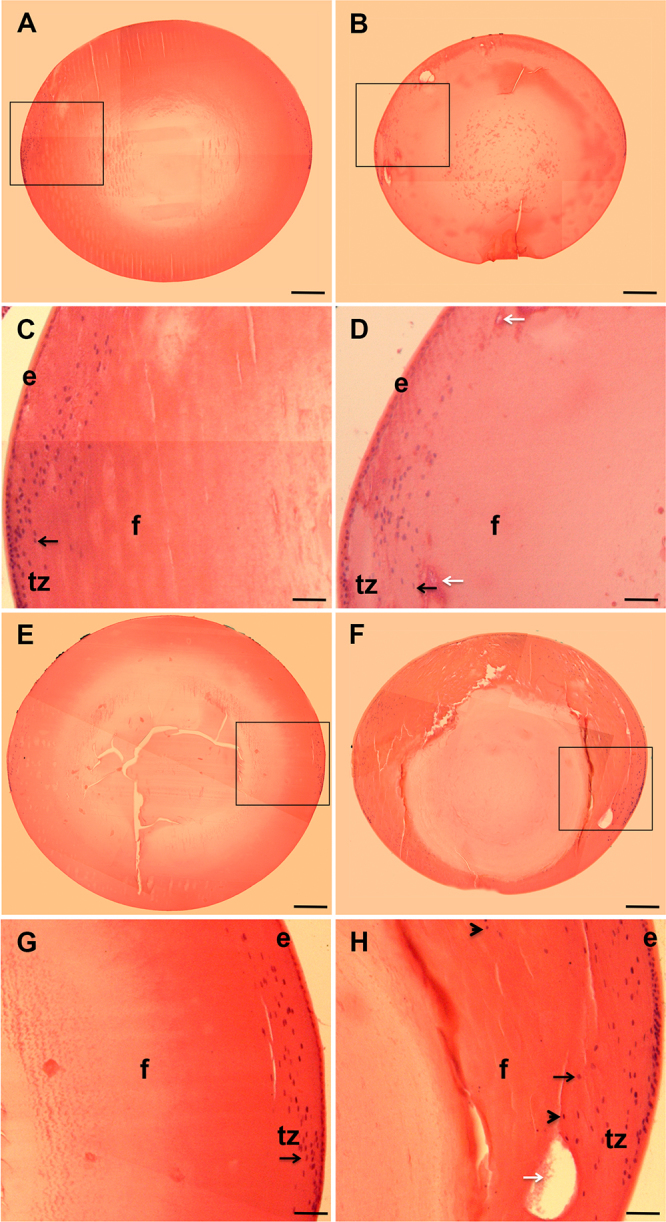
The absence of Dp71 is associated with histological alterations of the crystalline lens. Cross-sections of crystalline lenses from wt and KO-Dp71 mice eyes were stained with hematoxylin and eosin at 2 (A-D) and 7 (E-H) months of age. No major alterations in the histology of the crystalline lenses were observed with aging in wt mice (A and E); alternatively, a progressive disorganization was obvious in lenses from KO-Dp71 mice (B and F). Higher magnification images suggest alterations in the ultrastructure of the lenses in KO-Dp71 mice (D and H, white arrows) along with a disorganized transition zone at both 2 and 7 months of age (C versus D and G versus H, respectively; black arrows; e: epithelial cells; f: fiber cells; tz: transition zone). Scale bars A, B, E, and F: 300 μm; Scale bars C, D, G, and H: 80 μm.
Comparative expression and localization of DMD gene products in lenses from wt and KO-Dp71 mice
The DMD gene product expression was assessed by western blot analysis on whole lens extracts from wt and KO-Dp71 mice, and they were compared with whole retina extracts. As previously reported, all the DMD gene products except Dp116 were expressed in mouse retinas and only the Dp71 expression was disrupted in the retinas of KO-Dp71 mice (Figure 3). An analysis of lenses from wt mice demonstrated that Dp140, Dp71, and Dp45 were expressed at high levels in this tissue, as well as that Dp71 seemed to be significantly more abundant than the others were. As expected, Dp71 was undetectable in lens lysate from KO-Dp71 mice, whereas the expression of Dp140 was unchanged (Figure 3). The loss of the Dp45 expression observed in the lenses—but not in the retinas—of KO-Dp71 mice suggests that the isoform expressed in the lens comes from the same promoter as Dp71. Alternatively, the protein expressed in the retina may be from a different promoter (Figure 3).
Figure 3.
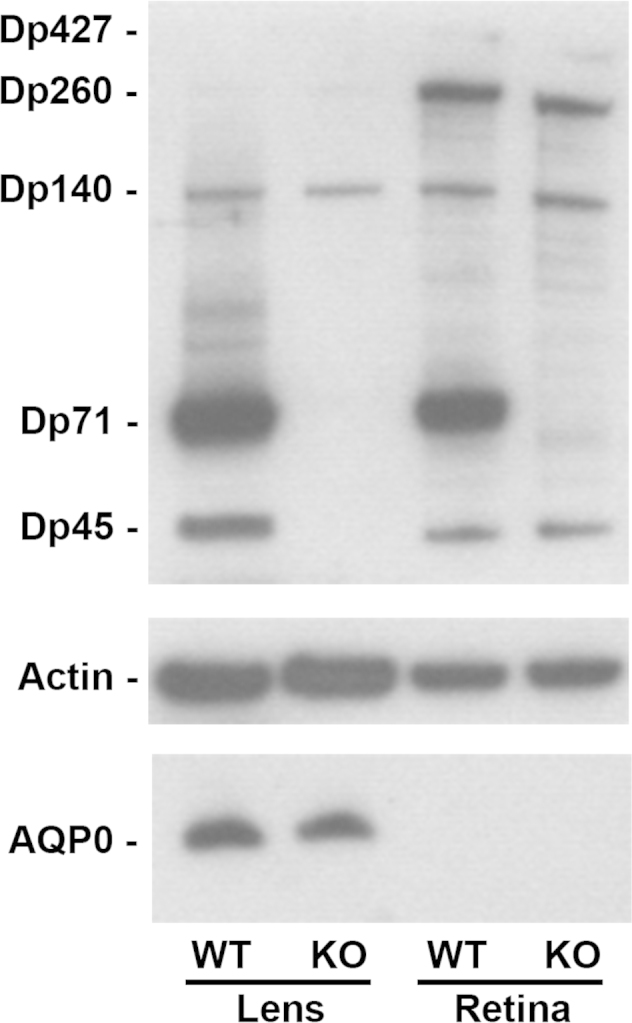
Dp71 is the major dystrophin isoform expressed in the crystalline lens. Levels of the expression of DMD gene products and AQP0 in lenses and retinas from wt and KO-Dp71 mice were analyzed by immunoblotting. Actin levels were used as a loading control. As in the retina, Dp71 is the main DMD gene product expressed in the lens. We also detected Dp427, Dp260, and Dp140 in the retinas, as well as Dp260 and Dp140 in the lenses from both strains.
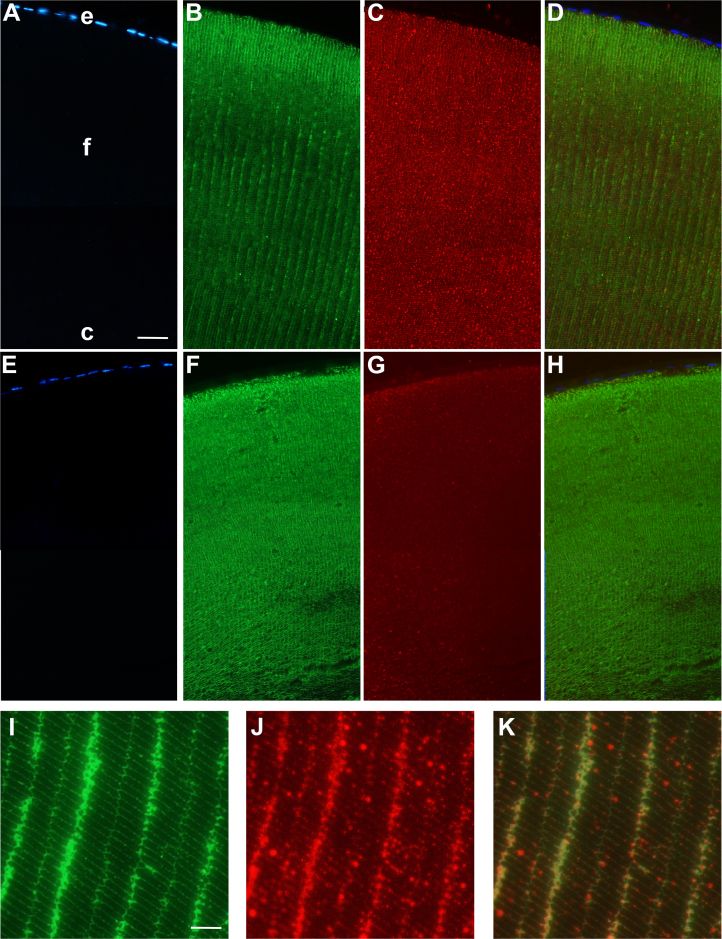
Discreet and specific localization of dystrophins and their associated complexes is critical for their role at the neuromuscular junction or in the central nervous system; thus, we assessed the specific cellular patterns of the expressions of dystrophins in lenses using immunohistochemistry. To this end, cross-sections of lenses from KO-Dp71 mice and their wt littermates were stained with a pan-specific antibody, recognizing all the DMD gene products. An analysis of the staining in tissues from wt animals revealed a wide expression throughout the lens, from the core to the epithelial cells (Figure 4C). When focusing on the anterior pole, the strongest staining was observed at the cell membranes of the secondary fibers; however, the basal side of the epithelial cells of the anterior pole was also positively stained for dystrophins (Figure 4). Of note, the latter was the only signal that remained when immunostaining was performed on KO-Dp71 mice (Figure 4G), suggesting that Dp71 was mainly localized in lens fiber cells, whereas the larger DMD gene products were present in lens epithelial cells. Higher magnification images clearly demonstrated the localization of dystrophin proteins at the membranes of the secondary fiber cells and its colocalization with AQP0 (Figures 4I–K).
Figure 4.
Dp71 localizes at the membrane of the lens fiber cells and its absence is associated with a loss of fiber cell organization. Immunolocalization of dystrophins (red) and AQP0 (green) in the anterior pole of the lens from wt (A-D) and KO-Dp71 (E-H) mice. Nuclei were counterstained with DAPI. Dystrophins are localized at the membrane of the secondary fiber together with the AQP0. As expected, total dystrophin staining in KO-Dp71 mice lenses was strongly decreased, confirming that Dp71 is the main product of the DMD gene in the crystalline lens. A comparison of the staining obtained for AQP0 in wt (B) and KO-Dp71 (F) mice confirms a strong disorganization of the ultrastructure of the lens fibers in the absence of Dp71. A weak staining remained in the epithelium of KO-Dp71 mice, suggesting that the other products of the DMD gene are mainly expressed by lens epithelial cells. Higher magnification images in wt crystalline lenses (I-K) clearly reveal a colocolalization of both proteins (K: merged) in the membranes of secondary fibers close to the anterior pole of the lens (e: epithelial cells; f: fiber cells; c: core). Scale bar A: 50 μm; Scale bar I:15 μm.
AQP0 expression and localization in lenses of wt and KO-Dp71 mice
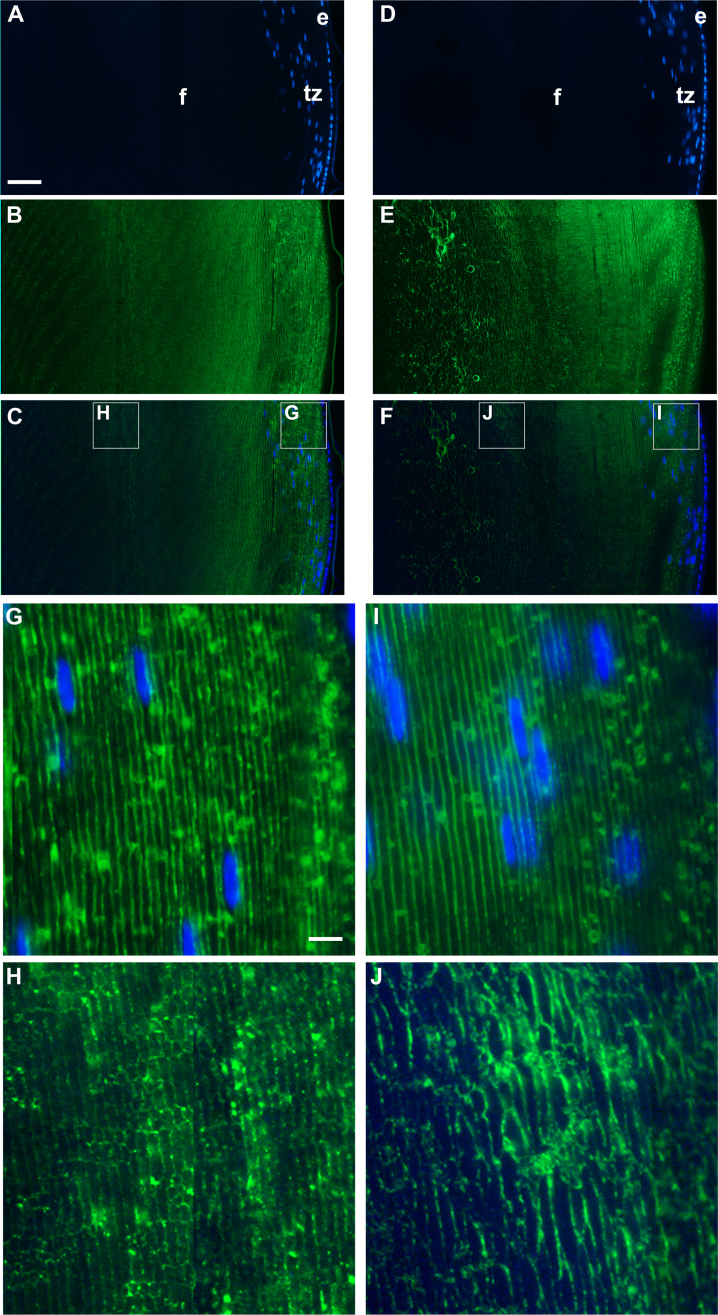
The progressive onset of cataracts combined with the histological findings suggested a progressive loss of structural integrity of the lens in the absence of Dp71. Because of its known central role in complexes necessary for the proper location of membrane transporters, channels, and receptors, we hypothesized that Dp71 could have a similar role in the lens. AQP0, also known as MIP-26, is a membrane-bound protein, abundantly and specifically expressed in lens fiber cells, and it regulates the transport of water. Neither the mRNA (data not shown) nor the protein levels of the AQP0 expression were significantly different between lenses from wt and KO-Dp71 mice (Figure 3). However, immunostaining against the AQP0 protein revealed a clear disorganization of the lens’ secondary fiber cells in the absence of Dp71 in both the anterior pole (Figure 4F) and the transition or germinative zone (Figure 5). Higher magnification images of the transition zones of KO-Dp71 mice lenses confirmed the relatively normal structural organization, but it also confirmed an increased number of nuclei deeper in the tissue (Figures 5G,I). A comparison of the AQP0 staining observed closer to the core of the lens also demonstrated more profound alterations in the structural organization of the lens fibers in the absence of Dp71 (Figures 5H,J). In addition to the vacuole-like structures also observed by histological analysis, staining for AQP0 revealed a disorganized pattern, an increased size of the fibers, and a more diffuse signal for AQP0, not as exclusively retained at the membrane as in wt animals.
Figure 5.
The absence of Dp71 leads to a dramatic disorganization of the secondary fibers close to the germinative zone of the lens. Immunolocalization of AQP0 (green) in the germinative zones of the lenses from wt (A-C) and KO-Dp71 (D-F) mice. Nuclei were counterstained with DAPI (blue). As expected, AQP0 is localized at the membrane of the secondary lens fibers in wt mice. In the absence of Dp71, the regular consistent staining of AQP0 is dramatically altered. Higher magnification images of the periphery of the germinative zone of wt (G) and KO-Dp71 (I) mice show limited disruptions to AQP0 staining. As well, images from the deeper regions of the KO-Dp71 (J) mice show an almost complete loss of organization of the secondary lens fibers when compared to similar regions in wt animals (H) (e: epithelial cells; f: fiber cells; tz: transition zone). Scale bar A: 50 μm; Scale bar I: 8 μm.
Actin network in lenses of wt and KO-Dp71 mice
The DMD gene products, including Dp71, participate in multi-protein complexes that are important for the proper localization of transmembrane proteins, such as ion and water channels, through their interactions with the extracellular matrix on one side and the actin cytoskeleton on the other. Since the integrity of the actin cytoskeleton is a key factor in keeping the lens transparent, we analyzed how it was affected by the absence of Dp71.
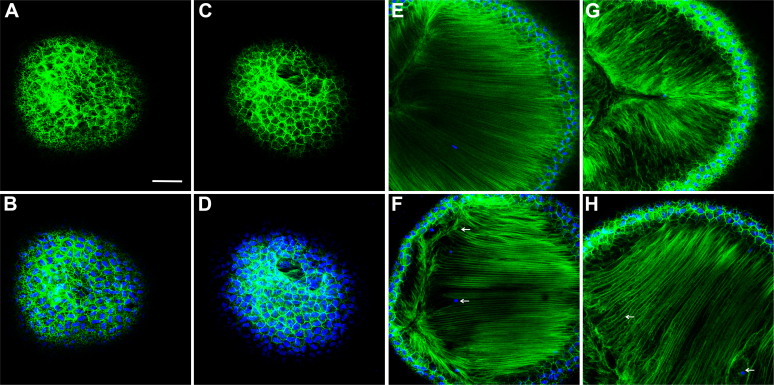
Consistent with the limited impact of the absence of Dp71 observed through other methods, an analysis of the actin filaments in the superficial epithelial cells did not reveal any profound alterations in KO-Dp71 mice, even at 9 months of age (Figures 6A–D). Interestingly, however, consistent with the alterations observed through histology and immunostaining, the actin filaments were noticeably disorganized starting at 3 months (Figure 6F), an effect that was enhanced at 9 months of age (Figure 6H).
Figure 6.
The absence of Dp71 leads to a profound disorganization of the actin filaments. Images of the F-actin network (green) and the cell nuclei (blue) at the level of the epithelium of 9-month-old wt (A-B) and KO-Dp71 mice (C-D). Similar to the results obtained by immunostaining, the network of actin filaments in lens epithelial cells was not different in wt (A-B) versus KO-Dp71 (C-D) mice. However, images of the actin network deep within the lens demonstrate subtle but noticeable changes in the lens fiber cell region at 3 months (E: wt-F:KO-Dp71), as well as changes that were exacerbated with aging, as demonstrated at 9 months (G: wt-H:KO-Dp71). Scale bar: 300 μm.
Discussion
The crystalline lens plays a crucial role in the transmission of visual information by focusing light rays onto the retina. To fulfill this function successfully, the lens must be completely transparent, implying a perfect organization of the cells and proteins constituting the crystalline lens. Proteins account for about 35% of the wet weight of the lens, which is twice as much as other tissues. Many proteins of the cytoskeleton have been described in the lens, reflecting the importance of cytoskeletal organization. To our knowledge, this is the first study of the expression and localization of dystrophins and, particularly, of Dp71. The slightest perturbation in the cellular or protein organizations can induce an opacification leading to the development of cataracts. Here we have shown that Dp71 was mainly expressed in fiber cells, while other dystrophin isoforms were mainly expressed in epithelial cells. In addition, we have shown that its absence induces the formation of juvenile congenital cataracts in mice.
Full-length dystrophin is responsible in muscle cells for maintaining membrane integrity during the contraction/relaxation cycle. We have shown that Dp71 was localized at the cell surface of the lens fibers. Between lens fibers, membranes form numerous interdigitations that are important for their proper organizations and juxtapositions. We have shown in this study that the absence of Dp71 induced a progressive disorganization of the lens fibers. Actin, myosin, tropomyosin, and other cytoskeletal proteins are highly expressed in the lens. Similar to Dp427 in muscles, Dp71 establishes in lens fibers a complex with β-dystroglycan (data not shown)—and other DAPs that remain to be fully identified—a complex that could participate in the linkage of the cytoskeleton to the extracellular matrix as well as play a critical role in maintaining the integrity of these interactions.
Intermediate filaments are also important in the lens, especially in fiber cells where they are mainly located at the cell surface [16]. In adult lenses, two proteins of this family are highly expressed: vimentin and beaded chain filaments [17]. Until now, there has been no evidence of the direct interactions between these two proteins and Dp71; however, their similar localizations and the already-known interactions between the DAP complexes and the intermediate filaments suggest a possible association between both proteins, directly or by the intermediation of DAPs. However, the cataract phenotype cannot be completely explained by such interactions, since it has been reported that disruptions in the beaded chain filament protein CP49 destabilized the lens’ fiber cell cytoskeleton, inducing perturbations of the lens’ optical quality without the formation of a cataract [18]. Similarly, no cataract formations were observed in the absence of vimentin, suggesting a potential functional redundancy between these filaments.
So far, only one report exists of a congenital cataract in a DMD patient, where one presented with a giant dystrophin deletion [19]. The authors found the expression in muscles of a product of about 200 kDa and the presence of a nuclear opacity since the first day of life, which evolved into an anterior subcapsular opacity and later into a complete anterior subcapsular cataract. The absence of additional reports of juvenile cataracts in DMD patients could be due to the short lifespans of those patients and might dramatically increase with increased survival rates. The critical role of Dp71 in the central nervous system could also prevent the onset or detection of cataracts in patients with mutations affecting all dystrophin isoforms as well as those presenting with more severe phenotypes and shorter lifespans.
Based on a previous work [7] that found the existence of congenital cataracts in mdx mice (strain mutant for the full-length dystrophin of 427 kDa), it has been postulated that congenital factors—along with an altered active transport in the epithelial cell membranes of the lens related to dystrophin abnormalities—may play a role in the formation of these cataracts. This hypothesis is consistent with our observation of the remaining labeling of dystrophins in the epithelial cells of KO-Dp71 mice (Figure 4). Our data also suggest that Dp71 plays a primary role in maintaining secondary fiber integrity rather than organizing them, since lenses from KO-Dp71 mice appear normal during the first few months of life. The progressive cataract observed in the absence of Dp71 could reflect a hypersensitivity to light exposure-induced stress—and associated increased oxidative environments—in a mechanism similar to what we previously described in the retina in response to ischemia [20]. Over time, the absence of Dp71 would induce the destabilization of the interaction between cells and the extracellular matrix or between the cells themselves. Because cytoskeletal disorganization does not seem to be sufficient to induce cataracts [18], we cannot rule out the possibility that the absence of Dp71 would also induce the delocalization of proteins implicated in intercellular communications, like the aquaporin channel AQP0. AQP0 is an important protein of the secondary lens fiber membranes, since it plays a central role in water transport in these cells [21,22]. Here we reported a disorganization of the membranes visualized using AQP0 as a marker. However, further studies are required to determine whether AQP0 is associated with Dp71–DAP complexes in the membranes of lens fiber cells, as well as whether the proper localization of this protein is itself affected by the absence of Dp71.
In summary, the findings reported here clearly show a novel role of Dp71 in a tissue in which its role had never been anticipated: the crystalline lens. The absence of Dp71 induces a progressive disorganization of the lens fiber cells, leading to degeneration and cataract formation. Further investigations are needed to establish the precise function of Dp71, as it could be important for the proper organization of the actin filament network or the appropriate localization of membrane proteins like AQP0.
Acknowledgments
This work was supported by INSERM (Institut National de la Santé Et de la Recherche Médicale) and by grants from the AFM (Association Française contre les Myopathies) to A.R. P.F. received financial support from FAF (Fédération des Aveugles et handicapés visuels de France) and the AFM is currently supported by R01EY020895. We are grateful to Melinda Duncan from the University of Delaware for her useful comments and suggestions and to Stephane Fouquet of the imaging core facility at the Institut de la Vision/INSERM/UPMC in Paris.
Bibliography
- 1.Wride MA. Lens fibre cell differentiation and organelle loss: many paths lead to clarity. Philos Trans R Soc Lond B Biol Sci. 2011;366:1219–33. doi: 10.1098/rstb.2010.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–17. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- 3.Gee SH, Madhavan R, Levinson SR, Caldwell JH, Sealock R, Froehner SC. Interaction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins. J Neurosci. 1998;18:128–37. doi: 10.1523/JNEUROSCI.18-01-00128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holt KH, Campbell KP. Assembly of the sarcoglycan complex. Insights for muscular dystrophy. J Biol Chem. 1998;273:34667–70. doi: 10.1074/jbc.273.52.34667. [DOI] [PubMed] [Google Scholar]
- 5.Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci USA. 2001;98:14108–13. doi: 10.1073/pnas.241508198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–52. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 7.Kurihara T, Kishi M, Saito N, Komoto M, Hidaka T, Kinoshita M. Electrical myotonia and cataract in X-linked muscular dystrophy (mdx) mouse. J Neurol Sci. 1990;99:83–92. doi: 10.1016/0022-510x(90)90202-x. [DOI] [PubMed] [Google Scholar]
- 8.Love DR, Byth BC, Tinsley JM, Blake DJ, Davies KE. Dystrophin and dystrophin-related proteins: a review of protein and RNA studies. Neuromuscular disorders. NMD. 1993;3:5–21. doi: 10.1016/0960-8966(93)90037-k. [DOI] [PubMed] [Google Scholar]
- 9.Tadayoni R, Rendon A, Soria-Jasso LE, Cisneros B. Dystrophin Dp71: the smallest but multifunctional product of the Duchenne muscular dystrophy gene. Mol Neurobiol. 2012;45:43–60. doi: 10.1007/s12035-011-8218-9. [DOI] [PubMed] [Google Scholar]
- 10.Rodius F, Claudepierre T, Rosas-Vargas H, Cisneros B, Montanez C, Dreyfus H, Mornet D, Rendon A. Dystrophins in developing retina: Dp260 expression correlates with synaptic maturation. Neuroreport. 1997;8:2383–7. doi: 10.1097/00001756-199707070-00056. [DOI] [PubMed] [Google Scholar]
- 11.Claudepierre T, Dalloz C, Mornet D, Matsumura K, Sahel J, Rendon A. Characterization of the intermolecular associations of the dystrophin-associated glycoprotein complex in retinal Muller glial cells. J Cell Sci. 2000;113:3409–17. doi: 10.1242/jcs.113.19.3409. [DOI] [PubMed] [Google Scholar]
- 12.Fort PE, Sene A, Pannicke T, Roux MJ, Forster V, Mornet D, Nudel U, Yaffe D, Reichenbach A, Sahel JA, Rendon A. Kir4.1 and AQP4 associate with Dp71- and utrophin-DAPs complexes in specific and defined microdomains of Muller retinal glial cell membrane. Glia. 2008;56:597–610. doi: 10.1002/glia.20633. [DOI] [PubMed] [Google Scholar]
- 13.Hernández-Gonzalez EO, Mornet D, Rendon A, Martinez-Rojas D. Absence of Dp71 in mdx3cv mouse spermatozoa alters flagellar morphology and the distribution of ion channels and nNOS. J Cell Sci. 2005;118:137–45. doi: 10.1242/jcs.01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarig R, Mezger-Lallemand V, Gitelman I, Davis C, Fuchs O, Yaffe D, Nudel U. Targeted inactivation of Dp71, the major non-muscle product of the DMD gene: differential activity of the Dp71 promoter during development. Hum Mol Genet. 1999;8:1–10. doi: 10.1093/hmg/8.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Shiels A, King JM, Mackay DS, Bassnett S. Refractive defects and cataracts in mice lacking lens intrinsic membrane protein-2. Invest Ophthalmol Vis Sci. 2007;48:500–8. doi: 10.1167/iovs.06-0947. [DOI] [PubMed] [Google Scholar]
- 16.Blankenship TN, Hess JF, FitzGerald PG. Development- and differentiation-dependent reorganization of intermediate filaments in fiber cells. Invest Ophthalmol Vis Sci. 2001;42:735–42. [PubMed] [Google Scholar]
- 17.Kamachi Y, Uchikawa M, Tanouchi A, Sekido R, Kondoh H. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev. 2001;15:1272–86. doi: 10.1101/gad.887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandilands A, Prescott AR, Wegener A, Zoltoski RK, Hutcheson AM, Masaki S, Kuszak JR, Quinlan RA. Knockout of the intermediate filament protein CP49 destabilises the lens fibre cell cytoskeleton and decreases lens optical quality, but does not induce cataract. Exp Eye Res. 2003;76:385–91. doi: 10.1016/s0014-4835(02)00330-5. [DOI] [PubMed] [Google Scholar]
- 19.Mirabella M, Galluzzi G, Manfredi G, Bertini E, Ricci E, De Leo R, Tonali P, Servidei S. Giant dystrophin deletion associated with congenital cataract and mild muscular dystrophy. Neurology. 1998;51:592–5. doi: 10.1212/wnl.51.2.592. [DOI] [PubMed] [Google Scholar]
- 20.Dalloz C, Sarig R, Fort P, Yaffe D, Bordais A, Pannicke T, Grosche J, Mornet D, Reichenbach A, Sahel J, Nudel U, Rendon A. Targeted inactivation of dystrophin gene product Dp71: phenotypic impact in mouse retina. Hum Mol Genet. 2003;12:1543–54. doi: 10.1093/hmg/ddg170. [DOI] [PubMed] [Google Scholar]
- 21.Gorin MB, Yancey SB, Cline J, Revel JP, Horwitz J. The major intrinsic protein (MIP) of the bovine lens fiber membrane: characterization and structure based on cDNA cloning. Cell. 1984;39:49–59. doi: 10.1016/0092-8674(84)90190-9. [DOI] [PubMed] [Google Scholar]
- 22.Varadaraj K, Kushmerick C, Baldo GJ, Bassnett S, Shiels A, Mathias RT. The role of MIP in lens fiber cell membrane transport. J Membr Biol. 1999;170:191–203. doi: 10.1007/s002329900549. [DOI] [PubMed] [Google Scholar]