Abstract
The Th1-associated chemokines CXCL-9, CXCL-10, CXCL-11 coordinate migration of CXCR3+Th1 cells. The objective of this study was to evaluate the role of the innate immune system in stimulating chemokine expression in an experimental model of dry eye and bridge the gap between innate and adaptive immunity. Desiccating stress (DS) induced very early (six hours) expression and production of Th1-associated chemokine in cornea and conjunctiva of C57BL/6 and recombination activating gene 1 (RAG1) knock out (KO) strains, demonstrating that chemokine expression does not require innate T cells. We then demonstrated that activating the innate immune system prior to adoptive transfer of T cells to RAG1KO increased disease severity. Interestingly, lack of induction of chemokines CXCL-9, CXCL-10, CXCL-11 in IFN-γKO mice, provided evidence that their expression requires IFN-γ for induction. Treatment of RAG1KO mice with anti-NK1.1 prevented the increase of CXCL9, CXCL10, and CXCL11 in response to DS, compared to isotype controls. Additionally, DS increased the expression of NKG2D in the conjunctiva. The expression of the NKG2D ligand, RAE-1, also increased at the ocular surface at both the protein and gene level. Neutralization of NKG2D at the ocular surface decreased the expression of CXCL-9, CXCL-10, CXCL-11 and IFN-γ. In summary, upregulation of CXCL9, CXCL-10, and CXCL-11 expression in experimental dry eye is T cell independent, requiring IFN-γ-producing NKG2D+ NK cells that are activated in response to DS induced stress signals. This work provides insight about the events that trigger the initial immune response in dry eye pathology.
Keywords: Dry eye, chemokines, NK cells, NK cell receptor ligands
Introduction
Previous studies in our laboratory have demonstrated that T lymphocytes, especially CD4+ T cells, are capable of causing pathologic changes of dry eye (1). Both Th1 and Th17 cells have been shown to modulate the immune response at the ocular surface (OS) (2,3). The prototypical cytokine of Th1 cells, IFN-γ, has been found to induce apoptosis in the corneal and conjunctival epithelium and goblet cell loss in conjunctiva (3,4). IFN-γ is increased in the tears and conjunctiva of aqueous deficient dry eye patients (5). Furthermore, IFN-γ has been implicated in the pathogenesis of conjunctival epithelial squamous metaplasia, progressive goblet cell loss and increased expression of the cornification marker small proline-rich protein-2a (3).
Homing of T cells to the OS is dependent on both the expression of chemokines by epithelial cells and the expression of chemokine receptors on migrating T cells. The chemokines CXCL9 (MIG), CXCL10 (IP-10), CXCL11 (I-TAC), which bind CXCR3, coordinate the migration of CXCR3+Th1 cells and are highly induced by IFN-γ (6). These chemokines are upregulated on the corneal and conjunctival epithelium in response to desiccating stress (DS) in mice and in dry eye patients (7,8). Our previous results indicate that CXCR3KO mice do not develop disease, as migration of Th1 cells to the OS is required for disease (9), thus suggesting that Th1-associated chemokines are vital to the pathogenesis of dry eye disease.
There are several types of lymphocytes involved in innate immunity. Natural killer T (NKT) cells express NK cell markers and conventional αβ T cell receptors (TCR). NKT cells are known to be involved in mucosal immunity and many autoimmune diseases, including psoriasis, asthma, and multiple sclerosis (10,11) and are an important source of IFN-γ. γΔ T cells are small subset of T cells that have γ and Δ chains that compose distinct TCRs differing from conventional αβ TCRs. γΔ T cells are important in innate immunity by regulating immune responses and producing various cytokines, including IFN-γ and IL-17 (12).
Natural killer (NK) cells are one of the earliest lines of defense in innate immunity that are activated quickly to respond to pathogens or tumors. NK cells lack antigen receptors like T and B cells, but instead express a series of activating or inhibitory receptors (13). Although there are many activating NK cell receptors, one of the major activating receptors expressed on all NK cells is NKG2D. The ligands for NKG2D are self-proteins related to MHC class I molecules that are induced under various conditions of stress, such as heat shock or inflammation. In humans the ligands for NKG2D are MHC class I chain-related protein A (MICA), MICB and six isoforms of UL16-binding protein (ULBP) (14,15). In mice there are several ligands of NKG2D: five isoforms of retinoic acid early inducible gene 1 (RAE-1) (α-ε), three minor histocompatibility antigen 60 (H60) molecules (a-c), and murine ULBP-like transcript 1 (MULT1) (16). After receiving an activation signal, NK cells produce perforin and granzymes or express ligands that induce apoptosis. Several studies have shown that NK cells are involved in the pathogenesis of dry eye disease (17,18). IFN-γ-producing NK cells have shown to increase in the early stages of experimental dry eye induction and depletion of NK cells led to decreased disease severity (18). NK cells play an active role in activating antigen presenting cells (APCs) through an early burst of cytokines, including IL-17A, IL-6 and IFN-γ (17).
Few studies have focused on the events that occur very early in the pathogenesis of dry eye disease. Critical to the design of new therapeutics is an understanding of the events that trigger the onset of the disease. Here, we describe events that occur very early in disease induction in our experimental model: 1) that IFN-γ produced by NK cells is responsible for upregulation of Th1 cell recruiting chemokines by conjunctival and corneal epithelial cells; 2) DS increases expression of activating NK cell receptor ligands; 3) and increased activation of innate immune pathways leads to increased T cell infiltration and function.
Materials and Methods
Mice
Six to eight week old female IFN-gamma KO (B6.129S7-Ifngtm1Ts/J), RAG1KO (B6.129S7-Rag1tm1Mom/J), and C57BL/6J were purchased from The Jackson Laboratory (Bar Harbor, ME) for establishment of mouse colonies in our vivarium. All knockout mice (KO) have a C57BL/6 background. All animal experiments were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine and adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Induction of desiccating stress in mice
C57BL/6J, IFN-γKO, IL-17KO and RAG1KO mice were exposed to desiccating stress (DS). DS was induced by subcutaneous injection of scopolamine hydrobromide (0.5 mg/0.2 ml; Sigma-Aldrich, St. Louis) four times a day (08:00, 12:00, 14:00, and 17:00 h), alternating flanks, for 6 hours (6H), 1, 5 or 10 consecutive days (DS1, DS5 or DS10), as previously described (3,19). Mice were placed in a cage with a perforated plastic screen on one side to allow airflow from a fan placed 6 inches in front of it for 16 h/day. Room humidity was maintained at 20-30%. Control mice were maintained in a non-stressed (NS) environment at 50-75% relative humidity without exposure to a forced air draft. DS and NS mice served as donors for adoptive transfer experiments.
RNA isolation and Real time PCR
Total RNA from conjunctiva and corneal epithelium was isolated using a QIAGEN RNeasy Plus Micro RNA isolation kit (Qiagen) following the manufacturer’s protocol. Corneal epithelium was scraped with a scalpel; conjunctiva was surgically excised. One sample equaled the tissue pooled from both eyes of each animal. After isolation, the concentration of RNA was measured and cDNA was synthesized using the Ready-To-Go™ You-Prime First-Strand kit (GE Healthcare). Real time PCR was performed using specific Taqman probes for CXCL9 (CXCL9) (Mm00434946_m1), CXCL10 (CXCL10) (Mm00445235_m1), CXCL11 (CXCL11) (Mm00444662_m1), IFN-γ (IFNG) (Mm00801778_m1), CCL20 (CCL20) (Mm01268754_m1), RAE-1α-ε (RAE1) (Mm04206137_gH), MULT1 (Ulbp1) (Mm01180648_m1), IL-17A (IL17A) (Mm0043918_m1), IL-13 (IL13) (Mm99999190_m1), IL-6 (IL6) (Mm99999064_m1), MMP-3 (MMP3) (Mm00440295_m1), MMP-9 (MMP9) (Mm00442991_m1), CD99 (Cd99) (Mm04214669_u1), Tbet (Tbx21) (Mm00450960_m1), and CD4 (Cd4) (Mm00442754_m1) genes (Taqman Universal PCR Master Mix AmpErase UNG) in a commercial thermocycling system (StepOnePlus™ Real-Time PCR System, Applied Biosystems), according to the manufacturer’s recommendations. The beta-2 microglobulin (β2m) (B2M) (Mm00437762_m1) gene was used as an endogenous reference for each reaction. The results of quantitative PCR were analyzed by the comparative Ct method in which the target of change = 2−ΔΔCt and were normalized by the Ct value of β2m and the mean Ct of relative mRNA level in the normal control group (non-stressed; NS) of each mouse strain. Gene expression of 3-4 mice per strain per time point in two independent experiments with a total of 6-8 mice was determined.
Immunodetection of Chemokines and RAE-1
Immunofluorescence staining was performed on experimental and control tissue sections to study the expression of CXCL9 (AF-492-NA, R&D Systems), CXCL10 (AF-466-NA, R&D Systems) and RAE-1 (C-20, sc-20333, Santa Cruz Biotechnology) in cornea and conjunctiva. Tissue sections (two slides per animal/strain/time point) for immunofluorescence staining were fixed in 4% paraformaldehyde at RT for 10 minutes. After blocking in 1% BSA in PBS for 1 hour, primary antibodies diluted 1:50 (from 0.2 mg/ml stock) in BSA were applied and sections were incubated for 1 hour at RT. Sections were washed with PBS and incubated with Alexa Fluor 488 diluted 1:250 (from 12.5 μg/ml stock) in PBS for 60 minutes at room temperature in a dark chamber, followed by counterstaining with propidium iodide (2 μg/mL in PBS) for 10 minutes. Negative controls were included by omitting primary antibodies. Sections were viewed and photographed with a laser scanning confocal microscope (LSM 510, with Krypton-argon and He-Ne laser; Carl Zeiss Meditec, Inc., Thornwood, NY) and were acquired with a 40x oil-immersion objective.
Isolation of murine CD4+ T cells
Superficial cervical lymph nodes (CLN) and spleens from donor mice were meshed gently between two frosted end slides, as previously described (1). Ammonium chloride tris was used to eliminate erythrocytes. Untouched CD4+ cells were isolated by negative selection using magnetic beads according to the manufacturer’s instructions (MACS system; Miltenyi Biotec, Auburn, CA). Isolated cells were used in adoptive transfer experiments. Purity of CD4+ T cells was determined to be greater than 90% by flow cytometry (data not shown). T cells were isolated from 3-4 mice per time point in two independent experiments using a total of 6-8 mice.
Adoptive transfer experiments
CD4+ cells (5 × 106 - approximately one donor-equivalent of cells) isolated from the spleens and cervical lymph nodes were transferred intraperitoneally (i.p.) to T cell deficient RAG1KO mice that were subjected to DS or not exposed to DS. Parameters of dry eye were evaluated three days after adoptive transfer of CD4+ T cells. Donor T cells were transferred to 3-4 recipient mice per time point in two independent experiments using a total of 6-8 mice.
Histology and Periodic Acid Schiff Staining and Goblet cell measurement
Enucleated mouse eyes were fixed in 10% formalin, and embedded in paraffin. Six-μm sections were stained with either hematoxylin and eosin or periodic acid-Schiff (PAS) reagent, as previously described (3). Goblet cell density in the superior and inferior conjunctiva was measured (n=3/strain/per time point) using NIS Elements Software (version 3.0, BR, Nikon, Melville, NY) using a ×10 objective. Three sections from three animals were examined (N = 9) for each time point/mouse for goblet cell density.
Immunohistochemistry
Immunohistochemistry was performed to detect and count the number of cells in conjunctival epithelium that stained positively for CD4 (clone H129.9, 10 μg/mL, BD Bioscience, San Diego, CA) and appropriate biotinylated secondary antibody (BD Pharmingen) and Vectastain Elite ABC using NovaRed reagents (Vector, Burlingame, CA) as previously described (3). Secondary antibody alone and appropriate anti-rat isotype (BD Biosciences) controls were also examined. Positively stained cells were counted in the goblet cell rich area of the conjunctiva using image-analysis software (NIS Elements Software, Nikon, Melville, NY) using a ×10 objective. Three sections from three animals were examined (N = 9) for each time point/mouse for CD4+ T cell. Immunohistochemistry was performed to detect the presence of NKG2D positive cells in the conjunctival epithelium with anti-NKG2D antibody (clone 191004, MAB1547, 10μg/mL, R&D Systems) and appropriate biotinylated secondary antibody (BD Pharmingen) and Vectastain Elite ABC using NovaRed reagents (Vector, Burlingame, CA) as previously described above.
NK cell and NKG2D depletion
Mice subjected to DS for 6 hours, 1 day, or 5 days received intraperitoneal injections (IP) of anti-NK1.1 antibody (PK136) (20) or i.p. injections of mouse-IgG isotype control (Sigma-Aldrich). Mice received a total of four i.p. injections at days -4,-2, 0 and +2 when subjected to DS for 5 days or three injections for NS, 6 hours, or 1 day. NK cells were depleted in 3-4 mice per strain per time point in two independent experiments using a total of 6-8 mice and gene expression studies were performed. NKG2D was depleted by topically applying neutralizing Ab with 2.5 μg/5μl in PBS (clone 191004, MAB1547, R&D Systems) to the eye three times per day starting two days prior to exposure to desiccating stress and continued up to DS1. As a control, eye were topically treated with rat-IgG isotype control.
Statistical Analysis
Sample size and power calculations were performed using Statmate software based on preliminary studies. Statistical analyses were performed with Graph Pad Prism software (Graph Pad, Inc, version 6). Data was first evaluated for normality with the Kolmogorov-Smirnov normality test. Appropriate parametric (two-way ANOVA or t-test) or non-parametric (Mann-Whitney U or Wilcoxon) statistical tests were used to make comparisons between 2 groups.
Results
Expression of Th1-associated chemokines is increased early after exposure to desiccating stress
We have previously reported chemokines are upregulated in the epithelium in response to DS in the experimental dry eye model (7) and also in dry eye patients (8). In order to determine the mechanism(s) responsible for the increase, C57BL/6 mice were exposed to six hours, one, five, or ten days of DS. A significant increase in CXCL10 (DS6H) and CXCL11 (DS1) mRNA transcripts in the conjunctiva was observed compared to NS controls (Figure 1B). Expression of Th1-associated chemokines did not occur early in the cornea, but was delayed until day five for CXCL9 and CXCL10. Th1-associated chemokines are highly induced by IFN-γ. Interestingly, IFN-γ was also upregulated very early after exposure to DS in both the conjunctiva and cornea (Figure 1A and B).
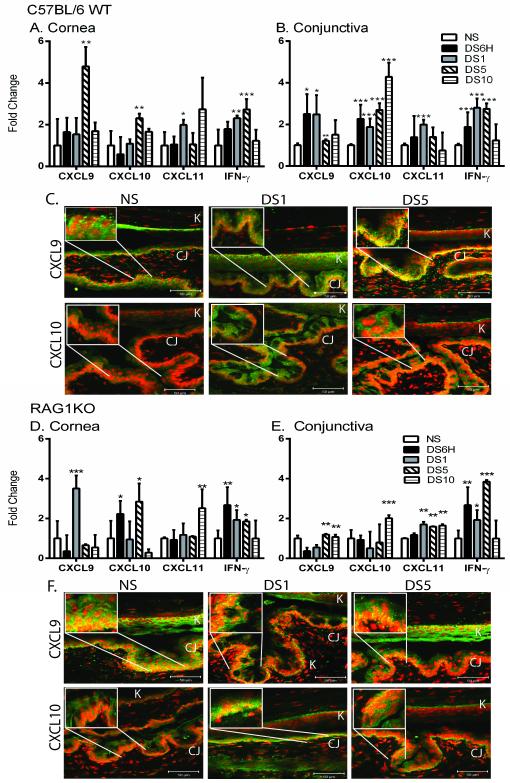
Figure 1. Induction of Th1-associated chemokines and IFN-γ occurs early in induction of dry eye disease.
A-B. Gene expression of CXCL9, CXCL10, CXCL11, and IFN-γ in cornea (A) and conjunctiva (B) of non-stressed (NS), six hours (DS6H) of desiccating stress (DS), one day of DS (DS1), five (DS5), or ten days (DS10) of DS C57BL/6 mice. C. Merged pictures of laser scanning immunofluorescent confocal microscopy of cornea and conjunctiva sections immunostained for CXCL9 (green) and CXCL10 (green) of NS, DS1, or DS5 of C57BL/6 WT mice with propidium iodide (PI) (red) nuclear counter staining. Yellow indicates strong double positive staining. D-E. Gene expression of CXCL9, CXCL10, CXCL11, and IFN-γ in cornea (E) and conjunctiva (F) of NS, DS6H, DS1, DS5, or DS10 of RAG1KO mice. F. Merged pictures of laser scanning immunofluorescent confocal microscopy of cornea and conjunctiva immunostained for CXCL9 (green) and CXCL10 (green) of NS, DS1, or DS5 of RAG1KO mice with propidium iodide (red) nuclear counter staining. Scale bar −50 μm. Results represent the mean ± SD expression of 6-8 animals per time point.*, p < 0.05; **, p < 0.01; ***, p <0.001 compared to NS. White boxes indicate magnified area.
In order to confirm that gene expression is translated to protein expression, immunofluorescence staining was performed. We previously demonstrated by ELISA that protein expression of these chemokines increases in response to DS (7). Increased expression of CXCL9 and CXCL10 expression in the basal layer of the conjunctiva was observed at DS1 (Figure 1C). A further increase in CXCL9 immunoreactivity was seen at DS5 as indicated by strong staining (yellow) in the merged picture. Immunofluoresce staining was not performed for CXCL11 due a point mutation that occurs in C57BL/6 mice that prevents translation of functional protein (21); however gene expression level can be determined. In our model, we demonstrate that an increase in Th1-associated chemokine expression occurs very early in the pathogenesis of disease.
Increased expression of Th1-associated chemokines does not require innate T cells
There are several possible sources for cellular source of IFN-γ very early in the progression of DED. To address the hypothesis that innate T cells (such as γΔ T cells or NKT cells) are the source of IFN-γ responsible for upregulation of Th1-associated chemokines, the expression of these chemokines was examined in T cell-deficient RAG1KO, as these do not have γΔ T cells, NKT cells, CD4+ or CD8+ T cells. The expression of CXCL9 and CXCL10 is upregulated in the cornea and conjunctiva in response to DS in RAG1KO mice (Figure 1D and E), demonstrating expression does not require the presence of T cells. The expression of IFN-γ was also increased in RAG1KO mice when subjected to DS. This is consistent with the hypothesis that IFN-γ is produced by a non-T cell source in response to DS. Protein expression of CXCL9 and CXCL10 also increased in the conjunctival epithelium of RAG1KO mice in response to DS (Figure 1F). These results indicated that increased expression of Th1-associated chemokines is not dependent on γΔ T cells, NKT T cells or acquired immunity.
Desiccating stress increases disease severity in RAG1KO CD4+T cell adoptive transfer recipient mice
We next sought to determine if DS treatment of T cell deficient RAG1KO recipient mice prior to adoptive transfer (AT) would increase disease severity. In order to examine this, recipient RAG1KO mice were exposed to DS prior to adoptive transfer of CD4+ T cells from DS5 C57BL/6 donor mice (DS5 CD4+ T). RAG1KO recipient mice were not stressed (NS RAG1KO) or exposed to DS for 1 (DS1 RAG1KO) or 5 (DS5 RAG1KO) days. As a control, RAG1KO recipient mice received CD4+ T cells from NS donor C57BL/6 mice (NS CD4+ T cells). RAG1KO recipient mice exposed to DS had significantly increased CD4+ T cell infiltration compared to NS RAG1KO recipients at each time point (Figure 2A). Recipients that received DS5 CD4+ T cells had increased T cell infiltration with increasing times of DS in recipient mice. Increased T cell infiltration was also observed between NS and DS5 RAG1KO recipients that received NS CD4+ T cells (Figure 2A).
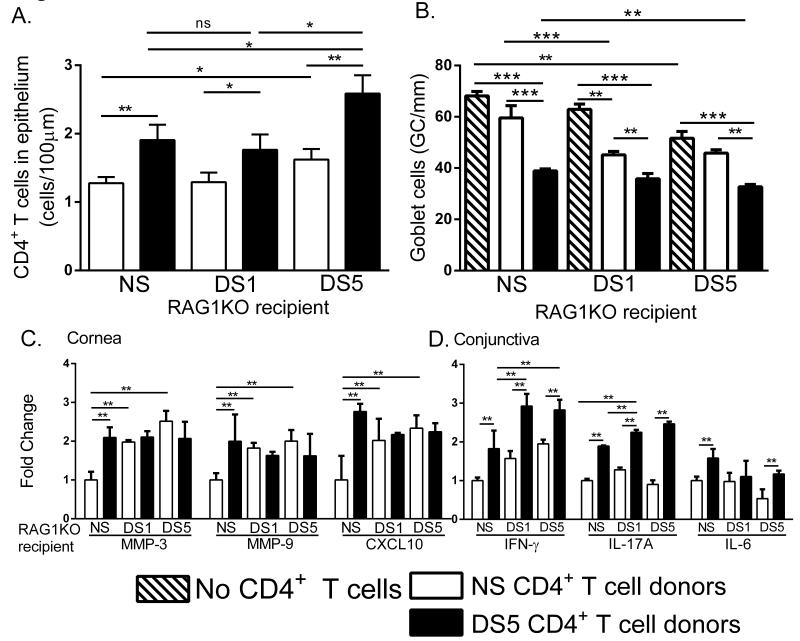
Figure 2. Desiccating stress increases disease severity in CD4+ T cell adoptive transfer recipients.
A. Increased T cell infiltration in recipient mice with increased DS and chemokine expression. B. Decreased numbers of goblet cells in the conjunctiva of RAG1KO recipients that were treated with DS prior to adoptive transfer. C. Gene expression of MMP-3, MMP-9 and CXCL10 in the cornea of recipient mice. D. Gene expression of IFN-γ, IL-17A and IL-6 in conjunctiva with increasing lengths of DS-treated RAG1KO recipient mice. Results represent the mean ± SEM expression of 6-8 animals per time point.*, p < 0.05; **, p < 0.01; ***, p <0.001; ns, not significant.
An important disease parameter in dry eye disease is goblet cell (GC) loss. In order to examine the effect of DS in GC loss the number of GCs in the conjunctiva of RAG1KO mice (that received no donor T cells) was determined. Significant GC loss was observed at DS5 and is presumably due the production of IFN-γ by innate immune cells. GC loss was observed in animals that received NS CD4+ T cells, however there were significantly more goblet cells than those that received DS5 CD4+ T cells. (Figure 2B) This loss can be attributed to IFN-γ produced by innate cells. Recipients that received DS5 CD4+ T cells had significantly greater GC loss compared to NS CD4+ T cell recipients (Figure 2B). Importantly, additional GC loss was observed when RAG1KO recipients were treated with DS, as a significant difference was observed between NS and DS5 recipients that received DS5 CD4+ T cells (Figure 2B). Overall, the most severe loss of GCs was observed in DS-treated recipients that received DS5 CD4+ T cells.
In order to characterize the inflammation in recipient mice, gene expression studies were performed. DS RAG1KO recipient mice, regardless of receiving NS or DS CD4+ T cells, have increased MMP-3 and MMP-9 expression in the cornea compared to NS recipient RAG1KO mice (Figure 2C). Thus, DS induces MMP-3 and MMP-9 expression independently of activated T cell adoptive transfer. In agreement, CXCL10 was upregulated in the cornea with adoptive transfer of NS CD4+ T cells. In the conjunctiva IFN-γ and IL-17A expression increased in mice receiving DS5 CD4+ T cells compared to mice receiving NS CD4+ T cells (Figure 2D). DS further increased IFN-γ in DS1 and DS5 recipients (as seen in Figure 1B) from IFN-γ produced by resident NK cells and not the transferred T cells (Figure 2D). Similarly, IL-17A increased only in mice receiving DS5 CD4+ T cells and further increased in DS exposed RAG1KO recipient mice. IL-6 expression only increased in NS RAG1KO recipient mice receiving DS5 CD4+ T cells (Figure 2D). Overall, these results indicate that prior activation of innate immunity induced by DS treatment correlates with increase dry eye disease severity in our CD4+ T cell adoptive transfer murine model.
Expression of Th1-associated chemokines requires IFN-γ
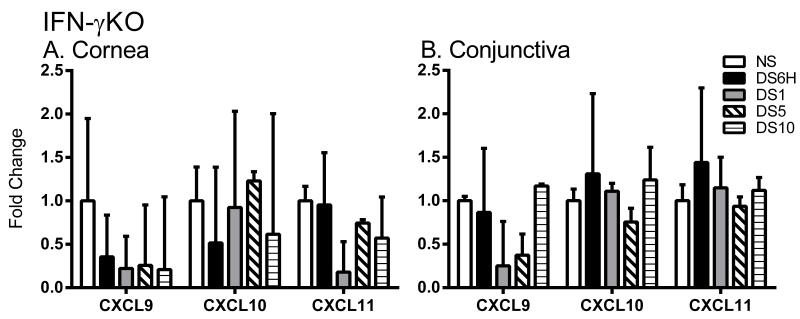
The expression of CXCL9, CXCL10, and CXCL11 is stimulated by IFN-γ (22). In order to address the hypothesis that IFN-γ is responsible of upregulation of these chemokines in DED, the expression of CXCL9, CXCL10, and CXCL11 was examined in IFN-γKO mice exposed to DS. As seen in figure 3 A and B, CXCL9, CXCL10, or CXCL1 are not upregulated in response to DS in IFN-γKO mice as a significant change in gene expression was not observed in the cornea or the conjunctiva. Thus, upregulation of Th1-associated chemokines in DED requires IFN-γ.
Figure 3. Induction of Th1-associated chemokines requires IFN-γ.
A-B. Gene expression of CXCL9, CXCL10, and CXCL11 in cornea (A) and conjunctiva (B) of NS, DS6H, DS1, DS5, or DS10 of IFN-γKO mice. Results represent the mean ± SD expression of 6-8 animals per time point.*, p < 0.05; **, p < 0.01; ***, p <0.001 compared to NS.
IL-17A is also involved in the pathogenesis of dry eye (2). In order to rule the possibility that IL-17 has a role in regulating Th1-associated chemokines, we determined expression of the chemokines in IL-17AKO mice. A significant increase in gene expression was observed in the conjunctiva for CXCL9 (1.9 ± 0.234, P<0.005), CXCL10 (2.9 ± 0.606, P<0.005) and CXCL11 (2.32 ± 0.329, P<0.005) in DS1 IL-17AKO mice compared to NS controls demonstrating that during short term desiccating stress lack of IL-17A does not influence the expression of Th1-associated chemokines (Supplementary Figure 1). The expression of CCL20, which binds CCR6 expressed on Th17 cells, in response to DS was also examined in wild type mice (increase of 2.25 ± 0.458 fold, P<0.005), RAG1KO mice (increase of 1.86 ± 0.109, P<0.005), IL-17AKO mice (no change), and IFN-γKO mice (increase of 3.76 ± 0.151, P<0.005) (data not shown). Thus, it appears that IFN-γ and IL-17A do not cross-regulate chemokine expression in experimental dry eye.
Depletion of NK cells prevents increased expression of Th1-associated chemokines and IFN-γ
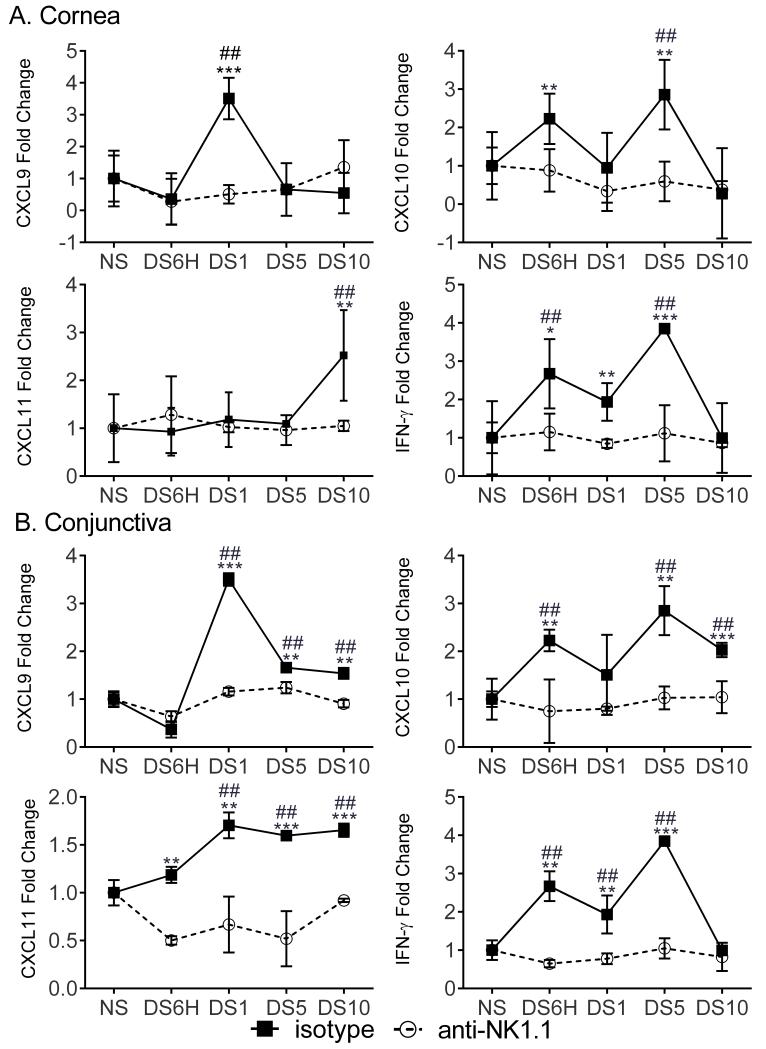
To test the hypothesis that the non-T cell source of IFN-γ was NK cells, we examined CXCL9, CXCL10, CXCL11, and IFN-γ expression in NK cell-depleted RAG1KO mice. As a control, RAG1KO mice were treated with isotype antibody. NK cell-depleted RAG1KO mice did not upregulate CXCL9, CXCL10, and CXCL11 expression in response to DS while isotype-treated mice did upregulate chemokines (Figure 4). Consistent with this hypothesis, the expression of IFN-γ is also decreased with the depletion of NK cells, but was not affected with isotype treatment (Figure 4). Importantly, compared to NS animals only isotype-treated RAG1KO mice had increased expression of Th-1-associated chemokine or IFN-γ, as increased expression was not observed in NK cell-depleted RAG1KO mice compared to NS controls (Figure 4). These results suggest that early induction of chemokines in DED is induced by IFN-γ-producing NK cells in response to DS. In order to examine the effect of scopolamine on NK cell-induced expression of IFN-γ and Th1-associated chemokines, groups of mice were exposed to DS with or without scopolamine or treated with scopolamine but not exposed to DS. The gene expression of IFN-γ, CXCL9, CXCL10, CXCL11 or RAE-1 was determined. Without scopolamine treatment IFN-γ was not induced at DS1 in the conjunctiva. Expression of CXCL9, CXCL10, and CXCL11 is also significantly reduced compared to the DS1 with scopolamine group (Supplementary Figure 2). Coupled with the reduction of IFN-γ and Th1-associated chemokines with NK1.1 Ab treatment these data suggest that scopolamine does not inhibit NK cell function.
Figure 4. Th1-associated chemokines and IFN-γ expression in DS mice requires NK cells.
Gene expression of CXCL9, CXCL10, CXCL11, and IFN-γ in non-stressed (NS), six hours (DS6H) of desiccating stress (DS), one day of DS (DS1), five days of DS (DS5), or ten days (DS10) of DS NK cell-depleted RAG1KO mice (○) or isotype-treated RAG1KO mice (■) in the cornea (A.) and conjunctiva (B.). Fold changes were determined by comparing to NS samples. P values were determined by comparing anti-NK1.1-treated to isotype-treated RAG1KO mice. Results represent the mean ± SD expression of 6-8 animals per time point.*, p < 0.05; **, p < 0.01; ***, p <0.001. *, compared to isotype controls. ##, p < 0.05 compared to NS animals of the same treatment.
Desiccating stress induces activating NK cell receptors in the cornea and conjunctiva
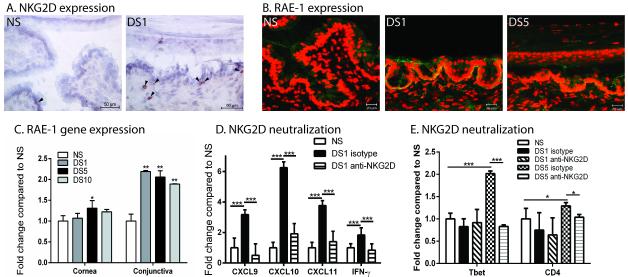
We hypothesized that DS induces NK cells expressing activating receptors and their receptor ligands. Immunohistochemical staining indicated an increase in NKG2D positive cells in DS1 mice in the conjunctiva compared to NS mice (Figure 5A). In order to examine the effect of DS on expression of NK cell receptor ligands, we determined protein and gene expression of the NKG2D ligand, RAE-1 α-ε. An increase in RAE-1 α-ε protein expression was observed in the cornea and conjunctiva of DS1 and DS5 mice (Figure 5B). Changes in gene expression of RAE-1 α-ε were determined in the cornea and conjunctiva of C57BL/6 mice exposed to DS for 1, 5 or 10 days compared to NS mice. RAE-1 expression increased in corneal epithelium after 5 days; however RAE-1 expression occurred very early at day 1 in the conjunctiva (Figure 5C). Similarly, RAG1KO mice also upregulate RAE-1 α-ε in response to DS (data not shown). Changes in gene expression of other NKG2D ligands, such as murine ULBP-Like Transcript 1 (MULT1) and CD99 (a ligand of PILR) was not observed in response to DS (data not shown). In order to confirm the importance of NKG2D/RAE-1 in induction of Th1-assoicated chemokines, mice were topically treated with anti-NKG2D antibody three times per day prior to exposure to DS and throughout the duration of exposure to DS. Mice treated with anti-NKG2D had significantly lower expression of CXCL9, CXCL10, CXCL11, and IFN-γ at DS1 compared to isotype control (Figure 5D) after one day of DS. This data suggests that DS induces the expression of activating NK cell receptor ligands in the epithelium that allows for the activation of NK cells. To confirm the role of NKG2D in the recruitment of CD4+ T cells to the ocular surface the expression of Th1 cell-specific transcription factor, Tbet, and CD4 was examined in isotype or anti-NKG2D treated in the conjunctiva from mice exposed to DS for one or five days. At DS5 Tbet expression significantly increases but this is diminished with anti-NKG2D Ab treatment, suggesting that expression of NKG2D promotes migration of Th1 to the ocular surface (Figure 5E). Similarly, CD4 expression is significantly decreased with NKG2D treatment suggesting a decrease in the total number of CD4+ T cells at the ocular surface (Figure 5E).
Figure 5. NKG2D and its ligand, RAE-1, increases with exposure to DS and NKG2D neutralization decreases expression of Th1-associated chemokines and IFN-γ.
A. Immunohistochemistry staining of NKG2D (red) with black arrows in the conjunctiva of NS and DS1 C57BL/6 mice B. Merged pictures of laser scanning immunofluorescent confocal microscopy of cornea and conjunctiva sections immunostained for RAE-1 (green) in conjunctiva of NS, DS1, or DS5 WT C57BL/6 mice. Counter stained with PI (red). Scale bar −20 μm. C. Gene expression of RAE-1 in cornea and conjunctiva of non-stressed (NS), one day (DS1) of desiccating stress (DS), five days of DS (DS5), or ten days (DS10) of DS WT C57BL/6 mice. D. Neutralization of NKG2D decreases conjunctival expression of CXCL9, CXCL10, CXCL11, and IFN-γ. E. Gene expression of Tbet and CD4 in the conjunctiva of non-stressed (NS), one day (DS1) of desiccating stress (DS), five days of DS (DS5), or ten days (DS10) of DS WT C57BL/6 mice. Results represent the mean ± SD expression of 6-8 animals per time point.*, p < 0.05; **, p < 0.01 compared to NS.
Discussion
Stress to the ocular surface can be a trigger for an inflammatory reaction leading to dry eye. Factors such as hyperosmolarity, ultraviolet light and desiccation can induce the release of proinflammatory mediators, such as cytokines, chemokines, and MMPs, by ocular surface epithelial cells possibly through stress signal transduction pathways (19,23-25). How these early epithelial stress responses initiate downstream T cell mediated immune responses is unclear. Stress responses by epithelial cells is not enough to mediate the disease, as CD4+ T cells and antigen presenting cells (APCs) have been shown to be required (1,26). Several antigens have suggested as autoantigens for the disease, such as type III muscarinic receptor (M3R) or Kallikrein 13 (27,28), that may be possibly liberated via a stress response and presented to T cells by APCs. However, the identity of the antigen(s) has not been confirmed and remains an important area for future investigation in the pathogenesis of dry eye. Important to understanding the pathogenesis of the disease is determining the immunological events that occur very early.
Here, we present evidence that production of Th1-associated chemokines by the ocular surface epithelial cells is a very early event following exposure to a desiccating environment. Our observation that CXCL9, CXCL10, and CXCL11 are upregulated in the corneal and conjunctival epithelium is in agreement with previous studies that show that CXCL9 and CXCL10 are increased in dry eye patients and mice (7,8); however, we demonstrate that this occurs as early as six hours after initial induction with DS. Chemokine expression in the cornea is delayed compared to the conjunctiva. This may be due to the lymphoid nature of the conjunctiva. Chemokine expression in the cornea may be a byproduct of the inflammatory response. Previous studies have demonstrated that mice lacking the chemokine receptors CXCR3 or CCR6 do not have an increase in CD4+ T cell infiltration, goblet cells loss or corneal disease when subjected to DS (9), demonstrating the importance of the chemokine-chemokine receptor system in recruitment of T cells to the ocular surface. In addition to recruiting Th1 cells, CXCL10 also functions to recruit macrophages, dendritic cells (DC), and NK cells that express CXCR3 (22). Thus, the initial induction of Th1-associated chemokines may be vital to additional recruitment of APCs to the ocular surface ushering the beginning of the adaptive immune response.
Seminal studies establishing the requirement of CD4+ T cell for pathogenesis of dry eye disease were performed using an adoptive transfer model (1,29). This model establishes the autoimmune nature of dry eye disease as pathogenic T cells can be transferred to naïve recipients to mediate disease. Modifying this model, we present evidence that pre-activating innate immunity in T cell-deficient RAG1KO mice prior to adoptive transfer increases severity of disease, as DS treated RAG1KO mice had increased T cell infiltration, decreased GC density and increased production of inflammatory cytokines. Increased T cell infiltration is observed with increased DS exposure and is consistent with increased chemokine expression induced by DS activated NK cells. Increased chemokine production even induced the influx of NS CD4+ T cells in DS-treated RAG1KO recipients. Similarly, DS treated CD4+ T cell recipient mice experienced increased GC loss than non-stressed mice. The significant decrease in goblet cells in DS treated RAG1KO mice receiving no T cells and NS CD4+ T cells can be attributed to the increase of IFN-γ in response to DS. DS treated RAG1KO mice receiving NS CD4+ T cells had an upregulation of MMP-3, MMP-9 that may induce acute corneal disease. As before, CXCL10 and IFN-γ were also upregulated and further increased with the transfer of DS CD4+ T cells. A small, but significant, increase in IL-17A expression was observed. This increase in IL-17A could be attributed to an increase in neutrophils that occurs in dry eye (28). Activating the innate immune system prior adoptive transfer may offer a more relevant model more closely mimicking a normal immune response by increasing T cell migration and NK cell function. As mentioned earlier, NK cells play a major role in activation of APCs and thus an important role in T cell activation.
Interestingly, but not surprising, IFN-γ expression also occurs very early following exposure to DS and it regulates Th1-associated chemokine expression. Th1-associated chemokines are predominately induced by IFN-γ. CXCL9 is solely induced by IFN-γ. CXCL10 is strongly induced by IFN-γ and IFN-α/β and weakly induced by TNF-α. (30) TNF-α also synergizes strongly with the IFNs for CXCL10 induction. CXCL11 is induced by IFN-γ and by IFN-β but not by IFN-α (30). Such early induction of chemokines and IFN-γ led us to the hypothesis that IFN-γ was responsible for chemokine upregulation at the ocular surface. To address this hypothesis we examined Th-1 associated chemokine expression in IFN-γ-deficient mice. In the face of DS, Th1-associated chemokine expression did not increase in IFN-γKO mice. Induction of IFN-γ so early in disease progression suggested an innate source of IFN-γ.
This led us to determine the cellular source of IFN-γ produced very early in the induction of disease. We first hypothesized that innate T cells were required for the increased chemokine expression. There are two possible candidates for innate T cells that produce IFN-γ: NKT cells and γΔ T cells. Both NKT cells and γΔ T cells are an important source of IFN-γ. To address this hypothesis we examined expression of Th1-associated chemokines and IFN-γ in T cell-deficient RAG1KO mice that lack both NKT cells and γΔ T cells. The demonstrated increase in chemokine and IFN-γ expression in the RAG1KO mice indicates this is a T cell independent process.
T cell independence of chemokine expression caused us to ask if the requirement of NK cells was needed for early expression of Th1-associated chemokines and IFN-γ in dry eye. NK cells can quickly respond to activation signals to produce cytokines, granzymes, and perforin without transcription or proliferation. NK1.1 positive cells produce a variety of cytokines in dry eye disease, including IL-6, IL-23, and IL-17A, in addition to IFN-γ and have been shown to increase DC maturation and Th17 cell function (17). Additionally, Chen et al. demonstrated that 1) NK1.1 expression increases in the very early phase of dry eye; 2) NK cells produce IFN-γ in dry eye; 3) APC maturation was inhibited in NK cell-depleted DED mice (18). This led us to hypothesize that NK cells produce IFN-γ that induce Th1-associated chemokines very early in the disease progression. Depletion of NK cells in T cell-deficient RAG1KO mice completely ablated both chemokine and IFN-γ expression. Increased chemokine expression leads to increased lymphocyte recruitment and bridges the gap between innate and adaptive immunity. Interestingly, IFN-γ has a vital role in the induction of both innate (produced by NK cells) and adaptive (produced by Th1 cells) immunity in the pathogenesis of dry eye. We do not suggest that NK cells are the only source of innate IFN-γ in DED. However, our data suggests that NK cells are the major source. The use of RAG1KO mice eliminated the IFN-γ produced by innate T cells. In figure 4, the depletion of NK cells ablates the expression of IFN-γ. Other possible sources include macrophages (in specific contexts) or neutrophils. However, macrophages are not a known major physiological source of IFN-γ. Neutrophils have been shown to produce IFN-γ in response to bacterial pathogens, however neutrophil infiltration has not been observed in this model of dry eye (data not shown). Overall, our data supports the hypothesis that NK cells (not innate T cells, macrophages or neutrophils) are the major early producers of IFN-γ as NK1.1 depletion decreases expression of IFN-γ.
The activating signal of the NK cell receptor, NKG2D, has been found to elicit cytokine production. NK cell production of MIP-1β, TNF-α and IFN-γ was enhanced upon treatment with soluble ULBP, an NKG2D ligand (14). A recent study demonstrated the increased expression of MICA, another NKG2D ligand, enhances the cytotoxicity of NK cell in human cornea epithelium (15). Thus, increased NKG2D ligand expression at the ocular surface suggests that DS increases NK cells activation very early in DED. It well understood that stress pathways regulate NKG2D ligands; however, what is determined as “stress” is difficult to determine, as ligands are induced by different stimuli (16). The stress response induced by DS in corneal epithelial and conjunctiva has not been completely described. Previous studies have shown that hyperosmolar stress and DS increases the production of IL-1β and TNF-α and MAPK signaling which induces NF-κB (31-34). It has been shown that NF-κB is induction leads to the expression of MICA in humans (35,36). One link between cellular stress and NK cell activation is increased RAE-1 expression via Toll-like receptor (TLR) stimulation (37). TLRs 2-4 and 9 are increased in the cornea and conjunctiva epithelial cells of dry eye mice (38). In our model, we observed an increase in RAE-1 ligands and did not detect the related MULT1 or CD99 ligand. Thus, it appears that hyperosmolarity and DS may induce expression of certain specific NK cell receptors, but not others. The involvement of NKG2D in our model is supported by data that depleting NKG2D function at the ocular surface ablates the gene expression of CXCL9, CXCL10, CXCL11 and IFN-γ very early in progression of the disease. Furthermore, depleting NKG2D decreased the expression of Tbet and CD4 at day 5 supporting the hypothesis that NKG2D-expressing NK cells are essential for the recruitment of Th1 cells to the ocular surface. Supporting the increased NK cell receptor and/or ligand hypothesis, a recent study by Rusakiewicz et al. showed that higher gene expression levels of the activating NK cell receptor, NCR3/NKp30, were found in primary Sjogren’s syndrome patients and a polymorphism in the promoter region for the gene encoding the NCR3/NKp30 receptor confers protection against the disease (39). Overall, our data supports the existence of an additional disease-promoting pathway in which increased NK cell activation occurs at mucosal sites.
Understanding how DS initiates these early events is crucial to understanding the factors that underlie the initial stress that triggers the autoimmune response. This is one of the first studies describing the relation of stress to activation of lymphocytes in dry eye. Overall, this work suggests that DS induces a cellular stress response in conjunctival and/or corneal epithelial cell that induces expression of RAE-1 leading to activation of NK cells. Activated NK cells produce IFN-γ that causes the upregulation of Th1-associated chemokines that induce the migration of Th1 cells from regional lymph nodes and serves in recruiting and activating dendritic cells. Blockade of chemokines or inhibitors of NKG2D ligands in DED may offer potentially very effective therapeutic targets.
Supplementary Material
Acknowledgments
Supported by NIH Grant EY018888 (CSDP), EY11915 (SCP), EY018090 (SCP), NIH Core Grants-EY002520 & EY020799, Research to Prevent Blindness, the Oshman Foundation, William Stamps Farish Fund, the Hamill Foundation and by the Cytometry and Cell Sorting Core at Baylor College of Medicine which is funded by the NIH National Institute of Allergy and Infectious Diseases grants P30AI036211, NCI P30CA125123, and NCRR S10RR024574
Reference List
- 1.Niederkorn JY, Stern ME, Pflugfelder SC, de Paiva CS, Corrales RM, Gao J, Siemasko K. Desiccating Stress Induces T Cell-Mediated Sjogren’s Syndrome-Like Lacrimal Keratoconjunctivitis. J. Immunol. 2006;176:3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 2.de Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JI, Fang B, Zheng X, Ma P, Farley WJ, Siemasko KS, Niederkorn JY, Stern ME, Li D-Q, Pflugfelder SC. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunology. 2009 May;2(3):243–53. doi: 10.1038/mi.2009.5. Epub 2009 Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Paiva CS, Villarreal AL, Corrales RM, Rahman HT, Chang VY, Farley WJ, Stern ME, Niederkorn JY, Li DQ, Pflugfelder SC. Dry Eye-Induced Conjunctival Epithelial Squamous Metaplasia Is Modulated by Interferon-{gamma} Invest Ophthalmol. Vis. Sci. 2007;48:2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Chen W, de Paiva CS, Volpe EA, Gandhi NB, Farley WJ, Li DQ, Niederkorn JY, Stern ME, Pflugfelder SC. Desiccating Stress Induces CD4(+) T-Cell-Mediated Sjogren’s Syndrome-Like Corneal Epithelial Apoptosis via Activation of the Extrinsic Apoptotic Pathway by Interferon-gamma. Am J Pathol. 2011;179:1807–1814. doi: 10.1016/j.ajpath.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am. J. Ophthalmol. 2009;147:198–205. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syrbe U, Siveke J, Hamann A. Th1/Th2 subsets: distinct differences in homing and chemokine receptor expression? Springer Semin. Immunopathol. 1999;21:263–285. doi: 10.1007/BF00812257. [DOI] [PubMed] [Google Scholar]
- 7.Yoon KC, de Paiva CS, Qi H, Chen Z, Farley WJ, Li DQ, Pflugfelder SC. Expression of th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol. Vis. Sci. 2007;48:2561–2569. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- 8.Yoon KC, Park CS, You IC, Choi HJ, Lee KH, Im SK, Park HY, Pflugfelder SC. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol. Vis. Sci. 2010;51:643–650. doi: 10.1167/iovs.09-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coursey TG, Gandhi NB, Volpe EA, Pflugfelder SC, de Paiva CS. Chemokine Receptors CCR6 and CXCR3 Are Necessary for CD4(+) T Cell Mediated Ocular Surface Disease in Experimental Dry Eye Disease. PLoS. One. 2013;8:e78508. doi: 10.1371/journal.pone.0078508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Middendorp S, Nieuwenhuis EE. NKT cells in mucosal immunity. Mucosal. Immunol. 2009;2:393–402. doi: 10.1038/mi.2009.99. [DOI] [PubMed] [Google Scholar]
- 11.Matangkasombut P, Pichavant M, Dekruyff RH, Umetsu DT. Natural killer T cells and the regulation of asthma. Mucosal. Immunol. 2009;2:383–392. doi: 10.1038/mi.2009.96. [DOI] [PubMed] [Google Scholar]
- 12.Huber S, Shi C, Budd RC. Gammadelta T cells promote a Th1 response during coxsackievirus B3 infection in vivo: role of Fas and Fas ligand. J. Virol. 2002;76:6487–6494. doi: 10.1128/JVI.76.13.6487-6494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 2011;89:216–224. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 14.Kubin M, Cassiano L, Chalupny J, Chin W, Cosman D, Fanslow W, Mullberg J, Rousseau AM, Ulrich D, Armitage R. ULBP1, 2, 3: novel MHC class I-related molecules that bind to human cytomegalovirus glycoprotein UL16, activate NK cells. Eur. J. Immunol. 2001;31:1428–1437. doi: 10.1002/1521-4141(200105)31:5<1428::AID-IMMU1428>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Hong J, Qiu T, Qian T, Li G, Yu X, Chen J, Le Q, Sun X, Xu J. Heightened expression of MICA enhances the cytotoxicity of NK cells or CD8+T cells to human corneal epithelium in vitro. BMC. Ophthalmol. 2012;12:6. doi: 10.1186/1471-2415-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu. Rev. Immunol. 2013;31:413–441. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Volpe EA, Gandhi NB, Schaumburg CS, Siemasko KF, Pangelinan SB, Kelly SD, Hayday AC, Li DQ, Stern ME, Niederkorn JY, Pflugfelder SC, de Paiva CS. NK cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoS. One. 2012;7:e36822. doi: 10.1371/journal.pone.0036822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Chauhan SK, Saban DR, Sadrai Z, Okanobo A, Dana R. Interferon-{gamma}-secreting NK cells promote induction of dry eye disease. J Leukoc. Biol. 2011;89:965–972. doi: 10.1189/jlb.1110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis. Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 20.Hornung M, Farkas SA, Sattler C, Schlitt HJ, Geissler EK. DX5+ NKT cells induce the death of colitis-associated cells: involvement of programmed death ligand-1. Eur. J Immunol. 2006;36:1210–1221. doi: 10.1002/eji.200535332. [DOI] [PubMed] [Google Scholar]
- 21.Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, Woehl B, Leung H, Groom J, Batten M, Harvey RP, Martinez A, Mackay CR, Mackay F. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacotte S, Brun S, Muller S, Dumortier H. CXCR3, inflammation, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2009;1173:310–317. doi: 10.1111/j.1749-6632.2009.04813.x. [DOI] [PubMed] [Google Scholar]
- 23.Corrales RM, Stern ME, de Paiva CS, Welch J, Li DQ, Pflugfelder SC. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol. Vis. Sci. 2006;47:3293–3302. doi: 10.1167/iovs.05-1382. [DOI] [PubMed] [Google Scholar]
- 24.Li DQ, Chen Z, Song XJ, Luo L, Pflugfelder SC. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol. Vis. Sci. 2004;45:4302–4311. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- 25.Luo L, Li DQ, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye & Contact Lens. 2005;31(5):186–93. doi: 10.1097/01.icl.0000162759.79740.46. [DOI] [PubMed] [Google Scholar]
- 26.Schaumburg CS, Siemasko KF, de Paiva CS, Wheeler LA, Niederkorn JY, Pflugfelder SC, Stern ME. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol. 2011;187:3653–3662. doi: 10.4049/jimmunol.1101442. [DOI] [PubMed] [Google Scholar]
- 27.Bacman S, Berra A, Sterin-Borda L, Borda E. Muscarinic acetylcholine receptor antibodies as a new marker of dry eye Sjogren syndrome. Invest Ophthalmol. Vis. Sci. 2001;42:321–327. [PubMed] [Google Scholar]
- 28.Stern ME, Schaumburg CS, Siemasko KF, Gao J, Wheeler LA, Grupe DA, De Paiva CS, Calder VL, Calonge M, Niederkorn JY, Pflugfelder SC. Autoantibodies contribute to the immunopathogenesis of experimental dry eye disease. Invest Ophthalmol. Vis. Sci. 2012;53:2062–2075. doi: 10.1167/iovs.11-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coursey TG, Gandhi NB, Volpe EA, Pflugfelder SC, de Paiva CS. Chemokine receptors CCR6 and CXCR3 are necessary for CD4(+) T cell mediated ocular surface disease in experimental dry eye disease. PLoS. One. 2013;8:e78508. doi: 10.1371/journal.pone.0078508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol. Cell Biol. 2011;89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Paiva CS, Corrales RM, Villarreal AL, Farley WJ, Li DQ, Stern ME, Pflugfelder SC. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp. Eye Res. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Q Li, D., Luo L, Chen Z, Kim HS, Song XJ, Pflugfelder SC. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp. Eye Res. 2006;82:588–596. doi: 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo MJ, Kim JM, Lee MJ, Sohn YS, Kang KK, Yoo M. The therapeutic effect of DA-6034 on ocular inflammation via suppression of MMP-9 and inflammatory cytokines and activation of the MAPK signaling pathway in an experimental dry eye model. Curr. Eye Res. 2010;35:165–175. doi: 10.3109/02713680903453494. [DOI] [PubMed] [Google Scholar]
- 34.Pan Z, Wang Z, Yang H, Zhang F, Reinach PS. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Invest Ophthalmol. Vis. Sci. 2011;52:485–493. doi: 10.1167/iovs.10-5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molinero LL, Fuertes MB, Girart MV, Fainboim L, Rabinovich GA, Costas MA, Zwirner NW. NF-kappa B regulates expression of the MHC class I-related chain A gene in activated T lymphocytes. J. Immunol. 2004;173:5583–5590. doi: 10.4049/jimmunol.173.9.5583. [DOI] [PubMed] [Google Scholar]
- 36.Lin D, Lavender H, Soilleux EJ, O’Callaghan CA. NF-kappaB regulates MICA gene transcription in endothelial cell through a genetically inhibitable control site. J. Biol. Chem. 2012;287:4299–4310. doi: 10.1074/jbc.M111.282152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamerman JA, Ogasawara K, Lanier LL. Cutting edge: Toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor. J. Immunol. 2004;172:2001–2005. doi: 10.4049/jimmunol.172.4.2001. [DOI] [PubMed] [Google Scholar]
- 38.Redfern RL, Patel N, Hanlon S, Farley W, Gondo M, Pflugfelder SC, McDermott AM. Toll-like receptor expression and activation in mice with experimental dry eye. Invest Ophthalmol. Vis. Sci. 2013;54:1554–1563. doi: 10.1167/iovs.12-10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rusakiewicz S, Nocturne G, Lazure T, Semeraro M, Flament C, Caillat-Zucman S, Sene D, Delahaye N, Vivier E, Chaba K, Poirier-Colame V, Nordmark G, Eloranta ML, Eriksson P, Theander E, Forsblad-d’Elia H, Omdal R, Wahren-Herlenius M, Jonsson R, Ronnblom L, Nititham J, Taylor KE, Lessard CJ, Sivils KL, Gottenberg JE, Criswell LA, Miceli-Richard C, Zitvogel L, Mariette X. NCR3/NKp30 contributes to pathogenesis in primary Sjogren’s syndrome. Sci. Transl. Med. 2013;5:195ra96. doi: 10.1126/scitranslmed.3005727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.