Abstract
The prevalence of type 2 diabetes (T2D) is greater in populations of African descent compared to European-descent populations. Genetic risk factors may underlie the disparity in disease prevalence. Genome-wide association studies (GWAS) have identified >60 common genetic variants that contribute to T2D risk in populations of European, Asian, African, and Hispanic descent. These studies have not comprehensively examined population differences in cumulative risk allele load. To investigate the relationship between risk allele load and T2D risk, 46 T2D single nucleotide polymorphisms (SNPs) in 43 loci from GWAS in European, Asian, and African derived populations were genotyped in 1,990 African Americans (n=963 T2D cases, n=1,027 controls) and 1,644 European Americans (n=719 T2D cases, n=925 controls) ascertained and recruited using a common protocol in the southeast United States. A genetic risk score (GRS) was constructed from the cumulative risk alleles for each individual. In African American subjects, risk allele frequencies ranged from 0.024 to 0.964. Risk alleles from 26 SNPs demonstrated directional consistency with previous studies, and 3 SNPs from ADAMTS9, TCF7L2, and ZFAND6 showed nominal evidence of association (p<0.05). African American individuals carried 38–67 (53.7 ± 4.0, mean ± SD) risk alleles. In European American subjects, risk allele frequencies ranged from 0.084 to 0.996. Risk alleles from 36 SNPs demonstrated directional consistency, and 10 SNPs from BCL11A, PSMD6, ADAMTS9, ZFAND3, ANK1, CDKN2A/B, TCF7L2, PRC1, FTO, and BCAR1 showed evidence of association (p<0.05). European American individuals carried 38–65 (50.9 ± 4.4) risk alleles. African Americans have a significantly greater burden of 2.9 risk alleles (p=3.97×10−89) compared to European Americans. However, GRS modeling showed that cumulative risk allele load was associated with risk of T2D in European Americans, but only marginally in African Americans. This result suggests that there are ethnic-specific differences in genetic architecture underlying T2D, and that these differences complicate our understanding of how risk allele load impacts disease susceptibility.
Keywords: diabetes type 2, African American, genetic association, genetic relationship analysis, age at onset
Introduction
Complex diseases, such as T2D, are influenced by a combination of genetic, lifestyle, and environmental risk factors (Qi et al. 2008; Lyssenko et al. 2008). In the United States, age-adjusted prevalence of diabetes is disproportionately greater among African American adults as compared to European Americans (12.6% vs. 7.1%) (Centers for Disease Control and Prevention 2012). The greater risk in African Americans persists even after adjustment for known environmental risk factors such as body mass index (BMI), physical activity, and socioeconomic status (Harris et al. 1998; Maskarinec et al. 2009; Cheng et al. 2012). In addition to lifestyle and environmental risks, genetic factors may contribute to the disparity in disease prevalence in African Americans. Genetic variation at T2D risk loci has been extensively characterized in populations of European descent, and less comprehensively in populations of African, Hispanic, East Asian, and South Asian descent. While heterogeneity in genetic architecture underlying T2D may exist between African Americans and European Americans, some recent evidence suggests that many T2D risk variants exhibit a consistent direction of effect across ethnicities (Waters et al. 2010; Haiman et al. 2012). It is unclear whether differences exist in cumulative risk allele loads between different populations which may partly explain ethnic disparities in T2D prevalence. We examined differences in cumulative risk allele load in African American and European American T2D cases and non-diabetic controls ascertained and examined in a common setting in a geographically defined region in an effort to provide a clear comparison of the allele loads.
Research Design and Methods
Subjects
Recruitment and sample collection procedures were approved by the Institutional Review Board at Wake Forest School of Medicine and written informed consent was obtained from all study participants. Case subjects consisted of unrelated individuals either without (T2D-only cases) or with end-stage renal disease (T2D-ESRD cases). A total of 1,990 African Americans (n=963 T2D-ESRD cases, n=1,027 controls) and 1,644 European Americans (n=151 T2D-only cases, n=568 T2D-ESRD cases, n=925 controls) were assessed. European Americans who have >9% African ancestry (n=10) were excluded from the analysis (Cooke et al. 2012a). T2D was diagnosed in case subjects who reported developing T2D after the age of 25 years and who did not receive only insulin therapy since diagnosis. In addition, T2D-ESRD cases had to have at least one of the following three criteria for inclusion: (i) T2D diagnosed at least 5 years before initiating renal replacement therapy, (ii) background or greater diabetic retinopathy and/or (iii) ≥100 mg/dl proteinuria on urinalysis in the absence of other causes of nephropathy. Control subjects included unrelated individuals without a current diagnosis of diabetes or renal disease. All subjects were recruited from the southeastern United States. Detailed ascertainment and recruitment criteria have been previously described (Yu et al. 1998; Freedman et al. 2000; Sale et al. 2004; Bento et al. 2008; McDonough et al. 2011; Palmer et al. 2012). African American subjects selected for this study are a subset of individuals from previous studies with available GWAS data (Cooke et al. 2012b; Ng et al. 2013).
SNP Selection
We selected SNPs from T2D risk loci with prior evidence of association (p<5×10−8) in GWAS of European, African American, and other ethnicities (Steinthorsdottir et al. 2007; Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research et al. 2007; Zeggini et al. 2007; Wellcome Trust Case Control Consortium 2007; Zeggini et al. 2008; Unoki et al. 2008; Timpson et al. 2009; Takeuchi et al. 2009; Rung et al. 2009; Tsai et al. 2010; Voight et al. 2010; Qi et al. 2010; Shu et al. 2010; Yamauchi et al. 2010; Cui et al. 2011; Sim et al. 2011; Parra et al. 2011; Kooner et al. 2011; Palmer et al. 2012; Cho et al. 2012; Perry et al. 2012; Huang et al. 2012; Imamura et al. 2012; Morris et al. 2012; Ng 2013). Linkage disequilibrium (LD) was examined in HapMap II CEU and YRI populations using SNAP (Johnson et al. 2008). For loci with multiple associated SNPs reported, SNPs were binned at r2>0.5 in HapMap II YRI population (release 22). In each bin, the SNP with the strongest evidence of association in the published literature was included in the analysis. Two independent SNPs were observed at 3 loci (CDKAL1, HHEX, and HNF1A). Of 93 selected SNPs, 22 were removed after LD pruning and 9 were removed for failed imputation in African American subjects. In European American subjects, 48 of 62 SNPs were successfully designed using Sequenom, and 2 SNPs failed genotyping quality control. A total of 46 SNPs in 43 T2D risk loci remained in both African Americans and European Americans for subsequent analyses.
Genotyping, imputation, and quality control
Genotyping in the European Americans was performed using the MassARRAY SNP Genotyping System (Sequenom Inc., San Diego, CA) as previously described (Buetow et al. 2001). Primers for PCR amplification and extension reactions were designed using the MassARRAY Assay Design Software (Sequenom). DNA samples were diluted to a final concentration of 5 ng/μl, and single-base extension reaction products were separated and scored using a matrix-assisted laser desorption ionization/time of flight mass spectrometer. Thirty-three blind duplicates were included in genotyping and had a concordance rate of 100%. SNPs had an average genotype call rate of 98.65% and Hardy-Weinberg p-values were >0.005 in all subjects.
Genotype data for African Americans was derived from (i) a GWAS using the Affymetrix® Genome-Wide Human SNP Array 6.0 (Affymetrix® Inc., Santa Clara, CA) (n=26 SNPs) or (ii) GWAS imputed data (n=20 SNPs). Genotyping was performed at the Center for Inherited Disease Research (CIDR). Genotypes were called using Birdseed version 2; APT 1.10.0 by grouping samples by DNA plate to determine the genotype cluster boundaries. To evaluate genotyping accuracy, 46 blind duplicates were included in genotyping and had a concordance rate of 99.59%. All genotyped SNPs passed quality controls with a genotype call rate ≥95%, Hardy-Weinberg p-values ≥0.0001 in cases and ≥0.01 in controls, no significant difference in missing data rate between cases and controls, and were polymorphic. Imputation was performed using MACH (version 1.0.16, http://www.sph.umich.edu/csg/abecasis/MaCH/) to obtain missing genotypes and replace genotypes inconsistent with reference haplotypes. SNPs that passed quality control and had a minor allele frequency (MAF) ≥1% were used for imputation. A 1:1 HapMap II (NCBI Build 36) CEU:YRI (European:African) consensus haplotype was used as reference. Imputed SNPs that had MAF ≥1%, minor allele count (MAC) ≥10, and RSQ ≥0.5 were included in subsequent data analyses. Detailed genotyping and imputation methods have been previously described (Palmer et al. 2012; Ng et al. 2013).
Principal Components Analysis
For African American subjects, principal components (PC) was computed as previously described (Palmer et al. 2012). The first PC was highly correlated (r2=0.87) with global African-European ancestry as measured by FRAPPE(Tang et al. 2005), was the only PC that accounted for substantial genetic variation at 22%, and was used as a covariate in single-SNP association and GRS analyses to adjust for African American population substructure.
Association Analysis
The association of genotyped and imputed SNPs with T2D was evaluated under an additive model adjusted for age, gender, and the first PC (African American subjects only) separately in African American and European American subjects. Association tests were performed using logistic regression in PLINK (http://pngu.mgh.harvard.edu/purcell/plink).
Genetic Risk Score Analysis
Genetic risk scores (GRS), both unweighted and weighted by published effect size, were calculated for each individual. The unweighted GRS was calculated by counting the number of risk alleles for a given individual. The weighted GRS was calculated by first multiplying the count of risk alleles for each individual at each SNP by the log of the published odds ratio and then summing this weighted value for all SNPs in the analysis for each individual. Missing genotypes for a given SNP were replaced with the average number of risk alleles across all samples within each ethnicity. The association of GRS with both T2D and age at diagnosis of T2D was evaluated under an unadjusted regression model (Model 1) and a regression model adjusted for age, gender, BMI, and the first PC (African American subjects only; Model 2) separately in African American and European American subjects.
Comparison of Genetic Risk Distributions
The total number of risk alleles for each individual (i.e. the unweighted GRS) was used to model the distribution of risk allele load in each population. Risk allele load distributions in African Americans and European Americans were compared using a two sided t-test and by analysis of variance (SAS 9.3, Cary, NC).
Results
Characteristics of study sample
The characteristics of case and control subjects by ethnicity are shown in Table 1. Mean age ranged from 49.0 (African American controls) to 64.9 years (European American cases). Compared to European Americans, the proportion of men was greater in African American controls and lower in cases. Mean body mass index (BMI) and weight were similar between cases and controls in both ethnicities.
Table 1.
Descriptive characteristics of diabetes case and control subjectsa
| Characteristic | African Americans | European Americans | ||
|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |
| Number | 963 | 1027 | 719 | 925 |
| Male (%) | 38.8 | 42.7 | 46.3 | 36.8 |
| Age (years) | 61.6 ± 10.4 | 49.0 ± 11.9 | 64.9 ± 10.3 | 53.9 ± 15.5 |
| Age at diagnosis (years) | 41.8 ± 12.3 | - | 46.7 ± 13.2 | - |
| BMI (kg/m2) | 29.7 ± 7.0 | 30.0 ± 7.1 | 30.1 ± 7.3 | 28.4 ± 5.7 |
| Weight (lbs) | 186 ± 46 | 190 ± 44 | 190 ± 47 | 179 ± 40 |
Data are shown as mean ± SD or percentage
Association of established T2D risk SNPs
The 46 T2D risk SNPs from 43 loci were polymorphic in both ethnicities, and a summary of the association analysis with T2D is shown in Table 2. The majority (n=35) of these SNPs were discovered in populations of European descent. Reported odds ratios (OR) for the risk alleles ranged from 1.06 to 1.54 In African American subjects, nominal evidence of association (p<0.05) was observed with 3 SNPs from ADAMTS9, TCF7L2, and ZFAND6 (3/46 SNPs; binomial p=0.41). In European American subjects, nominal evidence of association (p<0.05) was observed with 10 SNPs from BCL11A, PSMD6, ADAMTS9, ZFAND3, ANK1, CDKN2A/B, TCF7L2, PRC1, FTO, and BCAR1 (10/46 SNPs; binomial p=7.6×10−5).
Table 2.
Association of known T2D risk alleles
| African Americans | European Americans | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Nearest Gene | Chr | Positiona | RA/NRAb | RAFc | OR (95%CI)d | P-value | RAFc | OR (95%CI) d | P-value | Phete |
| rs10923931 | NOTCH2 | 1 | 120517959 | T/G | 0.35 | 1.04(0.88–1.22) | 0.641 | 0.10 | 1.19(0.89–1.59) | 0.243 | 0.628 |
| rs7578597 | THADA | 2 | 43732823 | T/C | 0.75 | 0.95(0.79–1.13) | 0.565 | 0.89 | 1.20(0.93–1.54) | 0.158 | 0.359 |
| rs243021 | BCL11A | 2 | 60584819 | A/G | 0.39 | 0.89(0.76–1.04) | 0.127 | 0.46 | 1.31(1.11–1.54) | 0.001 | 0.032 |
| rs7560163 | RND3 - FABP5L10 | 2 | 151637936 | C/G | 0.89 | 1.19(0.94–1.51) | 0.158 | 1.00 | 1.14(0.39–3.30) | 0.811 | 0.965 |
| rs7593730 | RBMS1 | 2 | 161171454 | C/T | 0.61 | 0.88(0.76–1.03) | 0.103 | 0.78 | 1.02(0.84–1.25) | 0.818 | 0.461 |
| rs831571 | PSMD6 | 3 | 64048297 | C/T | 0.81 | 1.01(0.83–1.23) | 0.907 | 0.81 | 0.80(0.65–0.99) | 0.044 | 0.320 |
| rs4607103 | ADAMTS9 | 3 | 64711904 | C/T | 0.71 | 0.79(0.67–0.93) | 0.005 | 0.78 | 0.79(0.64–0.98) | 0.035 | 0.991 |
| rs4402960 | IGF2BP2 | 3 | 185511687 | T/G | 0.53 | 0.96(0.82–1.12) | 0.590 | 0.32 | 0.94(0.79–1.12) | 0.475 | 0.911 |
| rs459193 | ANKRD55 | 5 | 55806751 | G/A | 0.58 | 0.99(0.85–1.16) | 0.909 | 0.75 | 1.02(0.85–1.23) | 0.820 | 0.875 |
| rs7754840 | CDKAL1 | 6 | 20661250 | C/G | 0.61 | 1.15(0.98–1.35) | 0.079 | 0.32 | 1.03(0.87–1.22) | 0.713 | 0.551 |
| rs10440833 | CDKAL1 | 6 | 20688121 | A/T | 0.22 | 1.13(0.94–1.35) | 0.199 | 0.27 | 1.05(0.88–1.26) | 0.587 | 0.731 |
| rs9470794 | ZFAND3 | 6 | 38106844 | C/T | 0.12 | 0.96(0.75–1.23) | 0.749 | 0.09 | 0.69(0.52–0.93) | 0.015 | 0.297 |
| rs1048886 | C6orf57 | 6 | 71289189 | G/A | 0.29 | 1.12(0.95–1.32) | 0.191 | 0.18 | 0.88(0.71–1.08) | 0.217 | 0.267 |
| rs849134 | JAZF1 | 7 | 28196222 | A/G | 0.77 | 1.17(0.97–1.40) | 0.095 | 0.51 | 1.08(0.92–1.27) | 0.355 | 0.679 |
| rs6467136 | ZNF800 - GCC1 | 7 | 127164958 | G/A | 0.63 | 1.02(0.87–1.20) | 0.821 | 0.53 | 0.91(0.77–1.07) | 0.248 | 0.532 |
| rs972283 | KLF14 | 7 | 130466854 | G/A | 0.88 | 1.29(1.00–1.67) | 0.054 | 0.52 | 0.98(0.84–1.15) | 0.798 | 0.232 |
| rs516946 | ANK1 | 8 | 41519248 | C/T | 0.78 | 1.11(0.92–1.32) | 0.273 | 0.76 | 1.25(1.04–1.50) | 0.020 | 0.558 |
| rs896854 | TP53INP1 | 8 | 95960511 | T/C | 0.71 | 1.12(0.94–1.32) | 0.204 | 0.51 | 1.12(0.95–1.31) | 0.179 | 1.000 |
| rs13266634 | SLC30A8 | 8 | 118184783 | C/T | 0.94 | 1.13(0.81–1.57) | 0.464 | 0.69 | 1.07(0.90–1.27) | 0.462 | 0.837 |
| rs7041847 | GLIS3 | 9 | 4287466 | A/G | 0.89 | 0.81(0.63–1.05) | 0.107 | 0.50 | 1.16(0.99–1.35) | 0.071 | 0.120 |
| rs2383208 | CDKN2A/B | 9 | 22132076 | A/G | 0.80 | 0.88(0.73–1.07) | 0.192 | 0.83 | 1.26(1.01–1.56) | 0.037 | 0.125 |
| rs13292136 | CHCHD9 | 9 | 81952128 | C/T | 0.91 | 0.97(0.75–1.26) | 0.822 | 0.94 | 1.02(0.72–1.43) | 0.917 | 0.892 |
| rs2796441 | TLE1 | 9 | 84308948 | G/A | 0.84 | 1.05(0.86–1.30) | 0.627 | 0.60 | 1.02(0.86–1.19) | 0.863 | 0.858 |
| rs10906115 | CDC123 - CAMK1D | 10 | 12314997 | A/G | 0.68 | 1.08(0.92–1.28) | 0.343 | 0.61 | 1.15(0.98–1.35) | 0.094 | 0.747 |
| rs1802295 | VPS26A | 10 | 70931474 | A/C | 0.95 | 0.97(0.69–1.38) | 0.883 | 0.30 | 0.96(0.81–1.15) | 0.689 | 0.832 |
| rs12571751 | ZMIZ1 | 10 | 80942631 | A/G | 0.54 | 0.93(0.80–1.08) | 0.352 | 0.53 | 1.17(1.00–1.38) | 0.056 | 0.198 |
| rs1111875 | HHEX | 10 | 94462882 | C/T | 0.78 | 1.10(0.91–1.32) | 0.323 | 0.61 | 1.07(0.91–1.26) | 0.428 | 0.899 |
| rs5015480 | HHEX | 10 | 94465559 | C/T | 0.62 | 0.92(0.79–1.08) | 0.325 | 0.60 | 1.09(0.92–1.29) | 0.300 | 0.363 |
| rs7901695 | TCF7L2 | 10 | 114754088 | C/T | 0.48 | 1.21(1.04–1.42) | 0.013 | 0.33 | 1.48(1.25–1.75) | 5.68E-06 | 0.285 |
| rs2237892 | KCNQ1 | 11 | 2839751 | C/T | 0.91 | 1.10(0.83–1.44) | 0.517 | 0.94 | 1.07(0.75–1.52) | 0.712 | 0.948 |
| rs5215 | KCNJ11 | 11 | 17408630 | C/T | 0.09 | 0.97(0.74–1.28) | 0.846 | 0.37 | 1.17(0.99–1.38) | 0.058 | 0.444 |
| rs1552224 | CENTD2 | 11 | 72433098 | A/C | 0.98 | 0.92(0.56–1.52) | 0.750 | 0.85 | 1.02(0.81–1.27) | 0.894 | 0.812 |
| rs10842994 | KLHDC5 | 12 | 27965150 | C/T | 0.96 | 1.13(0.75–1.68) | 0.567 | 0.82 | 1.07(0.86–1.32) | 0.547 | 0.877 |
| rs7961581 | TSPAN8/LGR5 | 12 | 71663102 | C/T | 0.19 | 1.03(0.85–1.25) | 0.755 | 0.28 | 1.03(0.86–1.24) | 0.727 | 0.993 |
| rs7305618 | HNF1A | 12 | 121402932 | C/T | 0.60 | 1.02(0.87–1.19) | 0.819 | 0.78 | 1.07(0.88–1.31) | 0.470 | 0.792 |
| rs7957197 | HNF1A | 12 | 121460686 | T/A | 0.89 | 1.22(0.94–1.59) | 0.133 | 0.82 | 1.16(0.94–1.43) | 0.172 | 0.841 |
| rs7172432 | C2CD4A/B | 15 | 62396389 | A/G | 0.31 | 0.97(0.82–1.15) | 0.721 | 0.58 | 0.95(0.81–1.12) | 0.530 | 0.907 |
| rs7177055 | HMG20A | 15 | 77832762 | A/G | 0.38 | 1.08(0.92–1.26) | 0.359 | 0.73 | 0.94(0.78–1.12) | 0.462 | 0.464 |
| rs11634397 | ZFAND6 | 15 | 80432222 | G/A | 0.44 | 0.84(0.72–0.98) | 0.031 | 0.66 | 1.17(0.99–1.39) | 0.072 | 0.078 |
| rs2028299 | AP3S2 | 15 | 90374257 | C/A | 0.29 | 1.18(1.00–1.40) | 0.057 | 0.28 | 1.17(0.98–1.40) | 0.080 | 0.976 |
| rs8042680 | PRC1 | 15 | 91521337 | A/C | 0.88 | 0.91(0.72–1.15) | 0.445 | 0.32 | 1.19(1.00–1.41) | 0.047 | 0.239 |
| rs9939609 | FTO | 16 | 53820527 | A/T | 0.49 | 0.95(0.82–1.10) | 0.509 | 0.43 | 1.34(1.14–1.58) | 3.66E-04 | 0.052 |
| rs7202877 | BCAR1 | 16 | 75247245 | T/G | 0.85 | 0.84(0.66–1.08) | 0.176 | 0.91 | 1.74(1.30–2.32) | 1.79E-04 | 0.019 |
| rs12970134 | MC4R | 18 | 57884750 | A/G | 0.14 | 1.15(0.93–1.42) | 0.213 | 0.27 | 1.19(0.99–1.42) | 0.059 | 0.866 |
| rs10401969 | CILP2 | 19 | 19407718 | C/T | 0.17 | 1.09(0.89–1.33) | 0.402 | 0.08 | 1.10(0.83–1.48) | 0.502 | 0.960 |
| rs4812829 | HNF4A | 20 | 42989267 | A/G | 0.10 | 1.15(0.89–1.49) | 0.284 | 0.17 | 1.04(0.83–1.30) | 0.726 | 0.702 |
NCBI build 37
Risk allele (RA) and non-risk allele (NRA) as previously reported. Alleles indexed to NCBI build 37 (forward strand)
Ethnic-specific risk allele frequencies (RAF) calculated for each SNP
Odds ratios (OR) and 95% confidence intervals (CI) under additive model are adjusted for age, gender, and population substructure (African Americans only)
Heterogeneity P-values between African Americans and European Americans for selected SNPs
21 SNPs that are directionally consistent with published results in both African Americans and European Americans are shown in bold
Compared to the null expectation that half of the previously reported risk alleles to show the same direction of association (OR>1), our study observed significant directional consistency in European American subjects (36/46 SNPs; binomial p=7.8×10−5) but not in African American subjects (26/46 SNPs, binomial p=0.23).
Comparison of Risk Allele Load
Risk allele load was used to compare the distribution of genetic risk between populations (Table 3, Figures 1 and 2, Supplementary Figure 1). African Americans had an average risk allele load of 53.7 ± 4.0 risk alleles compared to 50.9 ± 4.4 risk alleles in European Americans. African Americans carry, on average, 2.9 risk alleles more than their European American counterparts. This increase causes the distribution of genetic risk to be right-shifted in African Americans compared to European Americans (p=3.97×10−89; two sided t-test). To take into account the confounding effects of gender, age, BMI, and population substructure on differences in risk allele load between African Americans and European Americans, several analysis of variance (ANOVA) models were constructed. Adjustments for age, gender, and BMI did not change the inference of mean difference in risk allele load (p=3.2×10−72). A subset analysis further excluding African American individuals with a low degree of African ancestry (PC1<0.5, n=100) did not change the result (p=5.0×10−71). In European Americans, T2D-ESRD cases did not have a significant mean difference of risk alleles compared to T2D-only cases (p=0.72; two sided t-test).
Table 3.
Comparison of risk allele load
| Subjects | African Americans | European Americans | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Mean Difference | P-valuea | |
| Cases | 53.8 | 54 | 4.0 | 51.5 | 52 | 4.4 | 2.3 | 4.21E-28 |
| Controls | 53.7 | 54 | 3.9 | 50.4 | 50 | 4.3 | 3.3 | 3.09E-64 |
| All subjects | 53.7 | 54 | 4.0 | 50.9 | 51 | 4.4 | 2.9 | 3.97E-89 |
Two sided t-test
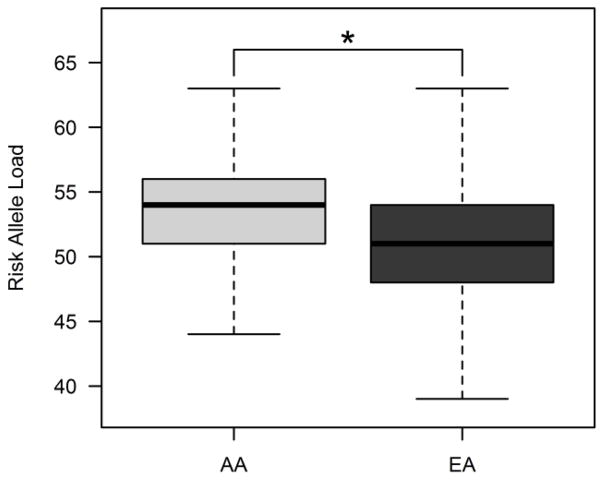
Fig. 1.
Comparison of risk allele distributions by ethnicity. African Americans (AA, light grey) show a highly significant mean increase in risk allele load compared to European Americans (EA, dark grey). *p=3.96×10−89
Fig. 2.
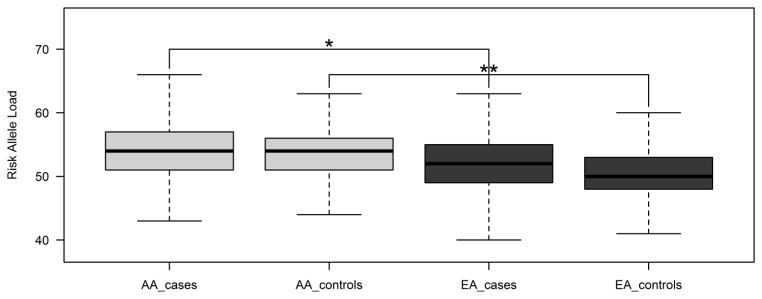
Comparison of risk allele distributions by ethnicity and case-control status. African American T2D cases (AA_cases, light grey) show a highly significant mean increase in risk allele load compared to European American T2D cases. (EA_cases, dark grey). African American controls (AA_controls, light grey) show a highly significant mean increase in risk allele load compared to European American controls. (EA_controls, dark grey). *p=4.21×10−28; **p=3.09×10−64
Genetic risk score association with T2D and T2D age at diagnosis
Both unweighted and weighted GRS incorporating all 46 SNPs were analyzed for association with T2D and with age at diagnosis of T2D as outlined in the methods. Unweighted scores ranged from 38 to 67 (53.7 ± 4.0, mean ± SD) in African Americans and 38 to 65 (50.9 ± 4.4) in European Americans. Effect sizes ranged from 0.06 to 0.43 (0.13 ± 0.08, Supplementary Table 1). Weighted scores ranged from 4.30 to 9.03 (6.91 ± 0.01) in African Americans and 4.40 to 8.83 (6.54 ± 0.02) in European Americans. Unweighted GRS was significantly associated with T2D in an unadjusted model in European Americans, with each risk allele increasing T2D risk by 6–8%. However, no significant association was observed in African Americans (Table 4). These results remained consistent when the model was adjusted for age, gender, BMI, and population substructure (African Americans only) (Table 4). Weighted GRS showed nominal evidence of association with T2D in African Americans (p=0.03); however, the association was lost when the model was adjusted for age, gender, BMI and population stratification (p=0.08) (Table 4). The association of weighted GRS with T2D in European Americans was consistent with the unweighted GRS results. Per-allele odds ratio estimates were greater for European Americans in both weighted and unweighted models (OR=1.06–1.69) compared to African Americans (OR=1.01–1.17) (Table 4). No association of GRS with T2D age at diagnosis was observed in either population in either the weighted or unweighted models (Supplementary Table 2).
Table 4.
Association of risk score with T2D by ethnicity
| African Americans | European Americans | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Unweighted | ||||
| Model 1a | 1.01(0.99–1.04) | 0.29 | 1.06(1.04–1.09) | 2.13E-07 |
| Model 2b | 1.01(0.99–1.04) | 0.36 | 1.08(1.05–1.11) | 1.05E-07 |
| Weighted | ||||
| Model 1a | 1.17(1.01–1.35) | 0.03 | 1.59(1.36–1.88) | 1.71E-08 |
| Model 2b | 1.16(0.98–1.38) | 0.08 | 1.69(1.39–2.06) | 1.65E-07 |
Model 1 is unadjusted
Model 2 is adjusted for age, gender, BMI and PC1 (African Americans only)
An additional GRS analysis restricted to the 21 SNPs that were directionally consistent with published results in both our African American and European American subjects did not change the inference of a greater odds ratio estimate in European Americans (Supplementary Table 3). Although per-allele odds ratio estimates were marginally greater for African Americans (OR=1.05–1.13) compared to European Americans (OR=1.04–1.11) in an unadjusted, unweighted model, estimates were higher in European Americans in subsequent models 1) adjusted for age, gender, BMI and population stratification, 2) incorporating a GRS weighted by the log of the published odds ratio, or 3) both. Again, no association of GRS with T2D age at diagnosis was observed in either population in any of the models.
Discussion
We evaluated 46 T2D risk SNPs from 43 loci identified in populations of European, Asian, or African descent. Our results show that the majority of these SNPs exhibit a consistent direction of effect in both African American and European American populations. A significant proportion of these SNPs were nominally associated with T2D in European Americans, but to a much more limited extent in African Americans. A comparison of risk allele load distributions shows that African Americans carry a greater load of T2D risk alleles. However, modeling of cumulative risk scores suggests that per allele effect estimates are relatively smaller compared to European Americans, and that association between the cumulative risk score and T2D is stronger in European Americans. Given these results, it is unclear whether the common risk variants examined in this study account for a portion of the observed disparity in T2D prevalence between these populations. Subsequent analyses including the major non-genetic influences of age, gender, and BMI do not support the relationship between genetic risk factors and ethnic disparities in T2D prevalence.
Several studies have examined the use of GRS to predict risk of T2D. In a previous study by Meigs et al., a GRS incorporating 18 common T2D risk variants was modestly but significantly associated (OR=1.12 per risk allele [95% CI 1.07–1.17]; p=0.01) with risk of diabetes in individuals of European descent, and this association was robust against adjustment for non-genetic risk factors (2008). In the Diabetes Prevention Program (DPP), a GRS integrating 34 T2D risk variants was more modestly associated (hazard ratio [HR]=1.02 per risk allele [95% CI 1.00–1.05]; p=0.03) with risk of progression to diabetes in a prospective, multi-ethnic cohort in an analysis adjusted for ancestry, lifestyle, and environmental risk factors (Hivert et al. 2011). In the Cooke et al. study of African Americans, a modest association (OR=1.06 [1.03–1.10], p=8.10×10−5) was observed between a GRS including 17 T2D risk variants and risk of diabetes, but the association was lost when the analysis was adjusted for the genotypic effects of the TCF7L2 risk variant rs7903146 (2012). Considering the increased haplotype diversity and different linkage disequilibrium structure in African-derived populations, common variants identified in primarily European studies are likely not the best predictors of diabetes risk in African Americans (Lewis et al. 2008). The identification of causal variants in T2D risk loci will be required to provide a clear picture of differences in risk allele load between African American and European American populations. Moreover, large longitudinal studies examining the discriminatory power of cumulative genetic risk factors for predicting disease risk as well as phenotypic associations of biochemical and anthropometric traits correlated with disease may be necessary to demonstrate a causal link between cumulative genetic risk and ethnic-specific differences in T2D prevalence.
A recent trans-ethnic T2D GWAS meta-analysis showed that 34 of 52 previously reported T2D SNPs (65.4%) showed the same direction of effect across European, East Asian, South Asian, and Mexican American populations (DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium et al. 2014). In our study, 21 of 46 tested SNPs (45.7%) were directionally consistent with published GWAS results and between African Americans and European Americans (Table 2). This result suggests that differences in the genetic architecture of T2D are greater between African Americans and European Americans than among other ethnic groups. However, it should be noted that our study focused on genetic burden, not transferability, and thus previously reported GWAS signals were not fine-mapped in either of our study populations to truly examine trans-ethnic directional consistency.
A limited number of studies have examined ethnic-specific differences in genetic risk of diabetes. In the study by Waters et al., a GRS incorporating 18 common T2D risk variants was associated with risk of diabetes in both African Americans (n=1,077 cases, 1,469 controls, OR=1.09 per risk allele [95% CI 1.05–1.12]; p=3.0×10−6) and European Americans (n=533 cases, 1,006 controls, OR=1.11 per risk allele [95% CI 1.06–1.17]; p=1.2×10−5) in an analysis adjusted for gender and quartiles of age and BMI, but no significant difference in risk allele load was observed between the two groups (2010). In the study by Haiman et al., a significant association between established T2D risk variants at five loci (WFS1, HHEX, CDNK2A/B, THADA, and KCNQ1) and risk of diabetes was detected in European Americans, but not in African Americans even though the study power was high (≥ 94% for all variants) (2012). This result suggests that heterogeneity in risk allele frequencies, effect size, and linkage disequilibrium between the established risk variant and the causal allele exists at some fraction of T2D risk loci.
This study had limitations. First, the 46 SNPs included in our analysis explain only a small proportion of T2D heritability. We did not account for exposure to epigenetic factors, structural variants, rare variants, gene-gene interactions, or gene-environment interactions, all of which may modify genetic risk and explain a proportion of the missing heritability. Additionally, we adjusted our analyses only for major phenotypic risk factors. Behavioral risk factors may modify an individual’s susceptibility to genetic risk of disease and to some degree explain the disparity in T2D prevalence between African Americans and European Americans. Finally, significant associations of individual SNPs with risk of T2D were scarce, but this result was expected considering the relatively small number of cases and controls used for this study as compared to the initial discovery populations.
In summary, African Americans carry a greater number of risk alleles at 46 established T2D risk loci than European Americans. Cumulatively, these variants are strong predictors of diabetes risk in European Americans, but poor predictors in African Americans. Differences in genetic variation between ethnicities create a complex pattern which complicates drawing clear conclusions regarding the relationship between genetic risk factors and ethnic disparities in T2D prevalence. Our results emphasize the need for further study of genetic variation underlying T2D in African Americans as a means to improve the overall quality of genetic research of this disease.
Supplementary Material
Acknowledgments
The authors would like to thank all study participants for their time and effort.
Funding
This work was supported by NIH grants R01 DK066358 and R01 DK053591 to DWB.
Footnotes
Duality of Interest
There are no conflicts of interest relevant to this article.
Author Contributions
J.M.K. wrote the manuscript, performed genotyping, and researched and analyzed the data. J.N.C.B. researched data, contributed to data analysis, and reviewed and edited the manuscript. N.D.P. and B.I.F. reviewed and edited the manuscript. C.D.L. contributed to data interpretation. M.C.Y.N. assisted with data analysis, designed the study, and reviewed and edited the manuscript. D.W.B. contributed to manuscript writing and study design, contributed to the discussion, and reviewed and edited the manuscript. D.W.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Bento JL, Palmer ND, Zhong M, et al. Heterogeneity in gene loci associated with type 2 diabetes on human chromosome 20q13.1. Genomics. 2008;92:226–234. doi: 10.1016/j.ygeno.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetow KH, Edmonson M, MacDonald R, et al. High-throughput development and characterization of a genomewide collection of gene-based single nucleotide polymorphism markers by chip-based matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Proc Natl Acad Sci U S A. 2001;98:581–584. doi: 10.1073/pnas.021506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Diabetes Report Card 2012. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services;; 2012. [Google Scholar]
- Cheng C-Y, Reich D, Haiman CA, et al. African ancestry and its correlation to type 2 diabetes in African Americans: a genetic admixture analysis in three U.S. population cohorts. PloS One. 2012;7:e32840. doi: 10.1371/journal.pone.0032840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YS, Chen C-H, Hu C, et al. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat Genet. 2012;44:67–72. doi: 10.1038/ng.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke JN, Bostrom MA, Hicks PJ, et al. Polymorphisms in MYH9 are associated with diabetic nephropathy in European Americans. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2012a;27:1505–1511. doi: 10.1093/ndt/gfr522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke JN, Ng MCY, Palmer ND, et al. Genetic Risk Assessment of Type 2 Diabetes-Associated Polymorphisms in African Americans. Diabetes Care. 2012b;35:287–292. doi: 10.2337/dc11-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B, Zhu X, Xu M, et al. A genome-wide association study confirms previously reported loci for type 2 diabetes in Han Chinese. PloS One. 2011;6:e22353. doi: 10.1371/journal.pone.0022353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research. Saxena R, Voight BF, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium, Asian Genetic Epidemiology Network Type 2 Diabetes (AGEN-T2D) Consortium, South Asian Type 2 Diabetes (SAT2D) Consortium, et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet. 2014;46:234–244. doi: 10.1038/ng.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman BI, Yu H, Anderson PJ, et al. Genetic analysis of nitric oxide and endothelin in end-stage renal disease. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2000;15:1794–1800. doi: 10.1093/ndt/15.11.1794. [DOI] [PubMed] [Google Scholar]
- Haiman CA, Fesinmeyer MD, Spencer KL, et al. Consistent directions of effect for established type 2 diabetes risk variants across populations: the population architecture using Genomics and Epidemiology (PAGE) Consortium. Diabetes. 2012;61:1642–1647. doi: 10.2337/db11-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- Hivert M-F, Jablonski KA, Perreault L, et al. Updated Genetic Score Based on 34 Confirmed Type 2 Diabetes Loci Is Associated With Diabetes Incidence and Regression to Normoglycemia in the Diabetes Prevention Program. Diabetes. 2011;60:1340–1348. doi: 10.2337/db10-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Ellinghaus D, Franke A, et al. 1000 Genomes-based imputation identifies novel and refined associations for the Wellcome Trust Case Control Consortium phase 1 Data. Eur J Hum Genet EJHG. 2012;20:801–805. doi: 10.1038/ejhg.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura M, Maeda S, Yamauchi T, et al. A single-nucleotide polymorphism in ANK1 is associated with susceptibility to type 2 diabetes in Japanese populations. Hum Mol Genet. 2012;21:3042–3049. doi: 10.1093/hmg/dds113. [DOI] [PubMed] [Google Scholar]
- Johnson AD, Handsaker RE, Pulit SL, et al. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinforma Oxf Engl. 2008;24:2938–2939. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooner JS, Saleheen D, Sim X, et al. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet. 2011;43:984–989. doi: 10.1038/ng.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JP, Palmer ND, Hicks PJ, et al. Association Analysis in African Americans of European-Derived Type 2 Diabetes Single Nucleotide Polymorphisms From Whole-Genome Association Studies. Diabetes. 2008;57:2220–2225. doi: 10.2337/db07-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssenko V, Jonsson A, Almgren P, et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med. 2008;359:2220–2232. doi: 10.1056/NEJMoa0801869. [DOI] [PubMed] [Google Scholar]
- Maskarinec G, Grandinetti A, Matsuura G, et al. Diabetes prevalence and body mass index differ by ethnicity: the Multiethnic Cohort. Ethn Dis. 2009;19:49–55. [PMC free article] [PubMed] [Google Scholar]
- McDonough CW, Palmer ND, Hicks PJ, et al. A GENOME WIDE ASSOCIATION STUDY FOR DIABETIC NEPHROPATHY GENES IN AFRICAN AMERICANS. Kidney Int. 2011;79:563–572. doi: 10.1038/ki.2010.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigs JB, Shrader P, Sullivan LM, et al. Genotype Score in Addition to Common Risk Factors for Prediction of Type 2 Diabetes. N Engl J Med. 2008;359:2208–2219. doi: 10.1056/NEJMoa0804742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AP, Voight BF, Teslovich TM, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981–990. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng MCY. Meta-analysis of genome-wide association studies in African Americans provides insights into the genetic architecture of type 2 diabetes. 2013 doi: 10.1371/journal.pgen.1004517. Manuscript in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng MCY, Saxena R, Li J, et al. Transferability and fine mapping of type 2 diabetes loci in African Americans: the Candidate Gene Association Resource Plus Study. Diabetes. 2013;62:965–976. doi: 10.2337/db12-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer ND, McDonough CW, Hicks PJ, et al. A genome-wide association search for type 2 diabetes genes in African Americans. PloS One. 2012;7:e29202. doi: 10.1371/journal.pone.0029202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra EJ, Below JE, Krithika S, et al. Genome-wide association study of type 2 diabetes in a sample from Mexico City and a meta-analysis of a Mexican-American sample from Starr County, Texas. Diabetologia. 2011;54:2038–2046. doi: 10.1007/s00125-011-2172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JRB, Voight BF, Yengo L, et al. Stratifying type 2 diabetes cases by BMI identifies genetic risk variants in LAMA1 and enrichment for risk variants in lean compared to obese cases. PLoS Genet. 2012;8:e1002741. doi: 10.1371/journal.pgen.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Cornelis MC, Kraft P, et al. Genetic variants at 2q24 are associated with susceptibility to type 2 diabetes. Hum Mol Genet. 2010;19:2706–2715. doi: 10.1093/hmg/ddq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Hu FB, Hu G. Genes, environment, and interactions in prevention of type 2 diabetes: a focus on physical activity and lifestyle changes. Curr Mol Med. 2008;8:519–532. doi: 10.2174/156652408785747915. [DOI] [PubMed] [Google Scholar]
- Rung J, Cauchi S, Albrechtsen A, et al. Genetic variant near IRS1 is associated with type 2 diabetes, insulin resistance and hyperinsulinemia. Nat Genet. 2009;41:1110–1115. doi: 10.1038/ng.443. [DOI] [PubMed] [Google Scholar]
- Sale MM, Freedman BI, Langefeld CD, et al. A genome-wide scan for type 2 diabetes in African-American families reveals evidence for a locus on chromosome 6q. Diabetes. 2004;53:830–837. doi: 10.2337/diabetes.53.3.830. [DOI] [PubMed] [Google Scholar]
- Shu XO, Long J, Cai Q, et al. Identification of new genetic risk variants for type 2 diabetes. PLoS Genet. 2010;6:e1001127. doi: 10.1371/journal.pgen.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim X, Ong RT-H, Suo C, et al. Transferability of type 2 diabetes implicated loci in multi-ethnic cohorts from Southeast Asia. PLoS Genet. 2011;7:e1001363. doi: 10.1371/journal.pgen.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinthorsdottir V, Thorleifsson G, Reynisdottir I, et al. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet. 2007;39:770–775. doi: 10.1038/ng2043. [DOI] [PubMed] [Google Scholar]
- Takeuchi F, Serizawa M, Yamamoto K, et al. Confirmation of multiple risk Loci and genetic impacts by a genome-wide association study of type 2 diabetes in the Japanese population. Diabetes. 2009;58:1690–1699. doi: 10.2337/db08-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Peng J, Wang P, Risch NJ. Estimation of individual admixture: analytical and study design considerations. Genet Epidemiol. 2005;28:289–301. doi: 10.1002/gepi.20064. [DOI] [PubMed] [Google Scholar]
- Timpson NJ, Lindgren CM, Weedon MN, et al. Adiposity-related heterogeneity in patterns of type 2 diabetes susceptibility observed in genome-wide association data. Diabetes. 2009;58:505–510. doi: 10.2337/db08-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai F-J, Yang C-F, Chen C-C, et al. A genome-wide association study identifies susceptibility variants for type 2 diabetes in Han Chinese. PLoS Genet. 2010;6:e1000847. doi: 10.1371/journal.pgen.1000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unoki H, Takahashi A, Kawaguchi T, et al. SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat Genet. 2008;40:1098–1102. doi: 10.1038/ng.208. [DOI] [PubMed] [Google Scholar]
- Voight BF, Scott LJ, Steinthorsdottir V, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010;42:579–589. doi: 10.1038/ng.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters KM, Stram DO, Hassanein MT, et al. Consistent association of type 2 diabetes risk variants found in europeans in diverse racial and ethnic groups. PLoS Genet. 2010 doi: 10.1371/journal.pgen.1001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Hara K, Maeda S, et al. A genome-wide association study in the Japanese population identifies susceptibility loci for type 2 diabetes at UBE2E2 and C2CD4A-C2CD4B. Nat Genet. 2010;42:864–868. doi: 10.1038/ng.660. [DOI] [PubMed] [Google Scholar]
- Yu H, Bowden DW, Spray BJ, et al. Identification of human plasma kallikrein gene polymorphisms and evaluation of their role in end-stage renal disease. Hypertension. 1998;31:906–911. doi: 10.1161/01.hyp.31.4.906. [DOI] [PubMed] [Google Scholar]
- Zeggini E, Scott LJ, Saxena R, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40:638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.