Abstract
Purpose
This study investigated the effects of the physicochemical properties of antibiotics on the morphology, loading efficiency, size, release kinetics, and antibiotic efficacy of loaded poly(DL-lactic-co-glycolic acid) (PLGA) microparticles (MPs) at different loading percentages.
Methods
Cefazolin, ciprofloxacin, clindamycin, colistin, doxycycline, and vancomycin were loaded at 10 and 20 weight percent into PLGA MPs using a water-in-oil-in water double emulsion fabrication protocol. Microparticle morphology, size, loading efficiency, release kinetics, and antibiotic efficacy were assessed.
Results
The results from this study demonstrate that the chemical nature of loaded antibiotics, especially charge and molecular weight, influence the incorporation into and release of antibiotics from PLGA MPs. Drugs with molecular weights less than 600 Da displayed biphasic release while those with molecular weights greater than 1000 Da displayed triphasic release kinetics. Large molecular weight drugs also had a longer delay before release than smaller molecular weight drugs. The negatively charged antibiotic cefazolin had lower loading efficiency than positively charged antibiotics. Microparticle size appeared to be mainly controlled by fabrication parameters, and partition and solubility coefficients did not appear to have an obvious effect on loading efficiency or release. Released antibiotics maintained their efficacy against susceptible strains over the duration of release. Duration of release varied between 17–49 days based on the type of antibiotic loaded.
Conclusions
The data from this study indicate that the chemical nature of antibiotics affects properties of antibiotic-loaded PLGA MPs and allows for general prediction of loading and release kinetics.
Keywords: Controlled release, PLGA, microparticles, antibiotic delivery, infection
1.0 Introduction
Infection remains a major complication of bone injury due to pathology or traumatic injury. The presence of infection is a serious deterrent to healing in bone tissue and may result in adverse outcomes including non-union and amputation [1]. The current standard of care relies on irrigation and debridement of infected tissue in combination with either systemic antibiotics over the course of several weeks to months or local delivery of antibiotic from poly(methylmethacrylate) (PMMA) beads, both of which have their disadvantages [2,3]. Systemic antibiotics come with a variety of undesirable side effects, including nephrotoxicity and ototoxicity, that can prove challenging when attempting to achieve sufficient concentration of antibiotic in the infected area to treat the infection. In addition, many antibiotics are only administered intravenously, and the cost of both inpatient and outpatient care is considerable [4]. Implantation of antibiotic-loaded PMMA beads achieves local delivery of antibiotics, but the majority of drug is released in the first few days, and the implantation of a foreign body in an infected area can cause further infectious complications via bacterial attachment to the biomaterial surface [3,5–9]. Additionally, the presence of sub-therapeutic levels of antibiotic through inadequate systemic or local delivery can contribute to the development of antibiotic resistance, which is a significant problem for both the patient and the public [1,7].
Poly(DL-lactic-co-glycoic acid) (PLGA) microparticles (MPs) have been used as a delivery vehicle for antibiotics of almost all types due to its tunable degradation profile and biocompatible degradation products [2,3,10]. Several studies have reported the release of antibiotics from PLGA MPs to occur over the span of weeks to months [4,11–14]. Antibiotic-loaded PLGA MPs have also been incorporated into tissue engineering scaffolds for the purpose of mitigating infection, and release has been reported to occur over 8 weeks in vitro and efficacy of the construct has been established in an infected in vivo model [3,5–9,11,15].
Bone infections are caused by of a variety of bacteria, most commonly Staphylococcus aureus, but also including coagulase-negative Staphyloccus spp., Streptococcus spp., E. coli, and Pseudomonas aeruginosa [16,17]. Because not all antibiotics are effective against all bacteria, antibiotic therapy must be carefully considered in order to avoid undertreating the infection or promoting antibiotic resistance. For this reason, many different classes and types of antibiotics must be able to be incorporated into PLGA MPs. As previous studies have shown, there are a multitude of fabrication parameters for PLGA MPs that affect drug loading and rate of release [18,19]. Additionally, the type of antibiotic may also play a role in the final characteristics of the loaded MPs due to polymer/drug interactions. The ability to use knowledge of the chemical nature of antibiotics to predict and manipulate the properties and release kinetics of PLGA MPs is advantageous for designing fabrication protocols that minimize antibiotic loss while ensuring a favorable antibiotic release profile for treating bone infections.
This study investigated six antibiotics of different classes and chemistries into PLGA MPs and evaluated the effects of the physicochemical properties of each antibiotic on the properties and release kinetics of antibiotic-loaded PLGA MPs. A single protocol was used to fabricate all MPs in order to isolate the effects of the drug on MP properties. It was hypothesized that charge, molecular weight, and partition and solubility coefficients of the incorporated antibiotic would influence the loading efficiency, size, and release kinetics of the resulting MPs, and that the antibiotic activity of the released drug would be maintained.
2.0 Materials and Methods
2.1 Materials
Poly(DL-lactic-co-glycolic acid) was obtained from Lakeshore Biomaterials (Birmingham, AL) and had a copolymer ratio of 50:50, a weight average molecular weight of 36 kDa, and a number average molecular weight of 21 kDa. Poly(vinyl) alcohol (PVA), Mueller-Hinton broth, ciprofloxacin, colistin sulfate, doxycycline hyclate, and vancomycin hydrochloride were obtained from Sigma Aldrich (St Louis, MO). Cefazolin sodium and clindamycin hydrochloride were obtained from Fisher Scientific (Waltham, MA). Properties of these antibiotics are detailed in Table 1. Colistin (polymyxin E) has two HPLC retention times due to this drug being a mixture of two drugs, colistin A and colistin B (Table 1). Experimental values for logP and logS were used when available, otherwise predicted values were calculated using ALOGPS (VCCLabs) [20,21]. The charge of antibiotics at pH 6.4–7.4 is shown, which encompasses the pH of aqueous phases during fabrication and the pH of the release medium. Ciprofloxacin was acidified with 5N HCl in order to create the water-soluble hydrochloride salt of ciprofloxacin. Bacterial strains Staphylococcus aureus (ATCC 29213), Escherichia coli (ATCC 25922), and Pseudomonas aeruginosa (ATCC 27853) were obtained from ATCC (Manassas, VA).
Table I.
Charge, pKa, molecular weight, HPLC retention time, logP, and logS of loaded antibiotics.
| Antibiotic | Antibiotic Class | Charge | pKa | Molecular Weight (g/mol) | Retention time (min) | logP | logS |
|---|---|---|---|---|---|---|---|
| Cefazolin sodium | Cephalosporin | −1 | 2.3 | 476.5 | 14.9 | −0.58 | −3.0 |
| Ciprofloxacin hydrochloride | Fluoroquinolone | +1 | 6.1, 8.7 | 331.3 | 15.1 | −0.57 | −2.4 |
| Clindamycin hydrochloride | Lincosamide | +1 | 7.79 | 461.4 | 18.4 | 2.16 | −2.1 |
| Colistin sulfate | Polymyxin | +5 | 10.3 and above | 1155.4 | 14.8/16.3 | −2.4 | −3.7 |
| Doxycycline hyclate | Tetracycline | 1 | 3.4, 7.7, 9.3 | 512.9 | 18.5 | −0.02 | −2.9 |
| Vancomycin hydrochloride | Glycopeptide | +1 | 2.6, 7.7, 8.6, 9.6, 10.5, 11.7 | 1485.7 | 11.9 | −3.1 | −3.8 |
2.2 Microparticle Fabrication
PLGA MPs containing various antibiotics were fabricated using a water-in-oil-in-water double emulsion solvent evaporation technique [11]. The internal water phase consisted of antibiotics dissolved in 0.3 wt% PVA in water at 10 wt% or 20 wt% of final microparticle weight, or a concentration of either 139 mg/mL or 312.5 mg/mL, respectively. All antibiotics were fully solubilized in the internal aqueous phase after 30 min on a rotary shaker table at 37°C. The oil phase consisted of PLGA dissolved in methylene chloride at a concentration of 222 mg/mL. The internal phase was added to the oil phase at a ratio of 1:5.6 v/v and emulsified using a Qsonica Q125 probe sonicator (Newtown, CT). The internal phase/oil phase emulsion was poured into a beaker containing 250 mL of outer phase comprising 0.3 wt% PVA and 4 wt% NaCl stirring at 700 rpm. The solvent was allowed to evaporate over 4 hrs, and the resulting microparticles were washed, frozen, and lyophilized. Microparticles were sieved to less than 300 μm and stored at −20°C. Blank microparticles were fabricated using an internal phase of 0.3 wt% PVA without antibiotic. Each formulation was synthesized in triplicate.
2.3 Scanning Electron Microscopy
SEM was used to examine the external and internal morphology of blank and antibiotic-loaded PLGA MPs. The internal morphology of the MPs was exposed by embedding them in HistoPrep, freezing, and creating 10 μm sections with a Leica cryotome (Allentown, NJ). The microparticles were sputter-coated with 20 nm of gold using a Torr International CrC-150 sputtering system (New Windsor, NY) and observed under a FEI Quanta 400 field emission scanning electron microscope (Hillsboro, OR) at an accelerating voltage of 5 kV.
2.4 Determination of Loading Efficiency
The loading efficiency of antibiotics into PLGA MPs was determined by dissolving MPs in methylene chloride and extracting the antibiotic into either phosphate-buffered saline (PBS) (pH 7.4) for cefazolin, ciprofloxacin, clindamycin, and doxycycline or potassium phosphate monobasic (pH 2.85) for colistin and vancomycin. Briefly, 20 mg of microparticles were dissolved in 2 mL of methylene chloride for 30 minutes. 20 mL of PBS (pH 7.4) was added and the mixture was stirred rapidly for 2 hrs to allow extraction of antibiotic into the PBS and evaporation of the organic solvent. Vancomycin and colistin could not be extracted with this method, so the protocol was modified. As before, 20 mg of MPs were dissolved in 2 mL methylene chloride for 30 min. To extract vancomycin and colistin, 20 mL of potassium phosphate monobasic was added, sonicated for 5 min, then stirred rapidly for 2 hrs. The concentration of antibiotic in the aqueous phase was determined by HPLC. Loading efficiency was calculated as
where Dout is the amount of drug recovered from Pout, a specified amount of microparticles and Din and Pin are the amounts of antibiotic and polymer used in the initial fabrication of the MPs, respectively.
2.5 Microparticle Size Determination
Microparticle size was determined using a Beckman Coulter Counter (Brea, CA). Briefly, 20 mg of MPs were mixed into Isotone II solution, and a 280 μm aperture was used to determine average particle diameter ± standard deviation. Microparticle diameter data was collected using n = 1000 particles per MP batch (n=3 per formulation). Each batch of MPs was tested in triplicate.
2.6 Antibiotic Release from PLGA Microparticles
Release kinetics of each of the antibiotics from PLGA MPs was determined by placing 50 mg of MPs into 5 mL of PBS (pH 7.4) under mild shaking (n=3). Each replicate was synthesized independently in order to assess inter-batch reliability. The supernatant was completely removed and replaced with fresh PBS at 12 hours and days 1, 4, 7, and biweekly until 49 days. The release medium was filtered with a 0.2 μm syringe filter, and the concentration of antibiotic was determined by HPLC. The HPLC system comprised a Waters 2695 separation module and 2996 photodiode array detector (Milford, MA) with a Waters XTerra RP 18 column (250mm × 4.6mm) at 35 °C. For cefazol in, ciprofloxacin, clindamycin, doxycycline, and vancomycin, the elution was performed with a flow rate of 1 mL/min in a mobile phase consisting of 25 mM KH2PO4 (Sigma, HPLC grade, pH 3) and acetonitrile (Sigma, HPLC grade). Peaks were eluted with a linear gradient of 5%–60% acetonitrile in water over 20 min. For colistin, the elution was performed with a flow rate of 0.5 mL/min in a mobile phase consisting of acetonitrile (HPLC grade) and water (0.22 μm filtered with 0.1 vol.% trifluoroacetic acid). Peaks were eluted with a linear gradient of 10%–65% acetonitrile in water over 20 min. Absorbance was monitored at λ = 270, 274, 204, 214, 350, and 274 nm for cefazolin, ciprofloxacin, clindamycin, colistin, doxycycline, and vancomycin, respectively. Standard solutions with antibiotic in PBS buffer (pH 7.4) were tested in the range of 5–1000 μg/mL. The percent cumulative release was calculated as amount of antibiotic released at each timepoint as a percentage of total antibiotic loaded. Release curves were divided into phases based on a line of best fit analysis.
2.7 Susceptibility Testing
The minimum inhibitory concentration (MIC) of released antibiotics against relevant strains of bacteria was tested using a broth microdilution assay as described in ISO standard 20776. Briefly, supernatant from the release study was sterile filtered and used as a stock solution in the broth microdilution assay. The stock solution was serially diluted with sterile Mueller-Hinton broth to 50 μL aliquots with concentrations ranging from 0 mg/L to 16 mg/L. Early and late timepoints were tested and were determined by using the earliest and latest timepoints at which there was at least 32 μg/mL of antibiotic in solution. Cefazolin was tested at days 7 and 17, ciprofloxacin at 0.5 and 14, clindamycin at 7 and 17, colistin at 14 and 31, doxycycline at 10 and 17, and vancomycin at 14 and 35. A 0.5 MacFarland standard of bacteria cultured in Mueller-Hinton broth was diluted 1:100 in sterile Mueller-Hinton broth, and 50 μL of the inoculum was added to each well. The lowest concentration well without growth after 18 h of culture at 37°C was denoted the MIC.
2.8 Statistical Analysis
All values are reported as mean ± standard deviation. Loading efficiency, MP size, release phases and cumulative release were compared using ANOVA with post-hoc analysis by Tukey’s honestly significant difference with an a priori level of significance set at α=0.05 (n = 3 samples). The MICs of each antibiotic were compared using Kruskal-Wallis with post-hoc analysis by Mann-Whitney U-test (n = 3). JMP (Version 7, SAS Institute Inc., Cary, NC) was used for ANOVA and post-hoc testing. R (R Development Core Team, 2010) was used for univariate and multivariate linear regression modeling [22].
3.0 Results
3.1 Scanning Electron Microscopy
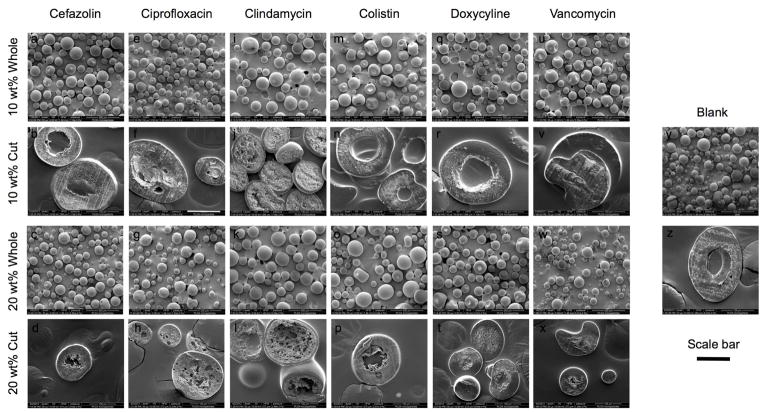
Representative SEM images of 10 wt% loaded and 20 wt% loaded MPs, both whole and sectioned, are shown in Fig. 1. The exterior morphology of the MPs is smooth, with few to no macropores noted. The MPs appear to have porous interiors surrounded by a thin shell of relatively non-porous PLGA, with the exception of 20 wt% ciprofloxacin, which appears to be uniformly porous throughout.
Figure 1.
Representative scanning electron micrographs of whole MPs and cut MPs to demonstrate external and internal morphology of cefazolin (a,b,c,d), ciprofloxacin (e,f,g,h), clindamycin (i,j,k,l), colistin (m,n,o,p), doxycycline (q,r,s,t), and vancomycin (u,v,w,x) of 10 wt% whole, 10 wt% cut, 20 wt% whole, and 20 wt% cut MPs, respectively. For comparison, blank MPs are shown whole and cut in (y) and (z), respectively. Whole MPs are shown at 100x magnification, and cut MPs are shown at 500x magnification. The scale bar represents 100 μm for rows 1 and 3 and (y), and 50 μm for rows 2 and 4 and (z).
3.2 Loading efficiency
Loading efficiencies for the various formulations of antibiotic-loaded PLGA MPs can be seen in Table 2. Ciprofloxacin loaded MPs show a decrease in loading efficiency when loading is increased from 10 wt% to 20 wt% due to precipitation of the antibiotic out of the primary water-in-oil emulsification during loading (p<0.05). While ciprofloxacin could be dissolved at 312.5 mg/mL in the internal aqueous phase, the solution was unstable and precipitated immediately upon addition of the polymer/dichloromethane solution. All other antibiotics show no significant difference in loading efficiency between 10 wt% and 20 wt% (p>0.05). At 10 wt% loading, the loading efficiency of cefazolin is significantly less than all other antibiotics. At 20 wt% loading, cefazolin and ciprofloxacin have decreased loading efficiencies compared to all other antibiotics.
Table II.
Loading efficiencies of microparticle formulations.
| Loading Efficiency of 10 wt% antibiotic-loaded PLGA MPs (%) | Loading Efficiency of 20 wt% antibiotic-loaded PLGA MPs (%) | |
|---|---|---|
| Cefazolin | 36.4 ± 3.3a | 51.3 ± 6.1d |
| Ciprofloxacin | 86.8 ± 10.9b,c,* | 38.9 ± 17.8d,* |
| Clindamycin | 84.9 ± 6.9b,c | 89.5 ± 4.0e,f |
| Colistin | 102.0 ± 5.0c | 105.3 ± 4.4f |
| Doxycyline | 71.4 ± 2.3b | 89.4 ± 0.9e,f |
| Vancomycin | 83.3 ± 4.8b,c | 76.9 ± 6.2e |
Indicates significant difference between 10wt% and 20wt% loaded for a given antibiotic.
Superscript letters indicate significant difference between antibiotics for a given loading wt%
Data is presented as mean ± standard deviation, n=3 per group.
3.3 Microparticle Size
Microparticle size for each formulation can be seen in Table 3. In the 10 wt% loaded groups, ciprofloxacin MPs are larger than vancomycin MPs (p<0.05). In the 20 wt% loaded group, there is no difference in average MP size between any of the antibiotics (p>0.05). There is no difference in MP size for any other antibiotic between 10 wt% loaded and 20 wt% loaded MPs, and there is no difference between any formulation and blank MPs (p>0.05). The reported distribution is based on number of particles.
Table III.
Microparticle sizes of each microparticle formulation.
| Size of 10 wt% antibiotic-loaded PLGA Microparticles (μm) | Size of 20 wt% antibiotic-loaded PLGA Microparticles (μm) | |
|---|---|---|
| Blank | 43.34 ± 41.93 | |
| Cefazolin | 41.9 ± 38.6 | 47.6 ± 40.3 |
| Ciprofloxacin | 60.3 ± 49.7a | 36.5 ± 31.9 |
| Clindamycin | 48.6 ± 33.4 | 51.0 ± 40.8 |
| Colistin | 41.3 ± 33.3 | 40.9 ± 34.9 |
| Doxycyline | 41.7 ± 33.1 | 41.5 ± 35.3 |
| Vancomycin | 33.1± 28.7b | 36.9 ± 28.1 |
Superscript letters indicate significant difference between groups.
Data is presented as mean ± standard deviation, n=3 per group.
3.4 Antibiotic Release
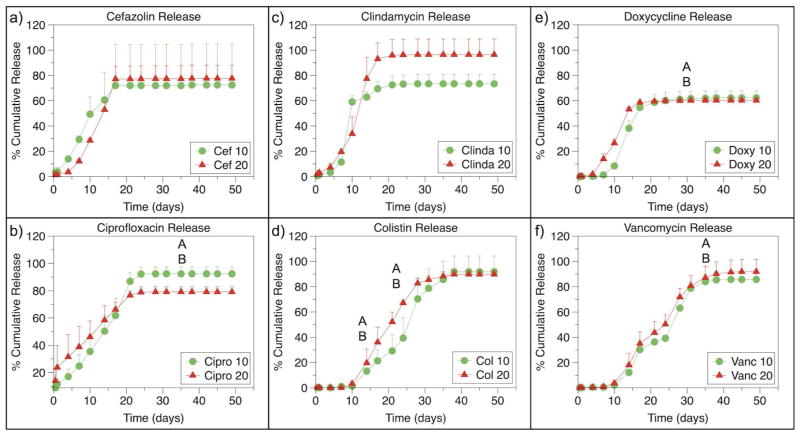
Release curves for each formulation of MP are shown in Fig. 2. The amount of antibiotic released at each time point was well below the solubility of each drug, indicating that the release kinetics seen are not a result of saturation of the medium. The release curves for each formulation were analyzed by comparing the rate of release during different phases of release. Rates of release are shown in Supplemental Tables SI and SII. Cefazolin, ciprofloxacin, clindamycin, and doxycycline shared a sigmoidal release curve with biphasic release while vancomycin and colistin appeared to share similar triphasic release kinetics.
Figure 2.
Release curves for a) cefazolin, b) ciprofloxacin, c) clindamycin, d) colistin, e) doxycycline, and f) vancomycin loaded PLGA microparticles at 10 wt% and 20 wt%. Significant differences between release rates during different phase are indicated by letters. For ciprofloxacin loaded MPs, there is a significant different between 10 wt% and 20 wt% loaded MPs during Phase 1 (p<0.05). For colistin loaded PLGA MPs, Phase 2 and Phase 3 are significantly different between 10 wt% and 20 wt% loaded MPs (p<0.05). Ciprofloxacin and doxycycline 10 wt% loaded MPs demonstrate increased release of antibiotic during Phase 3 compared to 20 wt% loaded MPs, and vancomycin loaded MPs demonstrate increased release from 20 wt% MPs compared to 10 wt% MPs in phase 4 (p<0.05). Cumulative percent release is the same between 10 wt% and 20 wt% for any antibiotic (p>0.05).
Of note, during Phase 1, 20 wt% ciprofloxacin loaded MPs have a significantly greater burst release than 20 wt% cefazolin, clindamycin, or doxycycline loaded MPs (p<0.05); there was no difference between 20 wt% cefazolin, clindamycin, or doxycycline loaded MPs (p>0.05). During Phase 2, the phase in which the majority of antibiotic is released, there is no difference in release rate between cefazolin, ciprofloxacin, clindamycin, and doxycycline when comparing either 10 wt% to 20 wt% loaded MPs or when comparing between cefazolin, ciprofloxacin, clindamycin, and doxycycline (p>0.05).
No significant differences between 10 wt% and 20 wt% loaded colistin and vancomycin loaded MPs were noted in Phase 1 or 2 (p>0.05). During Phase 2, 20 wt% loaded colistin MPs released more quickly than 10 wt% loaded colistin MPs (p<0.05). During Phase 3, the major release phase for colistin and vancomycin, there was no differences in release rates between colistin and vancomycin loaded MPs (p<0.05).
Cumulative percent release was not statistically different between the 10 wt% and 20 wt% loaded MPs for any antibiotic (p>0.05). In the 10 wt% loaded group, there was no difference in cumulative percent release between any antibiotics (p>0.05). In the 20 wt% loaded group, doxycycline MPs released less of the incorporated drug than clindamycin MPs (p<0.05).
3.5 Effects of Charge, Molecular Weight, Partition Coefficient, and Solubility Coefficient
The effects of charge, molecular weight, partition coefficient and solubility coefficient on loading efficiency, major release phase rate, and length of lag phase can be seen in Supplemental Figs. S1, S2, and S3, respectively. The correlation coefficients for the univariate linear regression can be found in Table 4. The length of lag phase was determined by the first timepoint at which the percent cumulative release increased more than 5%, as this increase was a precursor to the beginning of the release phase. The charge of the loaded antibiotic is strongly correlated to the loading efficiency in 10 wt% and 20 wt% loaded MPs (Table 4). When 20 wt% loaded ciprofloxacin MPs are excluded due to the precipitation of antibiotic during fabrication, the correlation coefficient between charge and loading efficiency for 20 wt% loaded MPs is 0.87. Correlations of loading efficiency and release rate to logP and logS are weak (<0.5) (Table 4). Length of lag phase is strongly correlated to logS and very strongly correlated to molecular weight (Table 4). Multivariate linear regression modeling showed no relationship between any of the explanatory variables (charge, molecular weight, logP, logS) and the response variables (loading efficiency, major release rate, length of lag phase).
Table IV.
Correlation coefficients between physicochemical properties and loading efficiency, dominant rate of release, and length of lag phase.
| Charge | Molecular Weight | logP | logS | |
|---|---|---|---|---|
| Loading Efficiency 10 wt% | 0.82 | 0.39 | −0.20 | −0.13 |
| Loading Efficiency 20 wt% | 0.68 | 0.45 | −0.05 | −0.34 |
| Release Rate 10 wt% | −0.15 | −0.14 | −0.02 | −0.03 |
| Release Rate 20 wt% | −0.26 | −0.19 | −0.47 | −0.18 |
| Length of Lag Phase 10 wt% | 0.64 | 0.85 | −0.57 | −0.75 |
| Length of Lag Phase 20 wt% | 0.55 | 0.93 | −0.68 | −0.86 |
3.6 Susceptibility
The MIC of both fresh antibiotic and antibiotic released from microparticles against standard strains of bacteria at early and late timepoints is shown in Table 5. Fresh antibiotic, antibiotic released at an early timepoint, and antibiotic released at a late timepoint have the same efficacy against the tested bacterial strain (p>0.05).
Table V.
MIC of fresh antibiotics and antibiotics released from PLGA microparticles against susceptible strains at early and late time points.
| Antibiotic loaded into PLGA MPs | Bacterial Strain | MIC of fresh antibiotic (μg/mL) | MIC of antibiotic from 10 wt% PLGA MPs (μg/mL) | MIC of antibiotic from 20 wt% PLGA MPs (μg/mL) | ||
|---|---|---|---|---|---|---|
| Early | Late | Early | Late | |||
| Cefazolin | S. aureus | 0.25 ± 0.00 | 0.25 ± 0.00 | 0.25 ± 0.00 | 0.25 ± 0.00 | 0.25 ± 0.00 |
| Ciprofloxacin | P. aeruginosa | 0.10 ± 0.04 | 0.13 ± 0.00 | 0.13 ± 0.00 | 0.13 ± 0.00 | 0.13 ± 0.00 |
| Clindamycin | S. aureus | 0.19 ± 0.00 | 0.19 ± 0.00 | 0.19 ± 0.00 | 0.19 ± 0.00 | 0.19 ± 0.00 |
| Colistin | E. coli | 5.33 ± 2.31 | 8.56 ± 7.04 | 6.67 ± 2.31 | 4.00 ± 0.00 | 5.33 ± 2.31 |
| Doxycyline | S. aureus | 0.13 ± 0.00 | 0.13 ± 0.00 | 0.13 ± 0.00 | 0.13 ± 0.00 | 0.13 ± 0.00 |
| Vancomycin | S. aureus | 0.25 ± 0.00 | 0.29 ±0.19 | 0.13 ± 0.00 | 0.33 ± 0.14 | 0.42 ± 0.14 |
Data is presented as mean ± standard deviation, n=3 per group
4.0 Discussion
Sustained local delivery of antibiotics via a PLGA particulate carrier is an attractive approach to the problem of implant-associated infections given the advantages of increased local concentrations, decreased systemic toxicity, and increased duration of antimicrobial coverage [15,23]. While antibiotic-loaded PLGA MPs have been widely investigated in the literature and have transitioned into the clinic on a limited basis, there is a relative dearth of studies that investigate the effect of drug characteristics on the properties and release kinetics of the resulting MPs.
In this study, six antibiotics of different classes were loaded at 10 wt% and 20 wt% into PLGA MPs using a single protocol, and the resulting MPs were assessed for morphology, loading efficiency, size, release kinetics, and activity against susceptible bacterial strains. It was hypothesized that the properties and release kinetics of the MPs could be predicted using known properties of the antibiotics being loaded such as charge, molecular weight, partition coefficient, and solubility coefficient. The results of this study indicate that certain antibiotic properties affect the characteristics of antibiotic-loaded PLGA MPs their release kinetics and that the chemical nature of antibiotics should be considered when designing fabrication protocols to achieve desired outcomes such as high loading efficiency and predictable release kinetics.
The external morphology of the MPs by SEM (Fig. 1) is consistent with previous studies that demonstrate that high polymer concentration results in decreased surface porosity. This occurs via the formation of micropores due to the increased viscosity of the water/oil emulsion and stabilization of the film at the oil/water interface [18,24]. The internal morphology of the MPs varies between antibiotics. The difference between the large pores seen in 20 wt% ciprofloxacin and 10 wt% and 20 wt% clindamycin MPs and the numerous small pores seen in ciprofloxacin 10 wt%, cefazolin, colistin, doxycycline, and vancomycin MPs may be due to interactions between the internal aqueous phase and the oil phase. While all antibiotics within their respective wt% loadings were dissolved at the same concentration (either 139 mg/mL or 312.5 mg/mL) in the same volume of internal phase, the aqueous antibiotic internal phase may either impart different viscosities to the drug/polymer emulsion or influence interfacial properties through interactions with the polymer, the PVA surfactant, or the external aqueous phase [25–27]. Mao et al. demonstrated that MPs fabricated with high viscosity polymer have small internal pores while MPs fabricated with low viscosity polymer have large internal pores, possibly due to the ability of the trapped aqueous internal phase to coalesce within a lower viscosity polymer solution [18]. The emulsion of clindamycin with PLGA may result in a lower viscosity solution than the emulsion of cefazolin, colistin, doxycycline, and vancomycin, resulting in the observed internal pore morphologies. Ciprofloxacin 20 wt% is unique in that it is the only formulation in which the dissolved drug solution precipitated during fabrication. This could contribute to the interesting internal morphology seen, where the large pores are either due to a lower viscosity with less ciprofloxacin entrapped or the presence of aggregates of ciprofloxacin precipitate.
The loading efficiencies of the MPs fabricated in this study are comparable to or better than available previous studies [11–13,28], especially given the relative hydrophilicity of these drugs. Only 10 and 20 wt% cefazolin-loaded MPs and 20 wt% ciprofloxacin-loaded MPs had entrapment efficiencies less than 70%. These results are likely due to a combination of two factors: polymer phase concentration and charge of the loaded antibiotic.
Previous studies have shown that increasing the polymer phase concentration increases the entrapment efficiency of loaded drug [18,19,24]. High polymer concentration can increase loading efficiency by stabilizing the interface between oil phase and external phase, resulting in the formation of particles with a dense polymer matrix, high surface area-to-volume ratio, and smaller, more tortuous pore networks, leading to decreased water penetration and decreased leaching of drug during the hardening phase [18,24]. Since the polymer concentration was kept constant for each formulation, charge is likely responsible for the difference between high loading efficiencies formulations and 10 wt% cefazolin, 20 wt% cefazolin, and 20 wt% ciprofloxacin.
Charge is another parameter than can affect loading efficiency. As seen in Table 4, loading efficiency is positively correlated with charge, with correlation coefficients of 0.82 and 0.68 for 10 wt% and 20 wt% loaded MPs, respectively. When ciprofloxacin 20 wt% is excluded due to precipitation of ciprofloxacin out of the emulsification phase, the correlation for 20 wt% loaded MPs is 0.87 (Table 4). Because the PLGA used in this study is uncapped and negatively charged, the low entrapment efficiency seen in the cefazolin groups is likely due to the negative charge of cefazolin at pH>3.6 (Table 1) [29–31]. In contrast, all other antibiotics are either zwitterionic or positively-charged at the pH of the internal and external aqueous phases. Colistin, a polypeptide antibiotic, demonstrated particularly high entrapment efficiency in both 10 wt% and 20 wt% formulations that could be attributed to the strong positive charge at pH 6.4. In the 10 wt% group, colistin loaded more efficiently than doxycycline, while in the 20 wt% group, colistin loaded more efficiently than vancomycin. Doxycycline, a tetracycline antibiotic, and vancomycin, a glycopeptide antibiotic, both had high entrapment efficiencies and are positively charged at pH 6.4, but due to the multitude of pKa values spanning a large pH range, both antibiotics also contain a negative charge that may have lowered its loading efficiency compared to colistin [32,33]. Negative-negative antibiotic-polymer charge interactions can promote the expulsion of cefazolin from the MPs, while favorable positive-negative interactions enhance the retention of zwitterionic and positively charged antibiotics. However, the presence of negative charges still appears to affect entrapment even when the overall charge is positive. These results demonstrate that while polymer concentration heavily influences the loading of PLGA MPs, the charge of the drug is also an important factor that can affect loading efficiency when using a w/o/w technique. Efficient incorporation of drug into the carrier is an important design criteria for local delivery systems given the expense of newer antibiotics necessary to treat common drug-resistant organisms[34].
Previous studies of PLGA w/o/w fabrication parameters indicate that MP size is largely a function of polymer concentration and stirring speed [18,19,24,35]. In this study, fabrication parameters, particularly temperature, internal and external phase volumes, and polymer concentration, were held constant between formulations, so differences in size may be attributed to characteristics of the incorporated drug. The MPs synthesized in this study were not statistically different in size compared to blank (unloaded) MPs, owing in part to the heterogeneity of MP sizes produced.
The fabrication parameters of antibiotic-loaded PLGA MPs can greatly affect release, and many previous studies have investigated the effects of the multitude of parameters that can be altered, such as internal phase volume, choice of polymer and polymer phase concentration, choice and concentration of surfactant, and drug loading [12,13,18,19,24,36]. Previous studies have also investigated the effects of different hydrophobic, minimally water soluble drugs on release from PLGA films and have found that the type of drug incorporated can affect release kinetics[37–39]. In this study of relatively hydrophilic and freely water soluble antibiotics, fabrication parameters were kept constant in order to examine the effects of different antibiotics on release kinetics.
The release of antibiotics from PLGA MPs in this study was characterized by little to no burst release during the lag phase, followed by daily release of drug above MIC for 17–49 days, depending on the type and loading of antibiotic. Similar to a previous study of antibiotic release from PLGA MPs from our laboratory, release from MPs in this study appears to be controlled by both diffusion and degradation [11]. The data suggest that molecular weight and solubility play a role in determining how quickly drug can begin to diffuse out from a dense polymer matrix, as suggested by the strong positive correlation between molecular weight and length of the lag phase and logS and length of the lag phase (Table 4). Solubility does not appear to significantly affect the rate of release during the release phases, as indicated by the very weak correlation between the two factors (Table 4). Relatively low molecular weight drugs (<600 Da), including cefazolin, ciprofloxacin, clindamycin, and doxycycline, displayed a biphasic release profile while larger molecular weight drugs (>1100 Da) displayed a triphasic release profile.
Typical release profiles for drug-loaded PLGA MPs demonstrate early off-loading of a large percentage of drug. In this study, early drug release from 0–1 days, which normally corresponds to diffusion-controlled release, is minimal due to a combination of decreased drug adsorption on the surface of the MPs and restriction of the pores due to swelling of the PLGA [18]. However, 20 wt% ciprofloxacin MPs displayed a greater initial burst release due to an increased amount of adsorbed drug on the surface of the MPs. This occurrence could be related to the precipitation of drug during fabrication (Fig. 1). For low molecular weight drugs, the bulk of the drug release occurs during days 1–21 with zero-order kinetics (Fig. 2). For large molecular weight drugs, release occurs in two bursts between 10 and 38 days, with the exception of 20 wt% loaded colistin MPs. The biphasic characteristic of drug release during this time period is indicative of release due to a combination of diffusion and degradation [11]. The PLGA used in this study, PLGA 50:50, degrades through bulk erosion, and is an amorphous polymer, leading to faster degradation than other copolymer ratios. Upon incubation in PBS pH 7.4, bulk degradation begins via swelling of the PLGA and hydrolysis of ester bonds, resulting in decreasing molecular weight and increasing polydispersity [40]. After initial immersion in PBS, bulk degradation may result in sufficient random chain scission to allow low molecular weight drugs to begin diffusing into the surrounding media. In contrast, the higher molecular weight drugs colistin and vancomycin cannot begin diffusing out until more significant degradation has occurred after 10 days (Fig. 2). This phenomenon could also be influenced by the fact that the larger molecular weight drugs in this study also tended to have lower solubility (correlation = −0.88), though the correlation of logS to lag phase is relatively weaker than that of molecular weight. The length of the lag phase and general release kinetics appear to be mainly influenced by molecular weight and solubility, but the rate of release appears to be mainly dependent on physical and material properties, such as diffusion and degradation, as supported by the lack of correlation between antibiotic properties and release rate (Table 4).
Surprisingly, neither charge nor solubility appears to have a strong effect on the rate of antibiotic release from PLGA MPs (Table 4). While colistin demonstrated similar triphasic release as vancomycin at 10 wt% loading, colistin appeared to take on the biphasic release profile of the small molecular weight drugs at 20 wt% loading. Change in release kinetics with increasing concentration of drug has been noted in other studies, possibly as a result of increased drug content contributing to faster degradation of the polymer and by creation of microporosity following drug dissolution [12,41]. It is unclear why this effect was seen with colistin and not with vancomycin. The large standard deviation seen in the release of cefazolin from 10 wt% loaded MPs was not seen in other formulations, and may indicate poor reproducibility in drug distribution within the MPs contributing to differing release kinetics.
The cumulative release of many antibiotics in this study did not reach 100% although the MPs had fully degraded by the end of the study. This could be due to degradation of the antibiotics between time points. Doxycycline is well-known for its photosensitivity and degrades in aqueous solution, especially when incubated at 37°C [42]. Although other antibiotics undergo some degradation in water, the PLGA matrix may have provided more protection from degradation while doxycycline was afforded less protection due to its particular sensitivity to light [43–46].
Loss of antibiotic activity is a serious concern when fabricating controlled delivery vehicles. Fabrication of PLGA MPs requires the use of an organic solvent and a method of emulsion, which may have deleterious effects on antibiotic efficacy. In addition, the MPs are incubated in conditions that may cause degradation of some antibiotics, as seen with doxycycline. All antibiotics in this study remained effective against susceptible strains at both early and late time points (Table 5), ranging from 12 hours to 35 days. The retention of efficacy is likely due to protection of the antibiotic within the polymer matrix until it is solubilized and released.
Some limitations of this study include the use of only one antibiotic with a negative charge and the lack of a standard statistical analysis to complete a main effects analysis on release curves. This study could also be expanded to include multiple antibiotics from each class. The relation between antibiotic chemistry and release kinetics could be further investigated by creating the relatively insoluble freebase counterparts of each antibiotic salt and evaluating incorporation into and release from PLGA MPs composed of terminally capped and uncapped polymer [37,39,47]. Prior et al. have investigated the relationship between gentamicin loading efficiency, end groups of PLGA, and pH of the aqueous phase, finding that differences in loading efficiency between capped and uncapped PLGA are likely due to a combination of terminal group and pH influence on exposed charges [31]. Further studies could elucidate the influence of end-capping on encapsulation by using antibiotics of different charges with the same fabrication protocol. While in vitro studies allow for the evaluation and comparison of the effects of drug loading on properties and release kinetics of PLGA MPs in a well-controlled environment, these results should be further expanded upon to evaluate release kinetics in vivo.
The information from this study indicates that consideration of antibiotic charge, molecular weight, and solubility is useful in predicting loading efficiency and in vitro release kinetics, respectively. This information could be useful in designing PLGA MPs for applications in which knowledge of expected release profile is necessary, such as early delivery of a small molecule antibiotic to an infected bone defect followed temporally by delivery of a large osteogenic protein such as bone morphogenetic protein-2. Furthermore, knowledge of the effect of drug properties can guide choice of polymer and fabrication parameters in order to minimize waste during processing and provide a basis for the rational design of PLGA MP drug release systems.
5.0 Conclusion
This study analyzed the effects of incorporation of various antibiotics on the properties and in vitro release kinetics of degradable, antibiotic-loaded PLGA MPs. Important characteristics of the drug-loaded MPs, such as loading efficiency and release kinetics, could be explained by the charge and molecular weight of the loaded antibiotic. The charge of loaded antibiotic was a significant factor in the loading efficiency, with negative charges conferring decreased efficiency compared to positive charges on the antibiotics. The time course of antibiotic release was variable between 3 weeks and 7 weeks, and small molecule drugs were released more quickly than large molecular weight drugs. Molecular weight also determine biphasic or triphasic release pattern, but solubility and partition coefficient did not appear to affect the rate of release. Microparticle size was mainly determined by fabrication parameters and did not appear to be significantly affected by the loaded drug. Importantly, the antibiotics loaded into the MPs retained their efficacy against bacteria both early and late in the release. The results of this study indicate that knowledge of the chemical nature of a drug can allow for prediction of important properties of drug-loaded MPs, particularly loading efficiency and release kinetics.
Supplementary Material
Acknowledgments
This work was supported by the Army, Navy, NIH, Air Force, VA and Health Affairs to support the AFIRM II effort, under Award No. W81XWH-14-2-0004. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. SRS and PPS would like to acknowledge the Baylor College of Medicine Medical Scientist Training Program.
Abbreviations
- MIC
Minimum Inhibitory Concentration
- MP
Microparticle
- PBS
Phosphate Buffered Saline
- PLGA
Poly(DL-lactic-co-glycolic acid)
- PMMA
Poly(methylmethacrylate)
- PVA
Poly(vinyl alcohol)
References
- 1.Somayaji SN, Ritchie S, Sahraei M, Marriott I, Hudson MC. Staphylococcus aureus Induces Expression of Receptor Activator of NF-κB Ligand and Prostaglandin E2 in Infected Murine Osteoblasts. Infect Immun. 2008 Oct 17;76:5120–6. doi: 10.1128/IAI.00228-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiemann AH, Hofmann GO. Principles of the therapy of bone infections in adult extremities. Strat Traum Limb Recon. 2009 Jul 3;4:57–64. doi: 10.1007/s11751-009-0059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kent ME, Rapp RP, Smith KM. Antibiotic beads and osteomyelitis: Here today, what’s coming tomorrow? Orthopedics. 2006;29:599–603. doi: 10.3928/01477447-20060701-02. [DOI] [PubMed] [Google Scholar]
- 4.Paladino JA, Poretz D. Outpatient parenteral antimicrobial therapy today. Clin Infect Dis. 2010 Sep 15;51(Suppl 2):S198–S208. doi: 10.1086/653520. [DOI] [PubMed] [Google Scholar]
- 5.Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987 Sep 25;237:1588–95. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 6.Gristina AG, Shibata Y, Giridhar G, Kreger A, Myrvik QN. The glycocalyx, biofilm, microbes, and resistant infection. Semin Arthroplasty. 1994 Oct 1;5:160–70. [PubMed] [Google Scholar]
- 7.Anagnostakos K, Hitzler P, Pape D, Kohn D, Kelm J. Persistence of bacterial growth on antibiotic-loaded beads: is it actually a problem? Acta Orthop. 2008 Apr 1;79:302–7. doi: 10.1080/17453670710015120. [DOI] [PubMed] [Google Scholar]
- 8.Shah SR, Tatara AM, D’Souza RN, Mikos AG, Kasper FK. Evolving strategies for preventing biofilm on implantable materials. Mater Today. 2013 May;16:177–82. [Google Scholar]
- 9.Miclau T, Edin ML, Lester GE, Lindsey RW, Dahners LE. Bone toxicity of locally applied aminoglycosides. J Orthop Trauma. 1995 Jan 1;9:401–6. doi: 10.1097/00005131-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Delivery Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 11.Shi M, Kretlow JD, Nguyen A, Young S, Baggett LS, Wong ME, et al. Antibiotic-releasing porous polymethylmethacrylate constructs for osseous space maintenance and infection control. Biomaterials. 2010 Mar 4;31:4146–56. doi: 10.1016/j.biomaterials.2010.01.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramchandani M, Robinson D. In vitro and in vivo release of ciprofloxacin from PLGA 50:50 implants. J Controlled Release. 1998 Jul 31;54:167–75. doi: 10.1016/s0168-3659(97)00113-2. [DOI] [PubMed] [Google Scholar]
- 13.Özalp Y, Özdemir N, Kocagöz S, Hasirci V. Controlled release of vancomycin from biodegradable microcapsules. J Microencapsul. 2001 Jan 1;18:89–110. doi: 10.1080/026520401750038638. [DOI] [PubMed] [Google Scholar]
- 14.Pillai RR, Somayaji SN, Rabinovich M, Hudson MC, Gonsalves KE. Nafcillin-loaded PLGA nanoparticles for treatment of osteomyelitis. Biomed Mater. 2008 Sep 1;3:034114–4. doi: 10.1088/1748-6041/3/3/034114. [DOI] [PubMed] [Google Scholar]
- 15.Spicer PP, Shah SR, Henslee AM, Watson BM, Kinard LA, Kretlow JD, et al. Evaluation of antibiotic releasing porous polymethylmethacrylate space maintainers in an infected composite tissue defect model. Acta Biomater. 2013 May;11:1–31. doi: 10.1016/j.actbio.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Lord CF, Gebhardt MC, Tomford WW, Mankin HJ. Infection in bone allografts. Incidence, nature, and treatment. J Bone Joint Surg Am. 1988 Mar 1;70:369–76. [PubMed] [Google Scholar]
- 17.Calhoun J, Manring MM, Shirtliff M. Osteomyelitis of the Long Bones. Seminars in Plastic Surgery. 2009 Apr 30;23:059–72. doi: 10.1055/s-0029-1214158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao S, Xu J, Cai C, Germershaus O, Schaper A, Kissel TT. Effect of WOW process parameters on morphology and burst release of FITC-dextran loaded PLGA microspheres. Int J Pharm. 2007 Apr 4;334:137–48. doi: 10.1016/j.ijpharm.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 19.Ghaderi R, Sturesson C, Carlfors J. Effect of preparative parameters on the characteristics of poly d, l-lactide-co-glycolide) microspheres made by the double emulsion method. Int J Pharm. 1996;141:205–16. [Google Scholar]
- 20.Wishart DS, Knox C, Guo AC, Cheng D. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36:D901–6. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tetko IV, Gasteiger J, Todeschini R, Mauri A, Livingstone D, Ertl P, et al. Virtual computational chemistry laboratory--design and description. J Comput Aided Mol Des. 2005 Jun 1;19:453–63. doi: 10.1007/s10822-005-8694-y. [DOI] [PubMed] [Google Scholar]
- 22.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: [Internet] Available from: http://www.R-project.org/ [Google Scholar]
- 23.Wahlig H, Dingeldein E, Buchholz HW, Buchholz M, Bachmann F. Pharmacokinetic study of gentamicin-loaded cement in total hip replacements. Comparative effects of varying dosage. J Bone Joint Surg Br. 1984 Mar 1;66:175–9. doi: 10.1302/0301-620X.66B2.6707051. [DOI] [PubMed] [Google Scholar]
- 24.Chaisri W, Ghassemi AH, Hennink WE, Okonogi S. Enhanced gentamicin loading and release of PLGA and PLHMGA microspheres by varying the formulation parameters. Colloids and Surfaces B: Biointerfaces. 2011 Jan 1;84:508–14. doi: 10.1016/j.colsurfb.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Pandey JD, Shukla A, Misra K, Rai RD. Ultrasonic, volumetric, and viscometric studies of tetracycline and its allied compound. J Chem Eng Data. 1989;34:29–31. [Google Scholar]
- 26.Weber A, Morlin G, Cohen M, Williams-Warren J, Ramsey B, SMITH A. Effect of nebulizer type and antibiotic concentration on device performance. Pediatr Pulmonol. 1997 Apr 1;23:249–60. doi: 10.1002/(sici)1099-0496(199704)23:4<249::aid-ppul2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 27.Sherman P. The influence of internal phase viscosity on the viscosity of concentrated water-in-oil emulsions. Kolloid-Zeitschrift. 1955;141:6–11. [Google Scholar]
- 28.Mundargi RC, Srirangarajan S, Agnihotri SA, Patil SA, Ravindra S, Setty SB, et al. Development and evaluation of novel biodegradable microspheres based on poly(d,l-lactide-co-glycolide) and poly(ε-caprolactone) for controlled delivery of doxycycline in the treatment of human periodontal pocket: In vitro and in vivo studies. J Controlled Release. 2007 May 14;119:59–68. doi: 10.1016/j.jconrel.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Singh M, Briones M, Ott G, O’Hagan D. Cationic microparticles: a potent delivery system for DNA vaccines. PNAS. 2000;97:811–6. doi: 10.1073/pnas.97.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chun KW, Yoo HS, Yoon JJ, Park TG. Biodegradable PLGA Microcarriers for Injectable Delivery of Chondrocytes: Effect of Surface Modification on Cell Attachment and Function. Biotechnol Prog. 2004 Dec 3;20:1797–801. doi: 10.1021/bp0496981. [DOI] [PubMed] [Google Scholar]
- 31.Prior S, Gamazo C, Irache JM, Merkle HP, Gander B. Gentamicin encapsulation in PLA/PLGA microspheres in view of treatingBrucellainfections. Int J Pharm. 2000 Jan 1;196:115–25. doi: 10.1016/s0378-5173(99)00448-2. [DOI] [PubMed] [Google Scholar]
- 32.Vijan LE. The interaction of vancomycin with DNA. Rev Roum Chem. 2009;54:807–13. [Google Scholar]
- 33.Giovagnoli S, Tsai T, DeLuca PP. Formulation and Release Behavior of Doxycycline–Alginate Hydrogel Microparticles Embedded into Pluronic F127 Thermogels as a Potential New Vehicle for Doxycycline Intradermal Sustained Delivery. AAPS PharmSciTech. 2010 Feb 2;11:212–20. doi: 10.1208/s12249-009-9361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falagas ME, Fragoulis KN, Karydis I. A comparative study on the cost of new antibiotics and drugs of other therapeutic categories. PLoS ONE. 2006;1:e11. doi: 10.1371/journal.pone.0000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravi S, Peh KK, Darwis Y, Murthy BK, Singh TRR, Mallikarjun C. Development and characterization of polymeric microspheres for controlled release protein loaded drug delivery system. Indian J Pharm Sci. 2008 May 1;70:303–9. doi: 10.4103/0250-474X.42978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaisri W, Hennink WE, Okonogi S. Preparation and characterization of cephalexin loaded PLGA microspheres. Curr Drug Delivery. 2009;6:69–75. doi: 10.2174/156720109787048186. [DOI] [PubMed] [Google Scholar]
- 37.Frank A, Rath SK, Venkatraman SS. Controlled release from bioerodible polymers: effect of drug type and polymer composition. J Controlled Release. 2005 Feb;102:333–44. doi: 10.1016/j.jconrel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Siegel SJ, Kahn JB, Metzger K, Winey KI, Werner K, Dan N. Effect of drug type on the degradation rate of PLGA matrices. Eur J Pharm Biopharm. 2006 Nov 1;64:287–93. doi: 10.1016/j.ejpb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 39.Maulding HV, Tice TR, Cowsar DR, Fong JW, Pearson JE, Nazareno JP. Biodegradable microcapsules: acceleration of polymeric excipient hydrolytic rate by incorporation of a basic medicament. J Controlled Release. 1986;3:103–17. [Google Scholar]
- 40.Lu L, Garcia CA, Mikos AG. In vitro degradation of thin poly(DL-lactic-co-glycolic acid) films. J Biomed Mater Res. 1999 Aug 1;46:236–44. doi: 10.1002/(sici)1097-4636(199908)46:2<236::aid-jbm13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 41.Sanders LM, McRae GI, Vitale KM, Kell BA. Controlled delivery of an LHRH analogue from biodegradable injectable microspheres. J Controlled Release. 1985 Nov;2:187–95. [Google Scholar]
- 42.Honnorat-Benabbou VC, Lebugle AA, Sallek B, Duffaut-Lagarrigue D. Stability study of tetracyclines with respect to their use in slow release systems. J Mater Sci: Mater Med. 2001;12:107–10. doi: 10.1023/a:1008909708650. [DOI] [PubMed] [Google Scholar]
- 43.Shah JC, Sadhale Y, Chilukuri DM. Cubic phase gels as drug delivery systems. Adv Drug Delivery Rev. 2001;47:229–50. doi: 10.1016/s0169-409x(01)00108-9. [DOI] [PubMed] [Google Scholar]
- 44.Donnelly RF. Stability of Pantoprazole Sodium in Glass Vials, Polyvinyl Chloride Minibags, and Polypropylene Syringes. Can J Hosp Pharm. 2011;64:252–6. doi: 10.4212/cjhp.v64i3.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orwa JA, Govaerts C, Gevers K, Roets E, Van Schepdael A, Hoogmartens J. Study of the stability of polymyxins B 1, E 1 and E 2 in aqueous solution using liquid chromatography and mass spectrometry. J Pharm Biom Anal. 2002;29:203–12. doi: 10.1016/s0731-7085(02)00016-x. [DOI] [PubMed] [Google Scholar]
- 46.Raverdy V, Ampe E, Hecq JD, Tulkens PM. Stability and compatibility of vancomycin for administration by continuous infusion. J Antimicrob Chemother. 2013 Apr 12;68:1179–82. doi: 10.1093/jac/dks510. [DOI] [PubMed] [Google Scholar]
- 47.Li B, Brown KV, Wenke JC, Guelcher SA. Sustained release of vancomycin from polyurethane scaffolds inhibits infection of bone wounds in a rat femoral segmental defect model. J Controlled Release. 2010;145:221–30. doi: 10.1016/j.jconrel.2010.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.