Abstract
Cardiovascular disease is the leading cause of death in the U.S. and other developed country. Metabolic syndrome, including obesity, diabetes/insulin resistance, hypertension and dyslipidemia is major threat for public health in the modern society. It is well established that metabolic syndrome contributes to the development of cardiovascular disease collective called as cardiometabolic disease. Despite documented studies in the research field of cardiometabolic disease, the underlying mechanisms are far from clear. Proteases are enzymes that break down proteins, many of which have been implicated in various diseases including cardiac disease. Matrix metalloproteinase (MMP), calpain, cathepsin and caspase are among the major proteases involved in cardiac remodeling. Recent studies have also implicated proteases in the pathogenesis of cardiometabolic disease. Elevated expression and activities of proteases in atherosclerosis, coronary heart disease, obesity/insulin-associated heart disease as well as hypertensive heart disease have been documented. Furthermore, transgenic animals that are deficient in or overexpress proteases allow scientists to understand the causal relationship between proteases and cardiometabolic disease. Mechanistically, MMPs and cathepsins exert their effect on cardiometabolic diseases mainly through modifying the extracellular matrix. However, MMP and cathepsin are also reported to affect intracellular proteins, by which they contribute to the development of cardiometabolic diseases. On the other hand, activation of calpain and caspases has been shown to influence intracellular signaling cascade including the NF-κB and apoptosis pathways. Clinically, proteases are reported to function as biomarkers of cardiometabolic diseases. More importantly, the inhibitors of proteases are credited with beneficial cardiometabolic profile, although the exact molecular mechanisms underlying these salutary effects are still under investigation. A better understanding of the role of MMPs, cathepsins, calpains and caspases in cardiometabolic diseases process may yield novel therapeutic targets for threating or controlling these diseases.
Keywords: Cardiometabolic diseases, MMP, calpain, cathepsin, caspase
1. Cardiometabolic diseases
Metabolic syndrome, a cluster of metabolic risk factors including obesity, diabetes/insulin resistance, hypertension as well as dyslipidemia, has been identified as a multiplex risk factor for cardiovascular disease [1, 2]. Although the diagnostic criteria for metabolic syndrome are still under debate, it is widely accepted that individuals with metabolic syndrome are at high risk for cardiovascular disease [3]. Cardiovascular disorders associated with metabolic syndrome are referred to as cardiometabolic diseases. Cardiometabolic diseases are multifactorial diseases with the involvement of a number of different factors including genetic, diets, lifestyle and living environment. Cardiac remodeling, coronary heart disease, even heart failure could result from metabolic syndrome. Along with the increased rates of obesity, diabetes and hypertension in the past decades, there has been an increase in the incidence of cardiometabolic diseases [4, 5]. Thus, recent research has targeted cardiometabolic diseases, with an aim to understand the pathogenesis of the disease and find potential clinical interventions to benefit subjects afflicted with these diseases. Recently, proteases have been implicated in the development and treatment of various disorders, especially cardiovascular disease. Given the increasing incidence of cardiometabolic diseases as well as the emerging role of proteases, this review summarizes the roles of major proteases including matrix metalloproteinase (MMP), calpain, cathepsin and caspase in cardiometabolic diseases.
2. Proteases
Proteins are the critical components for organisms and are involved in virtually all cellular functions. Besides protein production, the degradation of proteins is also important, as this is the way to recycle dysfunctional/damaged proteins and liberate the amino acids to form new proteins. Proteases are enzymes that perform protein catabolism by hydrolyzing peptide bonds that connects the amino acids to form the protein. To date, at least 500–600 proteases have been identified by using bioinformatic analysis [6]. Proteases are classified as serine, cysteine or threonine proteases, or as aspartic, –matrix metalloprotease and glutamic proteases based on their site of action [7]. Besides their traditional roles in protein turnover, proteases have been recently recognized as key - signaling molecules that participate in a number of vital physiological and pathological processes. The extracellular and intracellular protease including matrix metalloproteinase (MMP), calpain, cathepsin and caspase are among most extensively studied ones with respect to cardiovascular disease and remodeling. Research from our laboratory has focused on investigating the role of the cysteine proteinase cathepsin K in cardiometabolic diseases, including obesity, insulin resistance and hypertension-associated cardiac disease. Although emerging studies have shown the importance of proteases in cardiometabolic diseases, there are still some uncertainties remaining, and there is a paucity of review articles addressing the critical effects of proteases in cardiometabolic disease. Therefore, this review focuses on the role of proteases in the etiopathogenesis of cardiometabolic disease and attempts at addressing the potential molecular mechanisms involved in the process. To begin with, we shall briefly discuss the salient features of the various proteases that have documented role in cardiometabolic disease and subsequently address the role of each of these proteases in various disease conditions. In the subsequent section we shall address the potential clinical relevance of proteases and avenues for to harness them in the clinical setting, followed by a brief discussion on the future research questions which would further our understanding of the role of proteases in cardiometabolic disorders.
2.1. MMPs
MMPs are a class of metal-linked zinc-dependent proteases, whose biological activity requires calcium. MMPs cleave internal peptide bonds of proteins to degrade extracellular matrix (ECM). The MMP family is further classified as collagenases, gelatinases, stromelysins, elastases and membrane-type MMPs based on their enzyme characteristics. Collagenases, which include MMP-1, -8, and -13 cleave interstitial collagens I, II and III at specific sites and also cleave other ECM molecules such as gelatin and fibronectin. Collagen fragments are then degraded by gelatinases, which include MMP-2 and -9. Stromelysins, including MMP-3, -10 and -11 are responsible for gelatin, laminin and fibronectin degradation. Elastases, which include MMP-2, -9 and -12, degrade elastin in arterial wall [8, 9]. Membrane-type (MT)-MMPs are involved in the cleavage of types-I, -II and –III collagens and other components of ECM, which also activate proMMP to MMP [10]. The remarkable overlap in the activity of MMPs’ and the preferred substrate despite their different protein structure, suggests redundancy. MMPs are synthesized as a proenzyme form followed by the hydrolysis of the zinc-cysteine bond to the mature form [11]. Vascular wall smooth muscle cells, endothelial cells, monocytes, macrophages, and T-cells have been shown to secrete MMPs [11]. The –expression of MMPs proenzymes is highly regulated by transcriptional mechanisms. Cytokines such as tumor necrosis factor-α (TNF-α) and interleukins are potent stimulants of the MMP proenzymes. Platelet-derived growth factor (PDGF) and CD40 ligands are reported to enhance MMP production as well [12]. In addition to regulation at the transcriptional level, the activity of MMP is elevated by oxygen free radicals, thrombin, chymase and angiotensin-converting enzyme (ACE) at post-transcriptional level [13]. Conversely, nature has designed endogenous MMP inhibitors [tissue inhibitors of metalloproteinases (TIMPs)] to counter-balance MMP activity. Four members of TIMP family are currently known, which include TIMP-1 to -4. TIMP-1 inhibits MMP-1, -3, -7 and -9. TIMP-2 inhibits MMP-2, whereas TIMP-3 is reported to decrease activities of MMP-2 and -9. TIMP-4 on the other hand inhibits MT-MMP and MMP-2 activity [14]. The exogenous inhibitors to MMPs, such as the tetracycline family of antibiotics are artificial MMP inhibitors that can blunt the activity of MMPs [15].
2.2. Calpains
The calcium ion-dependent papain-like protease (calpain) is a group of calcium-dependent, non-lysosomal neutral cysteine proteases [16, 17]. So far, at least 16 calpains have been identified, most of them, including calpain 1, requiring micromolar concentrations of calcium for activity. Interestingly, calpain 2 requires millimolar calcium concentrations. Calpains are ubiquitously expressed on all types of cells [18]. However, some calpains, such as calpain 3, which is a skeletal muscle-specific protease are tissue specific [19]. Localized in cytosol, calpains mainly target intracellular proteins. A large number of proteins have been reported to be degraded by calpains, which include, but not limited to Bax [20, 21], calcineurin [22], caspases [23], calmodulin-protein kinase [24], G protein [25], IΚB [26, 27], p53 [28, 29] and protein kinase C (PKC) [30, 31]. Although the amino acid sequences targeted by calpains ill-defined, it is widely accepted that amino acid sequence rich in proline, glutamic acid, serine and threonine elevate calpain-binding and calpain-dependent proteolysis [32]. Calpains are primarily produced and localized in the cytosol as proenzymes, which are then activated by intracellular calcium influx. Calcium binding relieves restrictions that are enforced by domain interactions and thus leads to activation of calpains [16]. Additionally, calpains are activated through direct phosphorylation at serine 50 by extracellular signal-regulated kinases (Erk) even without cytosolic calcium flux [33]. Calpastatin, the endogenous inhibitor of calpain tightly regulates the activity of calpains 1 and 2. The inhibitory effect is achieved by reversibly binding of calpastatin domains to calpain domains. Calpain activity can be inhibited through post-translational modification of phosphorylation as well [16]. In addition to the endogenous inhibitors, exogenous inhibitors of calpain, such as calpeptin have also been designed and characterized [34]. Interestingly, recent studies have shown that calpains are also secreted by a variety of cells (endothelial cells, lymphocytes, chondrocytes and osteoblasts) to extracellular space of tissues, which suggests a potential role of calpain in ECM degradation [35, 36].
2.3. Cathepsins
Cathepsins are a family of lysosomal proteases that were originally found in the gastric juice. So far, 19 cathepsins have been identified in mice [37]. They are classified into serine, aspartic and cysteine cathepsins according to the different catalytic activity. Cathepsins A and G are serine cathepsins, cathepsins D and E are aspartic cathepsins, whereas other cathepsins are cysteinyl cathepsins [38]. Although cathepsins were initially thought to function in acidic environment only, recent studies have found that they can be activated in neutral environment including cytosol [39, 40], nucleus [41] and even secretory vesicles [42, 43] as well. Similar to calpains, some cathepsins show tissue and cell-specific expression. For example, cathepsin K is highly expressed on bone tissue, especially the osteoclasts [44] whereas cathepsin S is primarily expressed on immune cells [45]. Unlike MMPs and calpains, cathepsins own a broad range of substrates that include almost all intracellular and extracellular proteins. Nonetheless, cathepsins prefer to degrade specific proteins, and therefore are implicated in specific physiologic process, including protein turnover in bone and cartilage [46], neuropeptide and hormone processing [47], antigen presentation [48] and apoptosis [49]. Recent studies confirm that cathepsins are synthesized as pro-cathepsins with an N-terminal signal peptide targeting ER proteins, followed by N-linked glycosylation [50]. Cathepsins are then bound to the mannose-6-phosphate receptor so as to localize them in the target lysosomes. The N-terminal peptides in pro-cathepsins are cleaved to activate the cathepsins [51, 52]. The activity of cathepsins is regulated by several factors, such as pH, oxidation and the presence of inhibitors. It is well known that cathepsins function optimally under slightly acidic environment with exceptions of cathepsin S and K which have been shown to be active under physiological pH [53]. Human cathepsins are activated under acidic conditions and are inactivated at neutral pH and under alkaline conditions [54]. However, it was reported that human cysteine protease cathepsin L was also inactivated at acid pH by a first-order process [55]. Reactive oxygen species (ROS) is a notorious stimulus for cathepsin release and activation [56, 57]. In addition, ROS-activated cytokines such as TNF-α, PDGF and IFN-γ also activate and secrete cathepsin B and L in fibroblast-like cells [58]. Similar results have been reported in neonatal cardiomyocytes, suggesting the potential role of cathepsins in cardiac diseases [59]. Additionally, angiotensin II (Ang II) is reported to enhance cathepsin gene expression in hearts [43]. Similar to MMPs and calpains, activities of cathepsins are inhibited by their endogenous and exogenous inhibitors. Cystatins are the natural inhibitors for cathepsins. Cystatins are classified into three groups based on the distinct structure, tissue/organ distribution and physiological functions. These include, stefins, cystatins and kininogens [60]. Cystatin reversibly binds to cysteine cathepsins [61]. In addition to natural inhibitors, artificial inhibitors to cathepsins, such as E64 have been designed for research and clinical use [62]. Among the synthetic cathepsin inhibitors, odanacatib, a cathepsin K inhibitor is currently under clinical trial to treat osteoporosis [63].
2.4. Caspases
Caspases are a family of acid proteases that use cysteine residues as the catalytic tools and cleave their substrates at the aspartic acid residues [64]. This family of proteases was first observed to be critical in the proteolytic maturation of IL-1β [65], until several years later, a pioneer study reported that these proteases play crucial roles in the execution of apoptosis [66]. Following this important finding, a number of members of the caspases family, such as caspase-1, -2, -3, -8 and -9 were identified to be crucial for apoptosis [67–73]. So far, 15 members of the caspase family have been discovered, which are essential components for the apoptotic machinery [74]. As reviewed before [75], capases (caspases-2, -8, -9 and -10) with a long pro-domain are called initiator of apoptosis caspases or group-II caspases. Conversely, caspases containing short pro-domains are named as executioner caspases or group-III caspases [76–78]. Together, these two groups of caspases play a critical role in regulating apoptosis - and are focus of discussion here. Substrate specificity of caspases has been described in great detail in previous reviews [75]. Four main substrate pockets of caspases named S1 to S4 have been reported. Caspases are ubiquitously expressed in all type of cells, which are initially translated from caspase genes as inactive pro-forms. Pro-caspases are originally synthesized as catalytically-dormant tripartite proenzymes with a single polypeptide chain of 32–55 kDa commonly representing 3 domains. Advanced protein structure techniques have identified N-terminal pro-domains of pro-caspases that contain motifs called death domain superfamily, which is essential for apoptotic signaling transduction [79, 80]. Pro-caspases are converted to their active forms by proteolytic processing at specific aspartic acid residues under the stimulation of a number of signals. Caspase activation occurs through autoactivation via oligomerization [81, 82], death receptor or mitochondrial pathways induced transactivation [83], as well as proteolysis by other proteases including caspases, cathepsins, calpain and granzyme B [84, 85]. The activated caspases subsequently initiate the apoptotic cascade or directly execute apoptosis to destroy DNA and the cell. Two classes of endogenous caspase inhibitors including natural caspase inhibitors and dominant negative caspases have been documented [86, 87]. Natural caspase inhibitors are derived from a wide spectrum of viruses, such as cowpox virus [88] and baculovirus [89]. Dominant negative caspases are the alternatively splicing of the primary transcript, which are enzymatically inactive and expresses as modified mRNAs or truncated proteins [86]. Peptide caspase inhibitors are synthetic peptide designed to target specific sequence of substrates that can be cleaved by other proteases [90, 91].
3. Role of proteases in cardiometabolic diseases
Several lines of evidence have suggested that proteases participate in the pathogenesis of varieties of diseases, such as cancer [92], inflammation [93, 94], neurodegenerative diseases [95, 96], liver diseases [97], chronic kidney disease [98] and cardiovascular disease [99, 100]. During the recent years, our understandings of the potential mechanisms by which proteases contribute to the development of cardiometabolic diseases have improved substantially. With this in mind, the involvements of MMP, calpain, cathepsin and caspase in cardiometabolic diseases and the potential molecular mechanisms involved in the process are reviewed below.
3.4. MMPs in cardiometabolic diseases
ECM is essential for the maintenance of structural integrity, cell anchoring, cell-cell communication, force transmission and for mediating cell survival/apoptosis and cardiac remodeling [101]. Interestingly, MMPs have been shown to were shown to induce the release of growth factors anchored in the extracellular matrix, consequently leading to cell proliferation, suggesting a potential mechanism by which MMPs induce cardiac hypertrophy and remodeling [102]. As the major class of proteases targeting the ECM, MMPs have been reported to be involved in the pathogenesis of several cardiovascular diseases associated with metabolic syndrome [14]. In fact, each component of the metabolic syndrome has been found to enhance MMP expression and/or activity, suggesting a pivotal role of MMPs in the etiopathology of metabolic syndrome and cardiometabolic disease [103–107]. The subsection below provides highlights of the role of MMPs in some of these conditions.
3.4.1. MMPs in atherosclerosis and coronary heart disease
Atherosclerosis and coronary heart disease are the most common cardiac disease in adults [108]. The atherosclerotic plaque is composed of a lipid core, comprising a mixture of inflammatory cells, especially foam cells and an integument of fibrous cap. The fibrous cap is mainly formed from the extracellular matrix laid down by the smooth muscle cells. Collagen and elastin are the major components for the fibrous cap. The fibrous cap is prone to destruction under the proinflammatory conditions, which results in the release of thrombus into circulation leading to thrombosis [109]. Coronary artery is the main vessel to be affected by atherosclerosis, and myocardial infarction and stroke are the major complications of atherosclerosis [110].
Numerous studies have reported elevated levels of MMPs in atherosclerotic plaques. Cytokines, such as TNF-α have been reported to induce MMP expression in smooth muscle cells through ERK-NFΚB signaling pathway [111]. MMPs were also activated by mast cell proteases in atherosclerotic plaques [112]. In animal models of atherosclerosis as well as human coronary specimens, colocalization of MMP-9 and -3 at the edges of atherosclerotic plaque has been demonstrated [113]. An in vitro study suggests enhanced MMP-2 and -9 mRNA levels in oxidized-low density lipoprotein (ox-LDL) loaded macrophages, which was inhibited by aspirin [114]. Almost all the MMPs are capable of interfering with the atherosclerotic plaque development and stability, which has been extensively reviewed in a recent review [115]. Interestingly, serum MMP-9 concentrations have been positively correlated to total carotid artery plaque score and instability, suggesting that MMP-9 could serve as potential marker for atherosclerosis [116]. Pro-inflammatory mediators and reactive oxygen species lead to the activation of MMPs, which further degrade collagen and elastin to weaken the fibrous cap and cause plaque rupture. The process is accelerated by mobilized macrophages and T cells localized in advancing zone of atherosclerotic plaque [117]. The creation of transgenic animal models with either overexpression or knockout of MMPs, further aided in the understanding of the role of MMPs in atherogenesis and coronary heart disease. One earlier study reported that MMP-1 and ApoE double knockout mice transgenic for human MMP-1 gene in macrophages, exhibited smaller plaques with less collagen [118]. In contrast, overexpression of an auto-activated MMP-9 led to high levels of plaque instability in the same mouse model [119]. Similarly, overexpression of MMP-12 in rabbits enhanced plaque size and inflammation [120]. It was recently found that MMP-9 and ApoE double knockout mice displayed reduced atherosclerotic load despite being fed with a cholesterol rich food [121]. Similar results were obtained when using a MMP-2 and ApoE double knockout mouse model [122]. In contrast however, MMP-3 and ApoE double knockout mice showed increased plaque size [123]. Elevated level of MMP-9 in subjects with coronary artery disease with unstable angina has also been reported [124]. Thus, although the exact role of MMPs in atherosclerosis is remains controversial, based on the aforementioned studies it can be concluded that elevated levels of MMPs, especially MMP-2 and -9 is detrimental, in that, they enhance matrix destruction and cause inflammation, which possibly results in plaque rupture.
Endothelial erosion that occur in highly stenotic, fibrotic plaques without the presence of inflammation has been postulated to play a predominant role in the loss of endothelial cells, which is the other common trigger for coronary heart disease [125]. MMPs are expressed on the endothelial cells which express a variety of MMP substrates (including urokinase-type plasminogen activator receptor and TGF-beta) and activators such as tissue plasminogen activator [126–128]. Endothelial MMPs are also activated by cytokines including TNFα and IL-1 [129, 130]. Furthermore, endothelial MMP-9 expression can be up-regulated by oscillatory flow through activation of c-myc [131]. The elevated endothelial MMPs expression and activation may thus contribute to the development and progression of atherosclerosis and coronary heart disease.
It is well known that angiogenesis, the formation of new microvessels, occurs in atherosclerotic plaques leading to neovascularization and the growth of the lesion [132]. Although the mechanisms of involved in this process are not fully understood, MMPs, especially MMP-2 and -9, have shown to play a pivotal role in the process of angiogenesis. Elevated MMP-2 and -9 were reported in arteries from subjects with diabetic chronic kidney disease, which correlated to impaired angiogenesis and endothelial dysfunction [133]. A recent study demonstrated that MMP-8 plays an important role in angiogenesis as well. In this study, Fang and coworkers demonstrated that MMP-8/apoE double knockout mice displayed attenuated angiogenesis and smaller atherosclerotic plaque size compared to the apoE knockout mice, concluding that MMP- 8 triggers atherosclerotic formation, and targeting MMP-8 is as a potential therapeutic target of atherosclerosis [134]. In fact, numerous angiogenic signaling pathways, such as those that are induced by the growth factors FGF and VEGF have been shown to upregulate MMPs via Erk, JNK and Akt activation, which further consolidate the evidence that MMP-associated angiogenesis contribute to the development of atherosclerosis [135–137].
3.4.2. MMPs in obesity and insulin resistance-associated cardiac disease
Obesity, which is defined as an increased body mass index (> 30 kg/m2), is an important health issue for Americans in view of its increasing prevalence [138]. Obesity usually accompanies with insulin resistance, which is the fundamental pathological change that predisposes to type 2 diabetes. Both obesity and insulin resistance are important independent risk factors for cardiac disease [139]. Several mechanisms contribute to obesity and/or insulin resistance-induced cardiac disease although the exact mechanism involved is far from clear [140]. Interestingly, elevated plasma MMP-2 and -9 levels have been found in obese women and children [141, 142], which imply that MMPs are potentially involved in obesity-associated heart disease. This notion received support from the evidence of an animal study published in 2001 by Peterson and coworkers who found that the MMP-2 activity in the left ventricle was elevated in obese, spontaneously hypertensive heart failure (SHHF) rats, which paralleled impaired ventricular function. More importantly, pharmacological inhibition of MMP alleviated left ventricle dysfunction and remodeling in SHHF rats, suggesting that MMP activity contributed to left ventricle dysfunction in obese animals [143]. Sucrose-enriched diet feeding resulted in lipid accumulation accompanied by upregulation of cardiac MMP-2, MMP-9 and TIMP-1 expression in rats, which were reversed by l-arginine supplementation [144]. Another study showed that the activity of MMP-7, a critical regulator of cardiac fibrosis was increased in the diabetic heart as well [145]. Conflicting results were however found in a human study, in which authors demonstrated that abnormalities of left ventricle function in premenopausal obese women were related to the decreased plasma MMP-2 levels, possibly because the suppressed MMP system attenuates ECM degradation [146]. Results from an animal study further revealed that MMP-2 activity was decreased in a rat model that spontaneously develops diabetes mellitus. In this study, the authors observed that angiotensin II receptor blockade prevents left ventricular diastolic dysfunction by restoring MMP-2 activity [147]. Further research is necessary to ascertain the role of MMPs in obesity and insulin resistance-associated cardiac disease.
3.4.3. MMPs in hypertensive heart disease
Normal systolic blood pressure is within the range of 100–140 mmHg, whereas diastolic blood pressure is within the range of 60–90 mmHg. Hypertension is defined as a blood pressure that is persistent at or above 140/90 mmHg. Untreated or uncontrolled hypertension is an important risk factor for cardiac disease, which is called hypertensive heart disease. The role of MMPs has been extensively studied in hypertensive heart disease due to the involvement of ECM in cardiac remodeling that is commonly associated with the hypertensive heart. Elevated MMPs expression levels have been documented in hearts from different models of hypertensive animal, including angiotensin II-induced hypertension [148], pulmonary hypertension [149], renovascular hypertension [150], spontaneously hypertensive rats [151], volume overload [152] and pressure overload-induced hypertension [153]. Studies by using transgenic models further declare the contributions of MMP/TIMP in hypertensive heart disease. Overexpression of MMP-1 has been reported to correct the accumulation of cardiac fibrosis, cardiac dysfunction and remodeling in response to chronic pressure overload [154]. Conversely, MMP-2 knockout mouse was resistant to pressure overload-induced left ventricular hypertrophy and dysfunction [155]. MMP-7 inhibition by genetic knockout and pharmacological inhibitors displayed attenuated hypertrophy through correction of a disintegrin and metalloproteinase-12 (ADAM-12) overexpression [156]. Furthermore, angiotensin-converting enzyme inhibitor was shown to block MMP activity and thus protected left ventricle from remodeling and dysfunction [157]. Additionally, MMP-associated angiogenesis has been documented to be crucial in the transition from compensatory hypertrophic heart to decompensatory failing heart. In this study, increased MMP-2 as well as increased angiogenic factors were observed in hypertrophic heart, whereas an increase in MMP-9 and angiostatin were found in failing heart [158].
3.5. Calpains in cardiometabolic diseases
As discussed under section 2.2., calpain targets a wide spectrum of proteins, suggesting a key role of calpain in cardiac diseases. Accordingly, the involvement of calpain in cardiometabolic diseases has been well documented. The following section briefly discusses the role of calpain in various cardiometabolic diseases.
3.5.1. Calpain in atherosclerosis and coronary heart disease
It is widely accepted that calpains act in the endothelial cells and helps maintain vascular integrity. A number of factors such as VEGF and shear stress activate the calpain system under physiological condition [159, 160]. Mechanistically, calpain is able to regulate small GTPase [161] and lyse focal adhesion proteins [160]. Interestingly, it has been documented that nitric oxide production is mediated by calpain-induced proteolysis of HSP90 or PI3K/AMPK signaling cascade [159, 162]. In addition to its physiological role, calpain participates in the pathogenesis of various diseases. An earlier study revealed that sPLA2-modified LDL or oxidized LDL enhanced m-calpain expression in endothelial cells on atheroma [163, 164]. Activated calpain directly cleavages VE-cadherin and thus facilitates the extravasation of inflammatory cells and macromolecules into the vascular wall [165]. Silencing CAPN2 by siRNA technique further confirmed that knockdown of calpain induces pro-atherogenic hyperpermeability in the murine aorta [163]. It has been reported that calpain activity is also enhanced in endothelial cells from lipopolysaccharide-accelerated atherosclerosis [166]. Cytokines and inflammatory signals are involved into the development of atherosclerosis. Nuclear factor-κB (NF-κB) signal, a representative inflammatory signal, was modulated by calpain via degradation of IκB without affecting its phosphorylation [167]. Not surprisingly, NF-κB as well as cytokines levels in endothelial cells from pro-atherogenic aorta in LDLr−/− mice were remarkably decreased following the administration of calpain inhibitors [163]. Macrophage-derived foam cells are hallmark for atherosclerosis. Activated ATP-binding cassette transporter A1 (ABCA1) in macrophages is shown to be dampened by calpain-induced proteolysis [168]. Calpain has also been shown to interfere with cholesterol efflux from macrophage through the degradation of the ATP-binding cassette transporter G1 (ABCG1) [169]. In smooth muscle cells, activation of calpain was reported to precede the activation of caspase in response to degraded collagen, whereas, inhibition of calpain was observed to reduce apoptotic response, suggesting a potential role of calpain in atherosclerotic plaque rupture [170]. Evidence from a clinical study confirmed the association of calpain-10 with atherosclerosis and coronary heart disease in human [171].
3.5.2. Calpain in obesity and insulin resistance-associated cardiac disease
CAPN10 has been identified as a diabetic gene and a number of positional cloning studies have identified that genetic variation in CAPN10 accounted for 14% of the population-attributable risk to type 2 diabetes in Mexican Americans [172]. Several studies published subsequently, using a wide range of ethnic populations further confirmed the association of CAPN10 with type 2 diabetes [173–178]. This suggests that calpains may be potentially involved in insulin regulated pathway. The first paper in support of the role of calpain on insulin signaling published in 1990s, demonstrated that calpain regulates the expression of insulin receptor substrate-1 (IRS-1) [179]. A series of recent studies strongly support this notion that calpain regulates insulin signaling pathway. Calpain inhibitors prevented IRS-1 downregulation, while drug-induced overload of intracellular Ca2+ could restore the suppressed IRS-1 level [16]. A clinical study demonstrated that activation of calpain was detrimental to the diabetic myocardium, and blocking calpain activation protected heart from diabetes-associated cardiac injury [180]. Nonetheless, literature regarding the contribution of calpain in obesity, insulin resistance and associated cardiac disease are limited warranting further studies to understand the exact nature of the role of calpains in obesity and insulin resistance-associated cardiac disease.
3.5.3. Calpain in hypertensive heart disease
Several transcriptional factors, such as NF-κB, GATA binding protein 4 (GATA4) and nuclear factor of activated T cells (NFAT) participate in the development of hypertension-induced cardiac hypertrophy. Interestingly, calpain has been reported as a critical regulator for NF-κB, GATA4 and NFAT, implying the possible role of calpain in cardiac hypertrophy [167, 181, 182]. Enhanced calpain activity as well as decreased calpastatin expression was found in hearts from mice subjected to angiotensin II infusion [183]. In the same study, experiments performed on transgenic mice that constitutively express calpastatin revealed that infusion of angiotensin II failed to induce cardiac hypertrophy, although those mice did develop hypertension. Parallel to this finding, angiotensin II-induced NF-κB over-expression was blunted by calpastatin transgene in mice, which may represent the molecular mechanism by which calpastatin overexpressed mice exhibited resistance to hypertension-associated cardiac hypertrophy [183]. In line with these findings, pre-administration of calpeptin, a specific calpain inhibitor, to hypertensive β3-integrin-deficient mice alleviated cardiomyocyte apoptosis and prevented cardiac hypertrophy [184]. Another study suggested that elevated calpain activation in hypertrophic heart could lead to the degradation of focal adhesion kinase as well as calcineurin to worsen cardiac hypertrophic response [185]. Treatment with calpain inhibitor attenuated cardiac hypertrophy via inhibiting the degradation of these proteins. Indeed, most substrates of calpain are potentially involved in the pathogenesis of hypertensive heart disease. However, more conclusive studies are necessary to evaluate the molecular pathways by which calpains contribute to cardiac hypertrophy.
3.6. Cathepsins in cardiometabolic diseases
Studies in the recent years have implicated a role of cathepsins in the etiopathology of cardiometabolic disease. Several cathepsins, such as cathepsin S, K, B and L have been found to be expressed in cardiomyocyte, cultured cardiac fibroblasts and/or myofibroblasts [59, 186, 187]. Although the expression of cathepsins in heart is negligible at basal conditions, stimulation by cytokines, angiotensin II and superoxide can remarkably enhance their expression [43]. The following section provides an outline of our understanding of the role of cathepsin in various cardiometabolic diseases.
3.6.1. Cathepsins in atherosclerosis and coronary heart disease
Cathepsins exhibit potent collagenase and elastinase activity, by virtue of which they potentially participate in the formation and rupture of atheroma. Early studies documented that increased cathepsin activity was associated with experimental atherosclerosis [188]. Two decades ago, Reddy and the colleague observed that human macrophages secrete the active form of cathepsin S, B and L [189]. Subsequent studies showed that cathepsin D regulates ABCA1-mediated lipid efflux and decreased levels of cathepsin D levels results in low plasma HDL-C levels [190]. Platt and coworkers reported an increased expression of cathepsin K in the endothelium of human subjects with atherosclerosis [191]. Consistent with these observations, cathepsin levels were also found to be elevated in a diet-induced animal model of atherosclerosis [192]. A recent study investigated the relative expression of cathepsins at different sites of atherosclerotic plaque. Increased cathepsin expression was detected in sites prone to rupture, including macrophages bordering the lipid core and adjacent to the fibrous cap or macrophages/smooth muscle cells in the shoulder regions [193, 194]. Cathepsin L has been shown to be the major contributor to apoptosis of the macrophages resulting in necrotic core formation, leading to atherosclerotic plaque instability [195]. Interestingly, one recent study showed that cathepsin G deficiency attenuated the complexity of atherosclerotic lesions in apolipoprotein E-deficient mice via dampening apoptosis [196]. Cathepsin S, on the other hand, improves fibrous cap stabilization, and helps monocyte adhesion and migration in an in vitro system [197]. Cathepsin K levels were found positively correlated to the percent plaque volumes and negatively correlated to percent fibrous volumes. Additionally, cathepsin K blood levels have been claimed to be an independent predictor of coronary heart disease [198]. Similarly, serum cathepsin L contents were significantly increased in patients with coronary heart disease [199, 200]. However, Mirzaii-Dizgah and coworkers failed to observe any changes in serum and salivary cathepsin L levels in subjects with coronary heart disease [201]. Cystatin c, an endogenous inhibitor of cathepsin, has also been implicated in the development of coronary heart disease in a human study. The mutant haplotype of cystatin c gene was related to higher average number of stenosis per coronary artery segment [202]. Collectively these studies show that cathepsins contribute to the development of atherosclerosis and coronary heart disease. However different cathepsins may have different role in the pathogenesis – some of them being detrimental whereas some others being protective. Targeting cathepsins my thus represent an attractive strategy to treat atherosclerosis and coronary heart disease.
3.6.2. Cathepsins in obesity and insulin resistance-associated cardiac disease
In the year 2003, cathepsin K was identified as a novel marker of adiposity in white adipose tissue owing to its high levels of expression in white adipose tissue in the db/db mice [203]. Moreover, the expression of the transcription factors involved in the induction of cathepsin K were also elevated in the white adipose tissue from obese mice [203]. Subsequent studies ascertained the role of cathepsin K in adipocyte differentiation. Inhibition of cathepsin K by either pharmacological inhibitor or genetic ablation was sufficient to reverse adipocyte differentiation and lipid accumulation in response to high-fat diet feeding [204, 205]. Cathepsin K inhibition prevented high-fat diet or leptin deficiency-induced obesity as well as elevated serum glucose and insulin levels by degrading fibronectin [206]. Degradation of type I collagen has also been proposed as a potential mechanism by which cathepsin K participates in adipogenesis during the early differentiation phases [207]. Cathepsin S is another extensively studied cathepsin in terms of its role in the obesity. A number of studies have reported elevated levels of cathepsin S in adipose tissues from both human and animal models [208–210]. Interestingly, weight loss in morbidly obese women resulted in reduced expression of cathepsin S in the adipose tissue and lower circulating levels of this protease [211]. Recently, cathepsin D was also shown to be up-regulated in obese mouse and human adipose tissue [212]. In addition, activated cathepsin D associated with adipocyte hypertrophy triggered the activation of proapoptotic proteins [213].
Expression levels of cathepsin L in the muscle has been shown to be elevated in glucose intolerant mice [214]. In the skeletal muscle of diabetic subjects, reduced insulin-stimulated cathepsin L gene expression was also reported in this paper, suggesting that impaired cathepsin L expression is secondary to impaired glucose metabolism [214]. Cathepsin S levels strongly correlate with insulin resistance as reported by Jobs and coworkers, who in their study showed that subjects with higher cathepsin S levels had decreased insulin sensitivity and higher risk to develop type 2 diabetes [215]. This finding was further supported by a study from Chen and colleagues who showed a correlation between serum cathepsin S and insulin resistance, in type 2 diabetic subjects [216].
In our lab, we investigated the role of cathepsin K in obesity-associated cardiac dysfunction. We found that genetic ablation of cathepsin K in mice protected hearts from high-fat diet feeding-induced geometric and functional impairment, as evidenced by recovered fractional shortening as well as peak shortening of single cardiomyocytes. Mechanistically, insulin signaling pathway was dampened in the heart from high-fat diet feed mice, which was rescued by cathepsin K knockout. Moreover, activated apoptosis in mouse heart consequent to high-fat diet feeding was attenuated by cathepsin K knockout [187]. These studies substantiate the notion that cathepsin K participates in obesity-associated heart disease and targeting cathepsin K maybe a promising approach to counter obesity-associated complications in the heart.
3.6.3. Cathepsins in hypertensive heart disease
The early report linking cathepsin to hypertension was from a study by Saito and coworkers, wherein an increased cathepsin level in the serum of spontaneously hypertensive rats was observed [217]. A study published in the following year further by Wildenthal and coworkers confirmed the role of this lysosomal enzyme in the development of hypertension-induced cardiac hypertrophy [218], which was substantiated by another study by Rozek and colleagues [219]. Impaired cathepsin B activity was displayed in hypertensive heart, which was reversed by the treatment with ACE inhibitor. Additionally, the ACE inhibitor alleviated hypertension-associated cardiac hypertrophy in rats [220]. Cheng and coworkers investigated the role of cathepsin in hypertension-induced heart failure in rat [59]. They identified cathepsin S as the predominant cathepsin that is elevated in the failing heart. Furthermore, the expression and activity of cathepsin S was induced by IL-1β in cultured neonatal cardiomyocytes, suggesting that inflammatory mediators activate cathepsin S. A follow-up study from same group reported that superoxide anions activate cathepsin, which in turn triggers myocardial remodeling [43]. Blocking angiotensin II type 1 receptor attenuated myocardial remodeling and dysfunction via blunting superoxide-dependent induction of cathepsin. Additionally, the involvement of cathepsin G in the alternative pathways of angiotensin II biosynthesis makes cathepsin G a potential mediator in the development of hypertensive heart disease, although studies to this effect are lacking [221].
Despite the documented role of cathepsins in the pathophysiology of hypertensive heart disease, the underlying molecular mechanisms remain elusive. Most effects attributed to cathepsins in hypertensive heart disease are linked to the degradation of collagen or elastin. However, recent studies have suggested that cathepsins are involved in the crosstalk with several signaling pathways, which may contribute to their observed effects. The Akt/GSK3β pathways have been extensively studied in cardiac hypertrophy because of their effects on inflammation, fibrosis as well as apoptosis. By using a cathepsin L overexpressed mouse model, Tang and coworkers showed that cathepsin L protects against hypertension-induced cardiac hypertrophy via inactivation of the Akt/GSK3β signaling pathway [222]. In contrast, cathepsin L knockout in mice was shown to accentuate pressure overload-induced cardiac hypertrophy [223]. Impaired lysosomal protein degradation, increased sarcomere-associated protein aggregation, increased ubiquitin-proteasome system as well as altered endoplasmic reticulum homeostasis were found in hypertensive cathepsin L knockout mice, all of which resulted in the worsening of cardiac function and cardiac remodeling in response to pressure overload. We evaluated the role of cathepsin K knockout in mice subjected to pressure overload-induced cardiac hypertrophy [224]. Our results support the notion that cathepsin K knockout in mice prevents the development of cardiac hypertrophy and contractile dysfunction. Moreover, the stimulation of the mTOR and Erk signaling pathways which were induced in hypertrophic heart, were blunted by cathepsin K knockout and in cultured cardiomyocytes subjected to siRNA mediated silencing of cathepsin K. Furthermore, plasmid-mediated overexpression of cathepsin K in the cultured cardiomyocytes triggered cardiomyocyte hypertrophy, which was blocked by mTOR and Erk inhibitors. Collectively, our data suggest the important role of cathepsin K in cardiac hypertrophy induced by hypertension.
3.7. Caspases in cardiometabolic diseases
Caspases are essential players in the apoptotic cascade, playing a pivotal role in the initiation and execution of apoptosis. Volumes of literature have reported the involvement of apoptosis in cardiac disease, including cardiometabolic disease and a detailed discussion is beyond the scope of this review. The role of caspase in cardiometabolic disease will be the focus here.
3.7.1. Caspases in atherosclerosis and coronary heart disease
Macrophages are the most abundant inflammatory cells in atherosclerotic plaque. Macrophage-derived foam cells with lipid core represent the major component of the atherosclerotic plaque. Fas or other death receptor triggered apoptosis has been proposed to contribute to plaque macrophage death and even plaque development [225, 226]. Importantly, expression of c-FLIP, a dominant-negative inhibitor of caspase-8, has been reported to protect plaque macrophages from apoptotic cell death [227]. Role of caspase in macrophage apoptosis has been extensively reviewed in a previous literature [228]. Oxidized LDL and cholesterol accumulation participate in plaque cell death [226, 229]. Oxidized LDL-induced apoptosis involves both the death receptor and mitochondrial apoptotic pathways. Caspase cascade is in turn activated, which triggers apoptosis [230]. Besides macrophage, vascular cells such as endothelial cells and smooth muscle cells undergo apoptosis in atherosclerosis plaque as well. The role of apoptosis in plaque endothelial cells and smooth muscle cells has been reviewed elaborately in previous reports [231, 232].
3.7.2. Caspases in obesity and insulin resistance-associated cardiac disease
Lipoapoptosis has been observed in obesity and type 2 diabetes, which is an important feature that contributes to obesity-associated cardiac dysfunction. Accumulated unoxidized fatty acids in obese subjects trigger toxic pathways, such as ceramide pathway to initiate apoptosis in cardiomyocytes [233]. Increased cardiomyocyte fatty acid oxidation as well as inhibition of glucose uptake have been reported to induce apoptosis and result in dilated cardiomyopathy in mice expressing glycosylated-inositol (GPI)-anchored human lipoprotein lipase (hLpLGPI) [234]. Studies from our group have documented the involvement of caspase activation and apoptosis in cardiac dysfunction in response to high-fat diet feeding [187, 235]. Additionally, in genetically obese mice, enhanced apoptotic response in cardiomyocyte was accompanied by increased DNA damage and decreased survival rate [236]. Elevated cardiac Fas receptor-dependent apoptotic pathway has been observed in obese Zucker rats [237].. Increased protein levels of Fas ligand, Fas death receptors, Fas-associated death domain were significantly upregulated in obese rat hearts, which was paralleled with enhanced caspase-8 and -3 activities, suggesting the involvement of Fas receptor-dependent apoptosis in obesity-associated heart disease [237]. Interestingly, another study from the same group reported activated cardiac mitochondrial-dependent apoptotic pathway in obese Zucker rats. Increased Bcl-2/adenovirus E1B 19 kDa interacting protein (BNIP3), Bad expression as well as cytochrome c release were observed in hearts from obese rats. A reciprocal suppression of the antiapoptotic Bcl2 protein level was observed. Moreover, an increase in the expression of activated caspase-9 and -3 were reported, suggesting the involvement of mitochondrial-dependent apoptotic pathway in obesity-associated heart disease [238]. A follow-up study further confirmed the effect of mitochondrial apoptotic pathway in obesity-associated cardiac disease. Furthermore, aerobic exercise training was effective in blocking cardiac mitochondrial apoptotic signaling and rescuing cardiac dysfunction [239]. More importantly, reduction in body mass resulted in attenuated apoptosis, oxidative stress and inflammation, which rescued left ventricular remodeling as well as heart dysfunction in obese mice [240]. Cardiac remodeling caused by a high-carbohydrate, high-fat diet-induced metabolic change was alleviated by rutin via its antiapoptotic properties [241]. Collectively, the aforementioned studies provide strong evidence in support of the relationship between caspase-induced apoptosis and obesity/insulin resistance-induced heart disease.
3.7.3. Caspase in hypertensive heart disease
Ravassa and the colleagues reported the contribution of apoptosis in angiotensin II-induced ventricular cardiomyocyte dysfunction in the spontaneously hypertensive rats [242]. This finding was supported by the studies from the labs of Lopez-Farre and [243] and deBlois group [244]. Interestingly, the elevated apoptosis in hypertrophic hearts of spontaneously hypertensive rats was attenuated by exercise training [245]. Moreover, cardiomyocyte apoptosis was triggered in the failing hearts of hypertensive human subjects. On the other hand, the expression of gp130, a protein preventing cardiomyocyte apoptosis, was decreased in failing heart of hypertensive patients. There was a negative correlation between gp130 and cardiomyocyte apoptosis in hypertensive patients that develop heart failure [246]. Endoplasmic reticulum (ER) stress-induced apoptosis was reported in the hypertensive heart in high-salt diet fed Dahl salt-sensitive rats. Caspase-12 has been shown to be involved in the ER stress-associated cardiomyocyte apoptosis in hypertensive heart [247]. Interestingly, caspase-3 and calpain activities were elevated at the same time in the pressure overload-induced hypertrophic heart, whereas calpain inhibitor could block caspase-3 activation and prevent cardiac dysfunction. However, caspase inhibitor was not beneficial to cardiac function in the pressure overload model. Based on these findings the authors concluded that calpain may be the key mediator of cardiomyocyte death, although apoptosis cascade may be involved in the progression of end-stage hypertrophy to heart failure [34]. The exact role of caspase and apoptosis in hypertensive heart disease remains unclear although the activation of apoptosis under this condition is definite.
4. Potential clinical application of proteases
Due to the important role of proteases in ECM degradation, intracellular protein degradation and participation in a variety of singling pathways, it is reasonable to expect the possibility of harnessing proteases and their endogenous and synthetic inhibitors in for the diagnosis or treatment of cardiometabolic diseases. MMPs, cathepsins and caspases have been suggested as potential diagnostic markers for the detection and prognosis of cardiometabolic disease. Recent studies have shown that elevated levels of serum MMP-9 was found in atherosclerotic carotid artery, and is related to the plaque vulnerability, suggesting that cathepsins can also be potential markers of cardiovascular disease [248]. In another study, Shirakabe and coworkers investigated the change of serum MMP-2 levels in subjects with acute heart failure before and after treatment and its relationship to clinical prognosis was investigated. Following treatment for acute heart failure, the patients had decreased levels of MMP-2, indicating that prognosis of heart failure was markedly better among the patients with a altered MMP-2 levels [249]. Gene polymorphism of MMP-9 was found to increase the risk of clinical events in coronary heart disease patients. Human subjects with coronary artery disease exhibiting IL-18+183 AA/MMP-9-1562 CT/TT combined genotypes were shown to have a higher risk of clinical events [250]. In contrast, patients with 6A-1171-G-519 haplotype had a reduced risk of clinical events [251], suggesting that genotyping for MMP may represent a diagnostic tool for cardiac health. Cathepsins have also been suggested as potential biomarkers for cardiometabolic diseases [200]. Serum cathepsin L levels were higher in patients with acute myocardial infarction compared to those with unstable angina pectoris. Additionally, serum cathepsin L levels were positively related to number of coronary branch luminal narrowing and Gensini scores. Furthermore, cathepsin L levels have been negatively correlated to high-density lipoprotein and apolipoprotein, suggesting the potential diagnostic value of serum cathepsin L levels in coronary heart disease [200]. Cystatin C, the endogenous inhibitor of cathepsin, has also received attention as a biomarker for cardiometabolic diseases, including atherosclerosis [252], heart failure [253] and hypertensive cardiac hypertrophy [254]. As the direct executioner of apoptosis, caspases have also been evaluated as a potential biomarker of cardiometabolic diseases. Elevated serum caspase-1 content was observed in patients with acute angina [255]. Circulating caspase-3 p17 level has also been identified as a potential biomarker for cardiovascular disease [256].
During the past decade, several pharmaceutical companies have been engaged in designing and developing new drugs to target proteases for the treatment of cardiometabolic diseases. Two MMP inhibitors, KB-R7785 from Kanebo KK Company and SC-44463 from Pfizer are being developed for the treatment of cardiovascular disease [257]. Additionally, some widely used drugs like doxycycline has been tested for its efficacy in the treatment of coronary heart disease, by virtue of its effect on MMP [258]. Among the cathepsin family, pharmacological inhibition of cathepsin K has garnered the most interest because of its significant role in bone resorption. Currently, there are three cathepsin K inhibitors under clinical trial for treatment of osteoporosis. One such cathepsin K inhibitor Odanacitib, which is effective and without major side effects (following a 5-year trial), is currently under phase III clinical trials [259]. In view of the cardio-protective role of cathepsin K inhibition as evidenced by our lab and that of others, we can speculate the potential application of cathepsin K specific inhibitors in treating cardiovascular disease. Various caspase inhibitors have been designed to target a wide spectrum of diseases. Among the caspase inhibitors, pan-caspase inhibitors, IDN- 6734 and MX1013 have been tested [260].
5. Where are we in our understanding of the role of proteases in cardiometabolic disorders?
Although there are numerous proteases that have been identified the key proteases with demonstrated role in cardiometabolic diseases include the MMPs, calpains, cathepsins and caspases and their endogenous activators and inhibitors. There are a number of subtypes within these proteases, some of which have similar or opposing actions suggesting redundancy and counterregulatory functions. Despite growing evidence supporting a functional association between proteases and cardiometabolic diseases, it yet unclear if the upregulation of protease activity represent a cause or consequence (or both) of cardiometabolic disorders. It is likely that inflammatory mediators such as reactive oxygen species and cytokines activate proteases which can lead to proteolysis of substrates involved in key pathways regulating a variety of cardiometabolic functions. It is also likely that activated proteases upregulate inflammatory mediators resulting in a vicious cycle. Thus, the emerging consensus is that proteases play a critical role in cardiometabolic disease and targeting proteases may represent an important therapeutic strategy.
The proteases discussed above have all been strongly implicated in the progression of diabetic cardiac disease and heart failure. Among the proteases described above MMPs have been extensively studied for their role in diabetes induced cardiac remodeling and dysfunction. MMPs play a critical role in the cardiac remodeling event which is characterized by synthesis and degradation of extracellular matrix. Thus for MMPs, the etiopathology of cardiometabolic diseases may be attributed to their traditional proteolytic processing of signaling molecules. Whereas targeting of MMP activation is therefore a potential clinical strategy to treat cardiometabolic disease, altering TIMP may also serve as an important strategy to counterbalance the effects of MMP. On the other hand, being a calcium-activated protease calpain plays a major role in regulating calcium-regulated cellular process such as cardiac contractility, hypertrophy and apoptosis. However, calpains have also been shown to be important for maintaining protein homeostasis in the cardiac cells, and therefore a deficiency of calpain may also prove to be detrimental. This dichotomy suggests that calpains serve a regulatory role; whereas excessive calpain activity needs to be curbed, low levels of calpain activity may have to be boosted. Further studies are necessary to distinguish these diverse roles of calpain and to ascertain conditions wherein one strategy supersedes another. At the molecular level, calpains are thought to mediate their detrimental cardiovascular via the activation of NFΚB and the tumor TGF-beta signaling pathways. Calpains also play a critical role in cell death via activating the apoptosis pathway and/or proteolytic degradation of molecules involved in the apoptotic pathway. As discussed above, variation in calpain-10 gene has been shown to be associated with diabetes. However, understanding the implication of this genetic variation in cardiometabolic disease is a matter of great interest. Cathepsins on the other hand are recent entrants in the field. Among the cathepsins, the cysteinyl cathepsins that localize in the endosomes or lysosomes are thought to play a major role in cardiometabolic disease as lysosomal membrane instability caused by a variety of cellular stressors can lead to the release of these proteases. Although these proteases are optimally active in the acidic environment some of them may retain their activity in the neutral cytoplasmic or extracellular environment and mediate their potent elastolytic or collagenolytic activity. Caspases on the other hand have been shown to mediate apoptosis. MMPs, calpains and cathepsins can cause proteolytic activation of caspases leading to cell cellular apoptosis suggesting a cross-talk between different proteases.
Future research should be directed at studying the global changes in these proteases to address the following questions i) Is there a redundancy and counter-regulation among the proteases; and among proteases and their respective inhibitors? ii) In addition to their proteolytic activities are non-traditional mechanisms (such as autophagy), involved in mediating the activities attributed to these proteases? iii) What is the extent of cross-talk between these proteases? iv) What are the upstream regulators and downstream effectors of proteases, and can any of these be used as biomarkers of cardiometabolic syndrome and/or targets for treatment? Investigating these and other relevant questions would provide useful insights to our growing understanding of the role of proteases in cardiometabolic disease.
6. Conclusion
Emerging studies suggest a strong role of proteases especially MMPs, calpain, cathepsins and caspases in the development of cardiometabolic diseases, including atherosclerosis/coronary heart disease, obesity/insulin resistance-associated heart disease and hypertensive heart disease. Targeting these proteases would therefore represent a novel therapeutic approach for treating or controlling cardiometabolic diseases. Studies in human and animal models show that the expression of MMPs, calpain, cathepsins and caspases under cardiometabolic conditions are regulated by a variety of stimulants such as cytokines, ROS and angiotensin II. Traditionally, the activated proteases either degrade ECM or intracellular proteins to contribute to the pathogenesis of cardiometabolic diseases. Recently however, the nontraditional effects of protease have been elucidated. Proteases have been shown to regulate signaling cascades such as the Akt and mTOR signaling pathways. These novel insights not only add to our growing understanding of the role of proteases in cardiometabolic diseases but would also provide novel avenues of treatment of this disease (Fig. 1). More studies are warranted to understand the cross-talk between proteases and cellular signaling pathways. Such studies, together with already published work would provide a strong rationale for clinical trials which would allow the harnessing of protease inhibitors in treating cardiometabolic diseases.
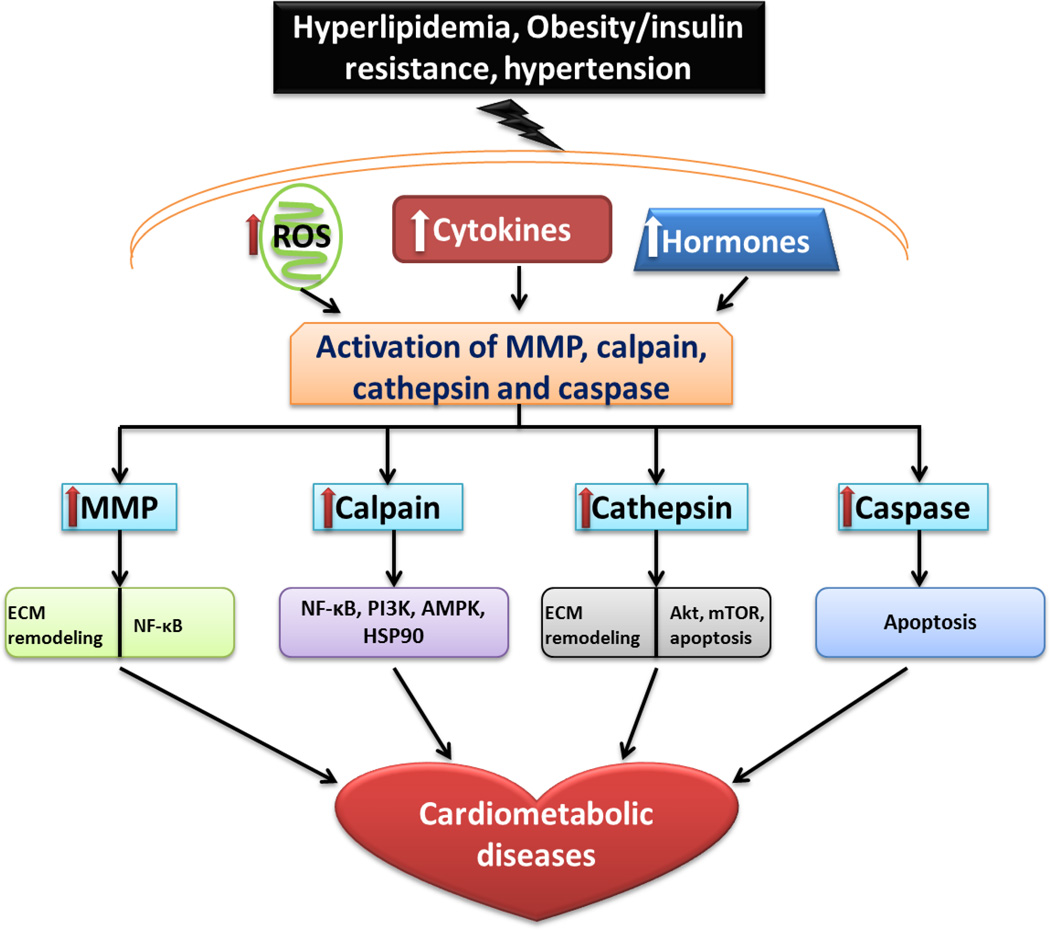
Figure 1.
Role of proteases in cardiometabolic disease. Proteases (MMP, calpain, cathepsin and caspase) affect ECM remodeling and participate in a variety of intracellular signaling pathways in response to elevated ROS, cytokines and hormones contributing to the pathogenesis of cardiometabolic diseases. MMP: matrix metalloproteinase. ECM: extracellular matrix. NF-κB: nuclear factor-κB. PI3K: phosphoinositide 3-kinase. AMPK: AMP-activated protein kinase. HSP90: heat shock protein 90. mTOR: mammalian target of rapamycin.
Highlights.
Proteases play a pivotal role in the pathophysiology of cardiometabolic diseases
Matrix metalloproteases, calpains, cathepsins and caspases are among the proteases that have been studied extensively
This review focuses on the functional and mechanistic studies of proteases in the context of cariometabolic disease
Proteases represent a potential target for treatment of cardiometabolic disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 2.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 4.Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- 5.Springer SC, Silverstein J, Copeland K, Moore KR, Prazar GE, Raymer T, Shiffman RN, Thaker VV, Anderson M, Spann SJ, Flinn SK. Management of type 2 diabetes mellitus in children and adolescents. Pediatrics. 2013;131:e648–e664. doi: 10.1542/peds.2012-3496. [DOI] [PubMed] [Google Scholar]
- 6.Puente XS, Sanchez LM, Overall CM, Lopez-Otin C. Human and mouse proteases: a comparative genomic approach. Nat Rev Genet. 2003;4:544–558. doi: 10.1038/nrg1111. [DOI] [PubMed] [Google Scholar]
- 7.Rawlings ND, Tolle DP, Barrett AJ. MEROPS: the peptidase database. Nucleic Acids Res. 2004;32:D160–D164. doi: 10.1093/nar/gkh071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasmin, McEniery CM, Wallace S, Dakham Z, Pulsalkar P, Maki-Petaja K, Ashby MJ, Cockcroft JR, Wilkinson IB. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:372. doi: 10.1161/01.ATV.0000151373.33830.41. [DOI] [PubMed] [Google Scholar]
- 9.Nenan S, Boichot E, Lagente V, Bertrand CP. Macrophage elastase (MMP-12): a pro-inflammatory mediator? Memorias do Instituto Oswaldo Cruz. 2005;100(Suppl 1):167–172. doi: 10.1590/s0074-02762005000900028. [DOI] [PubMed] [Google Scholar]
- 10.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: structures, evolution, and diversification. FASEB J. 1998;12:1075–1095. [PubMed] [Google Scholar]
- 12.Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation. 2000;102:1944–1949. doi: 10.1161/01.cir.102.16.1944. [DOI] [PubMed] [Google Scholar]
- 13.Stewart JA, Jr, Wei CC, Brower GL, Rynders PE, Hankes GH, Dillon AR, Lucchesi PA, Janicki JS, Dell'Italia LJ. Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardiol. 2003;35:311–319. doi: 10.1016/s0022-2828(03)00013-0. [DOI] [PubMed] [Google Scholar]
- 14.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- 15.Griffin MO, Fricovsky E, Ceballos G, Villarreal F. Tetracyclines: a pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am J Physiol Cell Physiol. 2010;299:C539–C548. doi: 10.1152/ajpcell.00047.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 17.Zatz M, Starling A. Calpains and disease. N Engl J Med. 2005;352:2413–2423. doi: 10.1056/NEJMra043361. [DOI] [PubMed] [Google Scholar]
- 18.Bukowska A, Lendeckel U, Bode-Boger SM, Goette A. Physiologic and pathophysiologic role of calpain: implications for the occurrence of atrial fibrillation. Cardiovasc Ther. 2010;30:e115–e127. doi: 10.1111/j.1755-5922.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- 19.Fougerousse F, Anderson LV, Delezoide AL, Suel L, Durand M, Beckmann JS. Calpain3 expression during human cardiogenesis. Neuromuscul Disord. 2000;10:251–256. doi: 10.1016/s0960-8966(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 20.Nozaki K, Das A, Ray SK, Banik NL. Calpain inhibition attenuates intracellular changes in muscle cells in response to extracellular inflammatory stimulation. Exp Neurol. 2010;225:430–435. doi: 10.1016/j.expneurol.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas A, El Rouby S, Reed JC, Krajewski S, Silber R, Potmesil M, Newcomb EW. Drug-induced apoptosis in B-cell chronic lymphocytic leukemia: relationship between p53 gene mutation and bcl-2/bax proteins in drug resistance. Oncogene. 1996;12:1055–1062. [PubMed] [Google Scholar]
- 22.Lakshmikuttyamma A, Selvakumar P, Sharma AR, Anderson DH, Sharma RK. In vitro proteolytic degradation of bovine brain calcineurin by m-calpain. Neurochem Res. 2004;29:1913–1921. doi: 10.1023/b:nere.0000042218.27842.79. [DOI] [PubMed] [Google Scholar]
- 23.Blomgren K, Zhu C, Wang X, Karlsson JO, Leverin AL, Bahr BA, Mallard C, Hagberg H. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of "pathological apoptosis"? J Biol Chem. 2001;276:10191–10198. doi: 10.1074/jbc.M007807200. [DOI] [PubMed] [Google Scholar]
- 24.McGinnis KM, Whitton MM, Gnegy ME, Wang KK. Calcium/calmodulin-dependent protein kinase IV is cleaved by caspase-3 and calpain in SH-SY5Y human neuroblastoma cells undergoing apoptosis. J Biol Chem. 1998;273:19993–20000. doi: 10.1074/jbc.273.32.19993. [DOI] [PubMed] [Google Scholar]
- 25.Greenwood AF, Jope RS. Brain G-protein proteolysis by calpain: enhancement by lithium. Brain Res. 1994;636:320–326. doi: 10.1016/0006-8993(94)91031-6. [DOI] [PubMed] [Google Scholar]
- 26.Han Y, Weinman S, Boldogh I, Walker RK, Brasier AR. Tumor necrosis factor-alpha-inducible IkappaBalpha proteolysis mediated by cytosolic m-calpain. A mechanism parallel to the ubiquitin-proteasome pathway for nuclear factor-kappab activation. J Biol Chem. 1999;274:787–794. doi: 10.1074/jbc.274.2.787. [DOI] [PubMed] [Google Scholar]
- 27.Lin YC, Brown K, Siebenlist U. Activation of NF-kappa B requires proteolysis of the inhibitor I kappa B-alpha: signal-induced phosphorylation of I kappa B-alpha alone does not release active NF-kappa B. Proc Natl Acad Sci U S A. 1995;92:552–556. doi: 10.1073/pnas.92.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kubbutat MH, Vousden KH. Proteolytic cleavage of human p53 by calpain: a potential regulator of protein stability. Mol Cell Biol. 1997;17:460–468. doi: 10.1128/mcb.17.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pariat M, Carillo S, Molinari M, Salvat C, Debussche L, Bracco L, Milner J, Piechaczyk M. Proteolysis by calpains: a possible contribution to degradation of p53. Mol Cell Biol. 1997;17:2806–2815. doi: 10.1128/mcb.17.5.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kishimoto A, Mikawa K, Hashimoto K, Yasuda I, Tanaka S, Tominaga M, Kuroda T, Nishizuka Y. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain) J Biol Chem. 1989;264:4088–4092. [PubMed] [Google Scholar]
- 31.Dwyer LD, Miller AC, Parks AL, Jaken S, Malkinson AM. Calpain-induced downregulation of activated protein kinase C-alpha affects lung epithelial cell morphology. Am J Physiol. 1994;266:L569–L576. doi: 10.1152/ajplung.1994.266.5.L569. [DOI] [PubMed] [Google Scholar]
- 32.Shumway SD, Maki M, Miyamoto S. The PEST domain of IkappaBalpha is necessary and sufficient for in vitro degradation by mu-calpain. J Biol Chem. 1999;274:30874–30881. doi: 10.1074/jbc.274.43.30874. [DOI] [PubMed] [Google Scholar]
- 33.Inserte J, Barba I, Hernando V, Garcia-Dorado D. Delayed recovery of intracellular acidosis during reperfusion prevents calpain activation and determines protection in postconditioned myocardium. Cardiovasc Res. 2009;81:116–122. doi: 10.1093/cvr/cvn260. [DOI] [PubMed] [Google Scholar]
- 34.Mani SK, Shiraishi H, Balasubramanian S, Yamane K, Chellaiah M, Cooper G, Banik N, Zile MR, Kuppuswamy D. In vivo administration of calpeptin attenuates calpain activation and cardiomyocyte loss in pressure-overloaded feline myocardium. Am J Physiol Heart Circ Physiol. 2008;295:H314–H326. doi: 10.1152/ajpheart.00085.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fushimi K, Nakashima S, Banno Y, Akaike A, Takigawa M, Shimizu K. Implication of prostaglandin E(2) in TNF-alpha-induced release of m-calpain from HCS-2/8 chondrocytes. Inhibition of m-calpain release by NSAIDs. Osteoarthritis Cartilage. 2004;12:895–903. doi: 10.1016/j.joca.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Letavernier B, Zafrani L, Nassar D, Perez J, Levi C, Bellocq A, Mesnard L, Sachon E, Haymann JP, Aractingi S, Faussat AM, Baud L, Letavernier E. Calpains contribute to vascular repair in rapidly progressive form of glomerulonephritis: potential role of their externalization. Arterioscler Thromb Vasc Biol. 2012;32:335–342. doi: 10.1161/ATVBAHA.111.240242. [DOI] [PubMed] [Google Scholar]
- 37.Sol-Church K, Picerno GN, Stabley DL, Frenck J, Xing S, Bertenshaw GP, Mason RW. Evolution of placentally expressed cathepsins. Biochem Biophys Res Commun. 2002;293:23–29. doi: 10.1016/S0006-291X(02)00167-5. [DOI] [PubMed] [Google Scholar]
- 38.Rawlings ND, Morton FR, Kok CY, Kong J, Barrett AJ. MEROPS: the peptidase database. Nucleic Acids Res. 2008;36:D320–D325. doi: 10.1093/nar/gkm954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sever S, Altintas MM, Nankoe SR, Moller CC, Ko D, Wei C, Henderson J, del Re EC, Hsing L, Erickson A, Cohen CD, Kretzler M, Kerjaschki D, Rudensky A, Nikolic B, Reiser J. Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J Clin Invest. 2007;117:2095–2104. doi: 10.1172/JCI32022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goulet B, Baruch A, Moon NS, Poirier M, Sansregret LL, Erickson A, Bogyo M, Nepveu A. A cathepsin L isoform that is devoid of a signal peptide localizes to the nucleus in S phase and processes the CDP/Cux transcription factor. Mol Cell. 2004;14:207–219. doi: 10.1016/s1097-2765(04)00209-6. [DOI] [PubMed] [Google Scholar]
- 42.Hook V, Funkelstein L, Wegrzyn J, Bark S, Kindy M, Hook G. Cysteine Cathepsins in the secretory vesicle produce active peptides: Cathepsin L generates peptide neurotransmitters and cathepsin B produces beta-amyloid of Alzheimer's disease. Biochim Biophys Acta. 2012;1824:89–104. doi: 10.1016/j.bbapap.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng XW, Murohara T, Kuzuya M, Izawa H, Sasaki T, Obata K, Nagata K, Nishizawa T, Kobayashi M, Yamada T, Kim W, Sato K, Shi GP, Okumura K, Yokota M. Superoxide-dependent cathepsin activation is associated with hypertensive myocardial remodeling and represents a target for angiotensin II type 1 receptor blocker treatment. Am J Pathol. 2008;173:358–369. doi: 10.2353/ajpath.2008.071126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drake FH, Dodds RA, James IE, Connor JR, Debouck C, Richardson S, Lee-Rykaczewski E, Coleman L, Rieman D, Barthlow R, Hastings G, Gowen M. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J Biol Chem. 1996;271:12511–12516. doi: 10.1074/jbc.271.21.12511. [DOI] [PubMed] [Google Scholar]
- 45.Staun-Ram E, Miller A. Cathepsins (S and B) and their inhibitor Cystatin C in immune cells: modulation by interferon-beta and role played in cell migration. J Neuroimmunol. 2011;232:200–206. doi: 10.1016/j.jneuroim.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Stoch SA, Wagner JA. Cathepsin K inhibitors: a novel target for osteoporosis therapy. Clin Pharmacol Ther. 2008;83:172–176. doi: 10.1038/sj.clpt.6100450. [DOI] [PubMed] [Google Scholar]
- 47.Friedrichs B, Tepel C, Reinheckel T, Deussing J, von Figura K, Herzog V, Peters C, Saftig P, Brix K. Thyroid functions of mouse cathepsins B, K, and L. J Clin Invest. 2003;111:1733–1745. doi: 10.1172/JCI15990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Honey K, Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol. 2003;3:472–482. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]
- 49.Chwieralski CE, Welte T, Buhling F. Cathepsin-regulated apoptosis. Apoptosis. 2006;11:143–149. doi: 10.1007/s10495-006-3486-y. [DOI] [PubMed] [Google Scholar]
- 50.Kominami E, Tsukahara T, Hara K, Katunuma N. Biosyntheses and processing of lysosomal cysteine proteinases in rat macrophages. FEBS Lett. 1988;231:225–228. doi: 10.1016/0014-5793(88)80736-1. [DOI] [PubMed] [Google Scholar]
- 51.Nishimura Y, Kawabata T, Yano S, Kato K. Inhibition of intracellular sorting and processing of lysosomal cathepsins H and L at reduced temperature in primary cultures of rat hepatocytes. Arch Biochem Biophys. 1990;283:458–463. doi: 10.1016/0003-9861(90)90667-n. [DOI] [PubMed] [Google Scholar]
- 52.Carmona E, Dufour E, Plouffe C, Takebe S, Mason P, Mort JS, Menard R. Potency and selectivity of the cathepsin L propeptide as an inhibitor of cysteine proteases. Biochemistry. 1996;35:8149–8157. doi: 10.1021/bi952736s. [DOI] [PubMed] [Google Scholar]
- 53.Lecaille F, Choe Y, Brandt W, Li Z, Craik CS, Bromme D. Selective inhibition of the collagenolytic activity of human cathepsin K by altering its S2 subsite specificity. Biochemistry. 2002;41:8447–8454. doi: 10.1021/bi025638x. [DOI] [PubMed] [Google Scholar]
- 54.Kirschke H, Schmidt I, Wiederanders B. Cathepsin S. The cysteine proteinase from bovine lymphoid tissue is distinct from cathepsin L (EC 3.4.22.15) Biochem J. 1986;240:455–459. doi: 10.1042/bj2400455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turk B, Dolenc I, Lenarcic B, Krizaj I, Turk V, Bieth JG, Bjork I. Acidic pH as a physiological regulator of human cathepsin L activity. Eur J Biochem. 1999;259:926–932. doi: 10.1046/j.1432-1327.1999.00145.x. [DOI] [PubMed] [Google Scholar]
- 56.Perdereau C, Godat E, Maurel MC, Hazouard E, Diot E, Lalmanach G. Cysteine cathepsins in human silicotic bronchoalveolar lavage fluids. Biochim Biophys Acta. 2006;1762:351–356. doi: 10.1016/j.bbadis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Serveau-Avesque C, Martino MF, Herve-Grepinet V, Hazouard E, Gauthier F, Diot E, Lalmanach G. Active cathepsins B, H, K, L and S in human inflammatory bronchoalveolar lavage fluids. Biol Cell. 2006;98:15–22. doi: 10.1042/BC20040512. [DOI] [PubMed] [Google Scholar]
- 58.Lemaire R, Huet G, Zerimech F, Grard G, Fontaine C, Duquesnoy B, Flipo RM. Selective induction of the secretion of cathepsins B and L by cytokines in synovial fibroblast-like cells. Br J Rheumatol. 1997;36:735–743. doi: 10.1093/rheumatology/36.7.735. [DOI] [PubMed] [Google Scholar]
- 59.Cheng XW, Obata K, Kuzuya M, Izawa H, Nakamura K, Asai E, Nagasaka T, Saka M, Kimata T, Noda A, Nagata K, Jin H, Shi GP, Iguchi A, Murohara T, Yokota M. Elastolytic cathepsin induction/activation system exists in myocardium and is upregulated in hypertensive heart failure. Hypertension. 2006;48:979–987. doi: 10.1161/01.HYP.0000242331.99369.2f. [DOI] [PubMed] [Google Scholar]
- 60.Lecaille F, Bromme D, Lalmanach G. Biochemical properties and regulation of cathepsin K activity. Biochimie. 2008;90:208–226. doi: 10.1016/j.biochi.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 61.Bode W, Engh R, Musil D, Laber B, Stubbs M, Huber R, Turk V. Mechanism of interaction of cysteine proteinases and their protein inhibitors as compared to the serine proteinase-inhibitor interaction. Biol Chem Hoppe Seyler. 1990;371(Suppl):111–118. [PubMed] [Google Scholar]
- 62.Matsumoto K, Mizoue K, Kitamura K, Tse WC, Huber CP, Ishida T. Structural basis of inhibition of cysteine proteases by E-64 and its derivatives. Biopolymers. 1999;51:99–107. doi: 10.1002/(SICI)1097-0282(1999)51:1<99::AID-BIP11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 63.Helali AM, Iti FM, Mohamed IN. Cathepsin K inhibitors: a novel target but promising approach in the treatment of osteoporosis. Current drug targets. 2013;14:1591–1600. doi: 10.2174/13894501113149990202. [DOI] [PubMed] [Google Scholar]
- 64.Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 65.Black RA, Kronheim SR, Sleath PR. Activation of interleukin-1 beta by a co-induced protease. FEBS Lett. 1989;247:386–390. doi: 10.1016/0014-5793(89)81376-6. [DOI] [PubMed] [Google Scholar]
- 66.Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 67.Walker NP, Talanian RV, Brady KD, Dang LC, Bump NJ, Ferenz CR, Franklin S, Ghayur T, Hackett MC, Hammill LD, et al. Crystal structure of the cysteine protease interleukin-1 beta-converting enzyme: a (p20/p10)2 homodimer. Cell. 1994;78:343–352. doi: 10.1016/0092-8674(94)90303-4. [DOI] [PubMed] [Google Scholar]
- 68.Wang L, Miura M, Bergeron L, Zhu H, Yuan J. Ich-1, an Ice/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell. 1994;78:739–750. doi: 10.1016/s0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 69.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 70.Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 71.Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 72.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 73.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 74.Stephanou A, Brar B, Liao Z, Scarabelli T, Knight RA, Latchman DS. Distinct initiator caspases are required for the induction of apoptosis in cardiac myocytes during ischaemia versus reperfusion injury. Cell Death Differ. 2001;8:434–435. doi: 10.1038/sj.cdd.4400846. [DOI] [PubMed] [Google Scholar]
- 75.Chowdhury I, Tharakan B, Bhat GK. Caspases - an update. Comp Biochem Physiol B Biochem Mol Biol. 2008;151:10–27. doi: 10.1016/j.cbpb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 76.Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu G, Cirilli M, Huang Y, Rich RL, Myszka DG, Wu H. Covalent inhibition revealed by the crystal structure of the caspase-8/p35 complex. Nature. 2001;410:494–497. doi: 10.1038/35068604. [DOI] [PubMed] [Google Scholar]
- 79.Martinon F, Hofmann K, Tschopp J. The pyrin domain: a possible member of the death domain-fold family implicated in apoptosis and inflammation. Curr Biol. 2001;11:R118–R120. doi: 10.1016/s0960-9822(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 80.Weber CH, Vincenz C. The death domain superfamily: a tale of two interfaces? Trends Biochem Sci. 2001;26:475–481. doi: 10.1016/s0968-0004(01)01905-3. [DOI] [PubMed] [Google Scholar]
- 81.Orth K, O'Rourke K, Salvesen GS, Dixit VM. Molecular ordering of apoptotic mammalian CED-3/ICE-like proteases. J Biol Chem. 1996;271:20977–20980. doi: 10.1074/jbc.271.35.20977. [DOI] [PubMed] [Google Scholar]
- 82.Yang X, Chang HY, Baltimore D. Autoproteolytic activation of pro-caspases by oligomerization. Mol Cell. 1998;1:319–325. doi: 10.1016/s1097-2765(00)80032-5. [DOI] [PubMed] [Google Scholar]
- 83.Muzio M, Salvesen GS, Dixit VM. FLICE induced apoptosis in a cell-free system. Cleavage of caspase zymogens. J Biol Chem. 1997;272:2952–2956. doi: 10.1074/jbc.272.5.2952. [DOI] [PubMed] [Google Scholar]
- 84.Duan H, Chinnaiyan AM, Hudson PL, Wing JP, He WW, Dixit VM. ICE-LAP3, a novel mammalian homologue of the Caenorhabditis elegans cell death protein Ced-3 is activated during Fas- and tumor necrosis factor-induced apoptosis. J Biol Chem. 1996;271:1621–1625. doi: 10.1074/jbc.271.3.1621. [DOI] [PubMed] [Google Scholar]
- 85.Zhou Q, Snipas S, Orth K, Muzio M, Dixit VM, Salvesen GS. Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J Biol Chem. 1997;272:7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]
- 86.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang HY, Yang X. Proteases for cell suicide: functions and regulation of caspases. Microbiol Mol Biol Rev. 2000;64:821–846. doi: 10.1128/mmbr.64.4.821-846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, Pickup DJ. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- 89.Tewari M, Dixit VM. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 90.Stegh AH, Peter ME. Apoptosis and caspases. Cardiol Clin. 2001;19:13–29. doi: 10.1016/s0733-8651(05)70192-2. [DOI] [PubMed] [Google Scholar]
- 91.Nalepa G, Zukowska-Szczechowska E. Caspases and apoptosis: die and let live. Wiad Lek. 2002;55:100–106. [PubMed] [Google Scholar]
- 92.Koblinski JE, Ahram M, Sloane BF. Unraveling the role of proteases in cancer. Clin Chim Acta. 2000;291:113–135. doi: 10.1016/s0009-8981(99)00224-7. [DOI] [PubMed] [Google Scholar]
- 93.Dai H, Korthuis RJ. Mast Cell Proteases and Inflammation. Drug Discov Today Dis Models. 2011;8:47–55. doi: 10.1016/j.ddmod.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 95.Samara C, Tavernarakis N. Calcium-dependent and aspartyl proteases in neurodegeneration and ageing in C. elegans. Ageing Res Rev. 2003;2:451–471. doi: 10.1016/s1568-1637(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 96.Artal-Sanz M, Tavernarakis N. Proteolytic mechanisms in necrotic cell death and neurodegeneration. FEBS Lett. 2005;579:3287–3296. doi: 10.1016/j.febslet.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 97.Han YP. Matrix metalloproteinases, the pros and cons, in liver fibrosis. J Gastroenterol Hepatol. 2006;21(Suppl 3):S88–S91. doi: 10.1111/j.1440-1746.2006.04586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eddy AA. Serine proteases, inhibitors and receptors in renal fibrosis. Thromb Haemost. 2009;101:656–664. [PMC free article] [PubMed] [Google Scholar]
- 99.Papazafiropoulou A, Tentolouris N. Matrix metalloproteinases and cardiovascular diseases. Hippokratia. 2009;13:76–82. [PMC free article] [PubMed] [Google Scholar]
- 100.Markmann A, Schafer S, Linz W, Lohn M, Busch AE, Wohlfart P. Downregulation of calpain 9 is linked to hypertensive heart and kidney disease. Cell Physiol Biochem. 2005;15:109–116. doi: 10.1159/000083643. [DOI] [PubMed] [Google Scholar]
- 101.Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5:15. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paez Pereda M, Ledda MF, Goldberg V, Chervin A, Carrizo G, Molina H, Muller A, Renner U, Podhajcer O, Arzt E, Stalla GK. High levels of matrix metalloproteinases regulate proliferation and hormone secretion in pituitary cells. J Clin Endocrinol Metab. 2000;85:263–269. doi: 10.1210/jcem.85.1.6248. [DOI] [PubMed] [Google Scholar]
- 103.Death AK, Fisher EJ, McGrath KC, Yue DK. High glucose alters matrix metalloproteinase expression in two key vascular cells: potential impact on atherosclerosis in diabetes. Atherosclerosis. 2003;168:263–269. doi: 10.1016/s0021-9150(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 104.Uemura S, Matsushita H, Li W, Glassford AJ, Asagami T, Lee KH, Harrison DG, Tsao PS. Diabetes mellitus enhances vascular matrix metalloproteinase activity: role of oxidative stress. Circ Res. 2001;88:1291–1298. doi: 10.1161/hh1201.092042. [DOI] [PubMed] [Google Scholar]
- 105.Bouvet C, Gilbert LA, Girardot D, deBlois D, Moreau P. Different involvement of extracellular matrix components in small and large arteries during chronic NO synthase inhibition. Hypertension. 2005;45:432–437. doi: 10.1161/01.HYP.0000154680.44184.01. [DOI] [PubMed] [Google Scholar]
- 106.Browatzki M, Larsen D, Pfeiffer CA, Gehrke SG, Schmidt J, Kranzhofer A, Katus HA, Kranzhofer R. Angiotensin II stimulates matrix metalloproteinase secretion in human vascular smooth muscle cells via nuclear factor-kappaB and activator protein 1 in a redox-sensitive manner. J Vasc Res. 2005;42:415–423. doi: 10.1159/000087451. [DOI] [PubMed] [Google Scholar]
- 107.Maquoi E, Munaut C, Colige A, Collen D, Lijnen HR. Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes. 2002;51:1093–1101. doi: 10.2337/diabetes.51.4.1093. [DOI] [PubMed] [Google Scholar]
- 108.Negi S, Anand A. Atherosclerotic coronary heart disease-epidemiology, classification and management. Cardiovasc Hematol Disord Drug Targets. 2010;10:257–261. doi: 10.2174/187152910793743832. [DOI] [PubMed] [Google Scholar]
- 109.Liu P, Sun M, Sader S. Matrix metalloproteinases in cardiovascular disease. Can J Cardiol. 2006;22(Suppl B):25B–30B. doi: 10.1016/s0828-282x(06)70983-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 111.Zhu X, Wang Z, Hu C, Li Z, Hu J. Honokiol suppresses TNF-alpha-induced migration and matrix metalloproteinase expression by blocking NF-kappaB activation via the ERK signaling pathway in rat aortic smooth muscle cells. Acta Histochem. 2013 doi: 10.1016/j.acthis.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 112.Johnson JL, Jackson CL, Angelini GD, George SJ. Activation of matrix-degrading metalloproteinases by mast cell proteases in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 1998;18:1707–1715. doi: 10.1161/01.atv.18.11.1707. [DOI] [PubMed] [Google Scholar]
- 113.Orbe J, Fernandez L, Rodriguez JA, Rabago G, Belzunce M, Monasterio A, Roncal C, Paramo JA. Different expression of MMPs/TIMP-1 in human atherosclerotic lesions. Relation to plaque features and vascular bed. Atherosclerosis. 2003;170:269–276. doi: 10.1016/s0021-9150(03)00251-x. [DOI] [PubMed] [Google Scholar]
- 114.Hua Y, Xue J, Sun F, Zhu LF, Xie M. Aspirin inhibits MMP-2 and MMP-9 expressions and activities through upregulation of PPARalpha/gamma and TIMP gene expressions in ox-LDL-stimulated macrophages derived from human monocytes. Pharmacology. 2009;83:18–25. doi: 10.1159/000166183. [DOI] [PubMed] [Google Scholar]
- 115.Newby AC. Matrix metalloproteinase inhibition therapy for vascular diseases. Vascul Pharmacol. 2012;56:232–244. doi: 10.1016/j.vph.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 116.Tan C, Liu Y, Li W, Deng F, Liu X, Wang X, Gui Y, Qin L, Hu C, Chen L. Associations of matrix metalloproteinase-9 and monocyte chemoattractant protein-1 concentrations with carotid atherosclerosis, based on measurements of plaque and intima-media thickness. Atherosclerosis. 2014;232:199–203. doi: 10.1016/j.atherosclerosis.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 117.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lemaitre V, O'Byrne TK, Borczuk AC, Okada Y, Tall AR, D'Armiento J. ApoE knockout mice expressing human matrix metalloproteinase-1 in macrophages have less advanced atherosclerosis. J Clin Invest. 2001;107:1227–1234. doi: 10.1172/JCI9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liang J, Liu E, Yu Y, Kitajima S, Koike T, Jin Y, Morimoto M, Hatakeyama K, Asada Y, Watanabe T, Sasaguri Y, Fan S, Fan J. Macrophage metalloelastase accelerates the progression of atherosclerosis in transgenic rabbits. Circulation. 2006;113:1993–2001. doi: 10.1161/CIRCULATIONAHA.105.596031. [DOI] [PubMed] [Google Scholar]
- 121.Luttun A, Lutgens E, Manderveld A, Maris K, Collen D, Carmeliet P, Moons L. Loss of matrix metalloproteinase-9 or matrix metalloproteinase-12 protects apolipoprotein E-deficient mice against atherosclerotic media destruction but differentially affects plaque growth. Circulation. 2004;109:1408–1414. doi: 10.1161/01.CIR.0000121728.14930.DE. [DOI] [PubMed] [Google Scholar]
- 122.Kuzuya M, Nakamura K, Sasaki T, Cheng XW, Itohara S, Iguchi A. Effect of MMP-2 deficiency on atherosclerotic lesion formation in apoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:1120–1125. doi: 10.1161/01.ATV.0000218496.60097.e0. [DOI] [PubMed] [Google Scholar]
- 123.Silence J, Lupu F, Collen D, Lijnen HR. Persistence of atherosclerotic plaque but reduced aneurysm formation in mice with stromelysin-1 (MMP-3) gene inactivation. Arterioscler Thromb Vasc Biol. 2001;21:1440–1445. doi: 10.1161/hq0901.097004. [DOI] [PubMed] [Google Scholar]
- 124.Tretjakovs P, Jurka A, Bormane I, Mikelsone I, Elksne K, Krievina G, Reihmane D, Verbovenko J, Bahs G. Circulating adhesion molecules, matrix metalloproteinase-9, plasminogen activator inhibitor-1, and myeloperoxidase in coronary artery disease patients with stable and unstable angina. Clin Chim Acta. 2012;413:25–29. doi: 10.1016/j.cca.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 125.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 126.Koolwijk P, Sidenius N, Peters E, Sier CF, Hanemaaijer R, Blasi F, van Hinsbergh VW. Proteolysis of the urokinase-type plasminogen activator receptor by metalloproteinase-12: implication for angiogenesis in fibrin matrices. Blood. 2001;97:3123–3131. doi: 10.1182/blood.v97.10.3123. [DOI] [PubMed] [Google Scholar]
- 127.Suzuki Y, Nagai N, Yamakawa K, Kawakami J, Lijnen HR, Umemura K. Tissue-type plasminogen activator (t-PA) induces stromelysin-1 (MMP-3) in endothelial cells through activation of lipoprotein receptor-related protein. Blood. 2009;114:3352–3358. doi: 10.1182/blood-2009-02-203919. [DOI] [PubMed] [Google Scholar]
- 128.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes & development. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 129.Wang BW, Chang H, Lin S, Kuan P, Shyu KG. Induction of matrix metalloproteinases-14 and -2 by cyclical mechanical stretch is mediated by tumor necrosis factor-alpha in cultured human umbilical vein endothelial cells. Cardiovasc Res. 2003;59:460–469. doi: 10.1016/s0008-6363(03)00428-0. [DOI] [PubMed] [Google Scholar]
- 130.Rajavashisth TB, Liao JK, Galis ZS, Tripathi S, Laufs U, Tripathi J, Chai NN, Xu XP, Jovinge S, Shah PK, Libby P. Inflammatory cytokines and oxidized low density lipoproteins increase endothelial cell expression of membrane type 1-matrix metalloproteinase. J Biol Chem. 1999;274:11924–11929. doi: 10.1074/jbc.274.17.11924. [DOI] [PubMed] [Google Scholar]
- 131.Magid R, Murphy TJ, Galis ZS. Expression of matrix metalloproteinase-9 in endothelial cells is differentially regulated by shear stress. Role of c-Myc. J Biol Chem. 2003;278:32994–32999. doi: 10.1074/jbc.M304799200. [DOI] [PubMed] [Google Scholar]
- 132.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation. 2005;112:1813–1824. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 133.Chung AW, Yang HH, Sigrist MK, Brin G, Chum E, Gourlay WA, Levin A. Matrix metalloproteinase-2 and -9 exacerbate arterial stiffening and angiogenesis in diabetes and chronic kidney disease. Cardiovasc Res. 2009;84:494–504. doi: 10.1093/cvr/cvp242. [DOI] [PubMed] [Google Scholar]
- 134.Fang C, Wen G, Zhang L, Lin L, Moore A, Wu S, Ye S, Xiao Q. An important role of matrix metalloproteinase-8 in angiogenesis in vitro and in vivo. Cardiovasc Res. 2013;99:146–155. doi: 10.1093/cvr/cvt060. [DOI] [PubMed] [Google Scholar]
- 135.Yao J, Xiong S, Klos K, Nguyen N, Grijalva R, Li P, Yu D. Multiple signaling pathways involved in activation of matrix metalloproteinase-9 (MMP-9) by heregulin-beta1 in human breast cancer cells. Oncogene. 2001;20:8066–8074. doi: 10.1038/sj.onc.1204944. [DOI] [PubMed] [Google Scholar]
- 136.Pintucci G, Yu PJ, Sharony R, Baumann FG, Saponara F, Frasca A, Galloway AC, Moscatelli D, Mignatti P. Induction of stromelysin-1 (MMP-3) by fibroblast growth factor-2 (FGF-2) in FGF-2−/− microvascular endothelial cells requires prolonged activation of extracellular signal-regulated kinases-1 and -2 (ERK-1/2) Journal of cellular biochemistry. 2003;90:1015–1025. doi: 10.1002/jcb.10721. [DOI] [PubMed] [Google Scholar]
- 137.Hollborn M, Stathopoulos C, Steffen A, Wiedemann P, Kohen L, Bringmann A. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Investigative ophthalmology & visual science. 2007;48:4360–4367. doi: 10.1167/iovs.06-1234. [DOI] [PubMed] [Google Scholar]
- 138.Barness LA, Opitz JM, Gilbert-Barness E. Obesity: genetic, molecular, and environmental aspects. Am J Med Genet A. 2007;143A:3016–3034. doi: 10.1002/ajmg.a.32035. [DOI] [PubMed] [Google Scholar]
- 139.Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 140.Xu X, Hua Y, Nair S, Zhang Y, Ren J. Akt2 knockout preserves cardiac function in high-fat diet-induced obesity by rescuing cardiac autophagosome maturation. Journal of molecular cell biology. 2013;5:61–63. doi: 10.1093/jmcb/mjs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Miksztowicz V, Siseles N, Fernandez Machulsky N, Schreier L, Berg G. Increase in MMP-2 activity in overweight and obese women is associated with menopausal status. Climacteric : the journal of the International Menopause Society. 2012;15:602–606. doi: 10.3109/13697137.2012.667174. [DOI] [PubMed] [Google Scholar]
- 142.Glowinska-Olszewska B, Urban M. Elevated matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 in obese children and adolescents. Metabolism: clinical and experimental. 2007;56:799–805. doi: 10.1016/j.metabol.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 143.Peterson JT, Hallak H, Johnson L, Li H, O'Brien PM, Sliskovic DR, Bocan TM, Coker ML, Etoh T, Spinale FG. Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation. 2001;103:2303–2309. doi: 10.1161/01.cir.103.18.2303. [DOI] [PubMed] [Google Scholar]
- 144.Monti LD, Galluccio E, Lucotti P, Setola E, Costa S, Fontana B, Oldani M, Merante D, Di Blasi P, Bosi E, Piatti PM. Beneficial role of L-arginine in cardiac matrix remodelling in insulin resistant rats. European journal of clinical investigation. 2008;38:849–856. doi: 10.1111/j.1365-2362.2008.02027.x. [DOI] [PubMed] [Google Scholar]
- 145.Ban CR, Twigg SM, Franjic B, Brooks BA, Celermajer D, Yue DK, McLennan SV. Serum MMP-7 is increased in diabetic renal disease and diabetic diastolic dysfunction. Diabetes research and clinical practice. 2010;87:335–341. doi: 10.1016/j.diabres.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 146.Kosmala W, Plaksej R, Przewlocka-Kosmala M, Kuliczkowska-Plaksej J, Bednarek-Tupikowska G, Mazurek W. Matrix metalloproteinases 2 and 9 and their tissue inhibitors 1 and 2 in premenopausal obese women: relationship to cardiac function. International journal of obesity. 2008;32:763–771. doi: 10.1038/sj.ijo.0803794. [DOI] [PubMed] [Google Scholar]
- 147.Hayashi T, Sohmiya K, Ukimura A, Endoh S, Mori T, Shimomura H, Okabe M, Terasaki F, Tsuda Y. Angiotensin II receptor blockade prevents microangiopathy and preserves diastolic function in the diabetic rat heart. Heart. 2003;89:1236–1242. doi: 10.1136/heart.89.10.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang H, Wu J, Dong H, Khan SA, Chu ML, Tsuda T. Fibulin-2 deficiency attenuates angiotensin II-induced cardiac hypertrophy by reducing transforming growth factor-beta signalling. Clinical science. 2014;126:275–288. doi: 10.1042/CS20120636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yao J, Xiong M, Tang B, Chen G, Liang M, Ma X, Wang Z, Wu Z. Simvastatin attenuates pulmonary vascular remodelling by down-regulating matrix metalloproteinase-1 and -9 expression in a carotid artery-jugular vein shunt pulmonary hypertension model in rats. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2012;42:e121–e127. doi: 10.1093/ejcts/ezs445. [DOI] [PubMed] [Google Scholar]
- 150.Rizzi E, Ceron CS, Guimaraes DA, Prado CM, Rossi MA, Gerlach RF, Tanus-Santos JE. Temporal changes in cardiac matrix metalloproteinase activity, oxidative stress, and TGF-beta in renovascular hypertension-induced cardiac hypertrophy. Experimental and molecular pathology. 2013;94:1–9. doi: 10.1016/j.yexmp.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 151.Liu W, Wang X, Feng W, Li S, Tian W, Xu T, Song Y, Zhang Z. Lentivirus mediated IL-17R blockade improves diastolic cardiac function in spontaneously hypertensive rats. Experimental and molecular pathology. 2011;91:362–367. doi: 10.1016/j.yexmp.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 152.Nagatomo Y, Carabello BA, Coker ML, McDermott PJ, Nemoto S, Hamawaki M, Spinale FG. Differential effects of pressure or volume overload on myocardial MMP levels and inhibitory control. Am J Physiol Heart Circ Physiol. 2000;278:H151–H161. doi: 10.1152/ajpheart.2000.278.1.H151. [DOI] [PubMed] [Google Scholar]
- 153.Lin J, Davis HB, Dai Q, Chou YM, Craig T, Hinojosa-Laborde C, Lindsey ML. Effects of early and late chronic pressure overload on extracellular matrix remodeling. Hypertension research : official journal of the Japanese Society of Hypertension. 2008;31:1225–1231. doi: 10.1291/hypres.31.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Foronjy RF, Sun J, Lemaitre V, D'Armiento JM. Transgenic expression of matrix metalloproteinase-1 inhibits myocardial fibrosis and prevents the transition to heart failure in a pressure overload mouse model. Hypertension research : official journal of the Japanese Society of Hypertension. 2008;31:725–735. doi: 10.1291/hypres.31.725. [DOI] [PubMed] [Google Scholar]
- 155.Matsusaka H, Ide T, Matsushima S, Ikeuchi M, Kubota T, Sunagawa K, Kinugawa S, Tsutsui H. Targeted deletion of matrix metalloproteinase 2 ameliorates myocardial remodeling in mice with chronic pressure overload. Hypertension. 2006;47:711–717. doi: 10.1161/01.HYP.0000208840.30778.00. [DOI] [PubMed] [Google Scholar]
- 156.Wang X, Chow FL, Oka T, Hao L, Lopez-Campistrous A, Kelly S, Cooper S, Odenbach J, Finegan BA, Schulz R, Kassiri Z, Lopaschuk GD, Fernandez-Patron C. Matrix metalloproteinase-7 and ADAM-12 (a disintegrin and metalloproteinase-12) define a signaling axis in agonist-induced hypertension and cardiac hypertrophy. Circulation. 2009;119:2480–2489. doi: 10.1161/CIRCULATIONAHA.108.835488. [DOI] [PubMed] [Google Scholar]
- 157.Sakata Y, Yamamoto K, Mano T, Nishikawa N, Yoshida J, Hori M, Miwa T, Masuyama T. Activation of matrix metalloproteinases precedes left ventricular remodeling in hypertensive heart failure rats: its inhibition as a primary effect of Angiotensin-converting enzyme inhibitor. Circulation. 2004;109:2143–2149. doi: 10.1161/01.CIR.0000125741.88712.77. [DOI] [PubMed] [Google Scholar]
- 158.Givvimani S, Tyagi N, Sen U, Mishra PK, Qipshidze N, Munjal C, Vacek JC, Abe OA, Tyagi SC. MMP-2/TIMP-2/TIMP-4 versus MMP-9/TIMP-3 in transition from compensatory hypertrophy and angiogenesis to decompensatory heart failure. Archives of physiology and biochemistry. 2010;116:63–72. doi: 10.3109/13813451003652997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Youn JY, Wang T, Cai H. An ezrin/calpain/PI3K/AMPK/eNOSs1179 signaling cascade mediating VEGF-dependent endothelial nitric oxide production. Circ Res. 2009;104:50–59. doi: 10.1161/CIRCRESAHA.108.178467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Miyazaki T, Honda K, Ohata H. Requirement of Ca2+ influx- and phosphatidylinositol 3-kinase-mediated m-calpain activity for shear stress-induced endothelial cell polarity. Am J Physiol Cell Physiol. 2007;293:C1216–C1225. doi: 10.1152/ajpcell.00083.2007. [DOI] [PubMed] [Google Scholar]
- 161.Gonscherowski V, Becker BF, Moroder L, Motrescu E, Gil-Parrado S, Gloe T, Keller M, Zahler S. Calpains: a physiological regulator of the endothelial barrier? Am J Physiol Heart Circ Physiol. 2006;290:H2035–H2042. doi: 10.1152/ajpheart.00772.2004. [DOI] [PubMed] [Google Scholar]
- 162.Averna M, Stifanese R, De Tullio R, Salamino F, Bertuccio M, Pontremoli S, Melloni E. Proteolytic degradation of nitric oxide synthase isoforms by calpain is modulated by the expression levels of HSP90. The FEBS journal. 2007;274:6116–6127. doi: 10.1111/j.1742-4658.2007.06133.x. [DOI] [PubMed] [Google Scholar]
- 163.Miyazaki T, Taketomi Y, Takimoto M, Lei XF, Arita S, Kim-Kaneyama JR, Arata S, Ohata H, Ota H, Murakami M, Miyazaki A. m-Calpain induction in vascular endothelial cells on human and mouse atheromas and its roles in VE-cadherin disorganization and atherosclerosis. Circulation. 2011;124:2522–2532. doi: 10.1161/CIRCULATIONAHA.111.021675. [DOI] [PubMed] [Google Scholar]
- 164.Porn-Ares MI, Saido TC, Andersson T, Ares MP. Oxidized low-density lipoprotein induces calpain-dependent cell death and ubiquitination of caspase 3 in HMEC-1 endothelial cells. Biochem J. 2003;374:403–411. doi: 10.1042/BJ20021955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cui GM, Zhao YX, Zhang NN, Liu ZS, Sun WC, Peng QS. Amiloride attenuates lipopolysaccharide-accelerated atherosclerosis via inhibition of NHE1-dependent endothelial cell apoptosis. Acta pharmacologica Sinica. 2013;34:231–238. doi: 10.1038/aps.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Milligan SA, Owens MW, Grisham MB. Inhibition of IkappaB-alpha and IkappaB-beta proteolysis by calpain inhibitor I blocks nitric oxide synthesis. Arch Biochem Biophys. 1996;335:388–395. doi: 10.1006/abbi.1996.9998. [DOI] [PubMed] [Google Scholar]
- 168.Wang N, Chen W, Linsel-Nitschke P, Martinez LO, Agerholm-Larsen B, Silver DL, Tall AR. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I. J Clin Invest. 2003;111:99–107. doi: 10.1172/JCI16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lin CY, Lee TS, Chen CC, Chang CA, Lin YJ, Hsu YP, Ho LT. Endothelin-1 exacerbates lipid accumulation by increasing the protein degradation of the ATP-binding cassette transporter G1 in macrophages. Journal of cellular physiology. 2011;226:2198–2205. doi: 10.1002/jcp.22556. [DOI] [PubMed] [Google Scholar]
- 170.von Wnuck Lipinski K, Keul P, Lucke S, Heusch G, Wohlschlaeger J, Baba HA, Levkau B. Degraded collagen induces calpain-mediated apoptosis and destruction of the X-chromosome-linked inhibitor of apoptosis (xIAP) in human vascular smooth muscle cells. Cardiovasc Res. 2006;69:697–705. doi: 10.1016/j.cardiores.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 171.Goodarzi MO, Taylor KD, Guo X, Quinones MJ, Cui J, Li Y, Saad MF, Yang H, Hsueh WA, Hodis HN, Rotter JI. Association of the diabetes gene calpain-10 with subclinical atherosclerosis: the Mexican-American Coronary Artery Disease Study. Diabetes. 2005;54:1228–1232. doi: 10.2337/diabetes.54.4.1228. [DOI] [PubMed] [Google Scholar]
- 172.Horikawa Y, Oda N, Cox NJ, Li X, Orho-Melander M, Hara M, Hinokio Y, Lindner TH, Mashima H, Schwarz PE, del Bosque-Plata L, Horikawa Y, Oda Y, Yoshiuchi I, Colilla S, Polonsky KS, Wei S, Concannon P, Iwasaki N, Schulze J, Baier LJ, Bogardus C, Groop L, Boerwinkle E, Hanis CL, Bell GI. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nature genetics. 2000;26:163–175. doi: 10.1038/79876. [DOI] [PubMed] [Google Scholar]
- 173.Garant MJ, Kao WH, Brancati F, Coresh J, Rami TM, Hanis CL, Boerwinkle E, Shuldiner AR. S. Atherosclerosis Risk in Communities, SNP43 of CAPN10 and the risk of type 2 Diabetes in African-Americans: the Atherosclerosis Risk in Communities Study. Diabetes. 2002;51:231–237. doi: 10.2337/diabetes.51.1.231. [DOI] [PubMed] [Google Scholar]
- 174.Leipold H, Knofler M, Gruber C, Haslinger P, Bancher-Todesca D, Worda C. Calpain-10 haplotype combination and association with gestational diabetes mellitus. Obstetrics and gynecology. 2004;103:1235–1240. doi: 10.1097/01.AOG.0000127790.15556.3d. [DOI] [PubMed] [Google Scholar]
- 175.Lynn S, Evans JC, White C, Frayling TM, Hattersley AT, Turnbull DM, Horikawa Y, Cox NJ, Bell GI, Walker M. Variation in the calpain-10 gene affects blood glucose levels in the British population. Diabetes. 2002;51:247–250. doi: 10.2337/diabetes.51.1.247. [DOI] [PubMed] [Google Scholar]
- 176.Orho-Melander M, Klannemark M, Svensson MK, Ridderstrale M, Lindgren CM, Groop L. Variants in the calpain-10 gene predispose to insulin resistance and elevated free fatty acid levels. Diabetes. 2002;51:2658–2664. doi: 10.2337/diabetes.51.8.2658. [DOI] [PubMed] [Google Scholar]
- 177.Cassell PG, Jackson AE, North BV, Evans JC, Syndercombe-Court D, Phillips C, Ramachandran A, Snehalatha C, Gelding SV, Vijayaravaghan S, Curtis D, Hitman GA. Haplotype combinations of calpain 10 gene polymorphisms associate with increased risk of impaired glucose tolerance and type 2 diabetes in South Indians. Diabetes. 2002;51:1622–1628. doi: 10.2337/diabetes.51.5.1622. [DOI] [PubMed] [Google Scholar]
- 178.Wang J, Xu J, Finnerty J, Furuta M, Steiner DF, Verchere CB. The prohormone convertase enzyme 2 (PC2) is essential for processing pro-islet amyloid polypeptide at the NH2-terminal cleavage site. Diabetes. 2001;50:534–539. doi: 10.2337/diabetes.50.3.534. [DOI] [PubMed] [Google Scholar]
- 179.Smith LK, Rice KM, Garner CW. The insulin-induced down-regulation of IRS-1 in 3T3-L1 adipocytes is mediated by a calcium-dependent thiol protease. Molecular and cellular endocrinology. 1996;122:81–92. doi: 10.1016/0303-7207(96)03875-0. [DOI] [PubMed] [Google Scholar]
- 180.Yang Y, Duan W, Zhou J, Yan J, Liu J, Zhang J, Jin Z, Yi D. Protective effects of adenosine on the diabetic myocardium against ischemia-reperfusion injury: role of calpain. Medical hypotheses. 2012;79:462–464. doi: 10.1016/j.mehy.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 181.Shioda N, Moriguchi S, Shirasaki Y, Fukunaga K. Generation of constitutively active calcineurin by calpain contributes to delayed neuronal death following mouse brain ischemia. Journal of neurochemistry. 2006;98:310–320. doi: 10.1111/j.1471-4159.2006.03874.x. [DOI] [PubMed] [Google Scholar]
- 182.Heidrich FM, Ehrlich BE. Calcium, calpains, and cardiac hypertrophy: a new link. Circ Res. 2009;104:e19–e20. doi: 10.1161/CIRCRESAHA.108.191072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Letavernier E, Perez J, Bellocq A, Mesnard L, de Castro Keller A, Haymann JP, Baud L. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ Res. 2008;102:720–728. doi: 10.1161/CIRCRESAHA.107.160077. [DOI] [PubMed] [Google Scholar]
- 184.Suryakumar G, Kasiganesan H, Balasubramanian S, Kuppuswamy D. Lack of beta3 integrin signaling contributes to calpain-mediated myocardial cell loss in pressure-overloaded myocardium. Journal of cardiovascular pharmacology. 2010;55:567–573. doi: 10.1097/FJC.0b013e3181d9f5d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Franchini KG, Clemente CF, Marin TM. Focal adhesion kinase signaling in cardiac hypertrophy and failure. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica … [et al.] 2009;42:44–52. doi: 10.1590/s0100-879x2009000100008. [DOI] [PubMed] [Google Scholar]
- 186.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 187.Hua Y, Zhang Y, Dolence J, Shi GP, Ren J, Nair S. Cathepsin k knockout mitigates high-fat diet-induced cardiac hypertrophy and contractile dysfunction. Diabetes. 2013;62:498–509. doi: 10.2337/db12-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Curreri PW, Kothari HV, Bonner MJ, Miller BF. Increased activity of lysosomal enzymes in experimental atherosclerosis, and the effect of cortisone. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 1969;130:1253–1256. doi: 10.3181/00379727-130-33766. [DOI] [PubMed] [Google Scholar]
- 189.Reddy VY, Zhang QY, Weiss SJ. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1995;92:3849–3853. doi: 10.1073/pnas.92.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Haidar B, Kiss RS, Sarov-Blat L, Brunet R, Harder C, McPherson R, Marcel YL. Cathepsin D, a lysosomal protease, regulates ABCA1-mediated lipid efflux. J Biol Chem. 2006;281:39971–39981. doi: 10.1074/jbc.M605095200. [DOI] [PubMed] [Google Scholar]
- 191.Platt MO, Ankeny RF, Shi GP, Weiss D, Vega JD, Taylor WR, Jo H. Expression of cathepsin K is regulated by shear stress in cultured endothelial cells and is increased in endothelium in human atherosclerosis. Am J Physiol Heart Circ Physiol. 2007;292:H1479–H1486. doi: 10.1152/ajpheart.00954.2006. [DOI] [PubMed] [Google Scholar]
- 192.Jormsjo S, Wuttge DM, Sirsjo A, Whatling C, Hamsten A, Stemme S, Eriksson P. Differential expression of cysteine and aspartic proteases during progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2002;161:939–945. doi: 10.1016/S0002-9440(10)64254-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sasaki T, Kuzuya M, Nakamura K, Cheng XW, Hayashi T, Song H, Hu L, Okumura K, Murohara T, Iguchi A, Sato K. AT1 blockade attenuates atherosclerotic plaque destabilization accompanied by the suppression of cathepsin S activity in apoE-deficient mice. Atherosclerosis. 2010;210:430–437. doi: 10.1016/j.atherosclerosis.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 194.Jaffer FA, Kim DE, Quinti L, Tung CH, Aikawa E, Pande AN, Kohler RH, Shi GP, Libby P, Weissleder R. Optical visualization of cathepsin K activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007;115:2292–2298. doi: 10.1161/CIRCULATIONAHA.106.660340. [DOI] [PubMed] [Google Scholar]
- 195.Li W, Kornmark L, Jonasson L, Forssell C, Yuan XM. Cathepsin L is significantly associated with apoptosis and plaque destabilization in human atherosclerosis. Atherosclerosis. 2009;202:92–102. doi: 10.1016/j.atherosclerosis.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 196.Rafatian N, Karunakaran D, Rayner KJ, Leenen FH, Milne RW, Whitman SC. Cathepsin G deficiency decreases complexity of atherosclerotic lesions in apolipoprotein E-deficient mice. Am J Physiol Heart Circ Physiol. 2013;305:H1141–H1148. doi: 10.1152/ajpheart.00618.2012. [DOI] [PubMed] [Google Scholar]
- 197.Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi GP. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cheng XW, Kikuchi R, Ishii H, Yoshikawa D, Hu L, Takahashi R, Shibata R, Ikeda N, Kuzuya M, Okumura K, Murohara T. Circulating cathepsin K as a potential novel biomarker of coronary artery disease. Atherosclerosis. 2013;228:211–216. doi: 10.1016/j.atherosclerosis.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 199.Wang J, Liu Y, Li X, Peng D, Tan Z, Liu H, Qin Y, Xue Y. Association of cathepsin L with coronary heart disease and its risk factors. Zhong nan da xue xue bao. Yi xue ban = Journal of Central South University. Medical sciences. 2009;34:130–134. [PubMed] [Google Scholar]
- 200.Liu Y, Li X, Peng D, Tan Z, Liu H, Qing Y, Xue Y, Shi GP. Usefulness of serum cathepsin L as an independent biomarker in patients with coronary heart disease. The American journal of cardiology. 2009;103:476–481. doi: 10.1016/j.amjcard.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mirzaii-Dizgah I, Riahi E. Serum and saliva levels of cathepsin L in patients with acute coronary syndrome. The journal of contemporary dental practice. 2011;12:114–119. doi: 10.5005/jp-journals-10024-1019. [DOI] [PubMed] [Google Scholar]
- 202.Eriksson P, Deguchi H, Samnegard A, Lundman P, Boquist S, Tornvall P, Ericsson CG, Bergstrand L, Hansson LO, Ye S, Hamsten A. Human evidence that the cystatin C gene is implicated in focal progression of coronary artery disease. Arterioscler Thromb Vasc Biol. 2004;24:551–557. doi: 10.1161/01.ATV.0000117180.57731.36. [DOI] [PubMed] [Google Scholar]
- 203.Chiellini C, Costa M, Novelli SE, Amri EZ, Benzi L, Bertacca A, Cohen P, Del Prato S, Friedman JM, Maffei M. Identification of cathepsin K as a novel marker of adiposity in white adipose tissue. Journal of cellular physiology. 2003;195:309–321. doi: 10.1002/jcp.10253. [DOI] [PubMed] [Google Scholar]
- 204.Xiao Y, Junfeng H, Tianhong L, Lu W, Shulin C, Yu Z, Xiaohua L, Weixia J, Sheng Z, Yanyun G, Guo L, Min L. Cathepsin K in adipocyte differentiation and its potential role in the pathogenesis of obesity. J Clin Endocrinol Metab. 2006;91:4520–4527. doi: 10.1210/jc.2005-2486. [DOI] [PubMed] [Google Scholar]
- 205.Funicello M, Novelli M, Ragni M, Vottari T, Cocuzza C, Soriano-Lopez J, Chiellini C, Boschi F, Marzola P, Masiello P, Saftig P, Santini F, St-Jacques R, Desmarais S, Morin N, Mancini J, Percival MD, Pinchera A, Maffei M. Cathepsin K null mice show reduced adiposity during the rapid accumulation of fat stores. PloS one. 2007;2:e683. doi: 10.1371/journal.pone.0000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yang M, Sun J, Zhang T, Liu J, Zhang J, Shi MA, Darakhshan F, Guerre-Millo M, Clement K, Gelb BD, Dolgnov G, Shi GP. Deficiency and inhibition of cathepsin K reduce body weight gain and increase glucose metabolism in mice. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:2202–2208. doi: 10.1161/ATVBAHA.108.172320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Han J, Luo T, Gu Y, Li G, Jia W, Luo M. Cathepsin K regulates adipocyte differentiation: possible involvement of type I collagen degradation. Endocrine journal. 2009;56:55–63. doi: 10.1507/endocrj.k08e-143. [DOI] [PubMed] [Google Scholar]
- 208.Taleb S, Lacasa D, Bastard JP, Poitou C, Cancello R, Pelloux V, Viguerie N, Benis A, Zucker JD, Bouillot JL, Coussieu C, Basdevant A, Langin D, Clement K. Cathepsin S, a novel biomarker of adiposity: relevance to atherogenesis. FASEB J. 2005;19:1540–1542. doi: 10.1096/fj.05-3673fje. [DOI] [PubMed] [Google Scholar]
- 209.Jobs E, Riserus U, Ingelsson E, Helmersson J, Nerpin E, Jobs M, Sundstrom J, Lind L, Larsson A, Basu S, Arnlov J. Serum cathepsin S is associated with serum C-reactive protein and interleukin-6 independently of obesity in elderly men. J Clin Endocrinol Metab. 2010;95:4460–4464. doi: 10.1210/jc.2010-0328. [DOI] [PubMed] [Google Scholar]
- 210.Arnlov J. Cathepsin S as a biomarker: where are we now and what are the future challenges? Biomarkers in medicine. 2012;6:9–11. doi: 10.2217/bmm.11.102. [DOI] [PubMed] [Google Scholar]
- 211.Taleb S, Cancello R, Poitou C, Rouault C, Sellam P, Levy P, Bouillot JL, Coussieu C, Basdevant A, Guerre-Millo M, Lacasa D, Clement K. Weight loss reduces adipose tissue cathepsin S and its circulating levels in morbidly obese women. J Clin Endocrinol Metab. 2006;91:1042–1047. doi: 10.1210/jc.2005-1601. [DOI] [PubMed] [Google Scholar]
- 212.Huvenne VA, Tyler PA, Masson DG, Fisher EH, Hauton C, Huhnerbach V, Le Bas TP, Wolff GA. A picture on the wall: innovative mapping reveals cold-water coral refuge in submarine canyon. PloS one. 2011;6:e28755. doi: 10.1371/journal.pone.0028755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Eguchi A, Feldstein AE. Lysosomal Cathepsin D contributes to cell death during adipocyte hypertrophy. Adipocyte. 2013;2:170–175. doi: 10.4161/adip.24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Huang X, Vaag A, Carlsson E, Hansson M, Ahren B, Groop L. Impaired cathepsin L gene expression in skeletal muscle is associated with type 2 diabetes. Diabetes. 2003;52:2411–2418. doi: 10.2337/diabetes.52.9.2411. [DOI] [PubMed] [Google Scholar]
- 215.Jobs E, Riserus U, Ingelsson E, Sundstrom J, Jobs M, Nerpin E, Iggman D, Basu S, Larsson A, Lind L, Arnlov J. Serum cathepsin S is associated with decreased insulin sensitivity and the development of type 2 diabetes in a community-based cohort of elderly men. Diabetes care. 2013;36:163–165. doi: 10.2337/dc12-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chen RP, Ren A, Ye SD. Correlation between serum cathepsin S and insulin resistance in type 2 diabetes. Experimental and therapeutic medicine. 2013;6:1237–1242. doi: 10.3892/etm.2013.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Saito N, Mukaino S, Ogino K. Proceedings: Triglyceride, lipid peroxidation, and cathepsin in the serum of spontaneously hypertensive rats. Japanese heart journal. 1976;17:345–347. doi: 10.1536/ihj.17.345. [DOI] [PubMed] [Google Scholar]
- 218.Wildenthal K, Mueller EA. Lysosomal enzymes in the development and regression of myocardial hypertrophy induced by systemic hypertension. J Mol Cell Cardiol. 1977;9:121–130. doi: 10.1016/0022-2828(77)90044-x. [DOI] [PubMed] [Google Scholar]
- 219.Rozek RJ, Kuo TH, Giacomelli F, Wiener J. Proteolytic activities in hypertensive cardiomyopathy of rats. J Mol Cell Cardiol. 1983;15:173–187. doi: 10.1016/0022-2828(83)90297-3. [DOI] [PubMed] [Google Scholar]
- 220.Suzuki H, Schaefer L, Ling H, Schaefer RM, Dammrich J, Teschner M, Heidland A. Prevention of cardiac hypertrophy in experimental chronic renal failure by long-term ACE inhibitor administration: potential role of lysosomal proteinases. American journal of nephrology. 1995;15:129–136. doi: 10.1159/000168817. [DOI] [PubMed] [Google Scholar]
- 221.Kirimura K, Takai S, Jin D, Muramatsu M, Kishi K, Yoshikawa K, Nakabayashi M, Mino Y, Miyazaki M. Role of chymase-dependent angiotensin II formation in regulating blood pressure in spontaneously hypertensive rats. Hypertension research : official journal of the Japanese Society of Hypertension. 2005;28:457–464. doi: 10.1291/hypres.28.457. [DOI] [PubMed] [Google Scholar]
- 222.Tang Q, Cai J, Shen D, Bian Z, Yan L, Wang YX, Lan J, Zhuang GQ, Ma WZ, Wang W. Lysosomal cysteine peptidase cathepsin L protects against cardiac hypertrophy through blocking AKT/GSK3beta signaling. Journal of molecular medicine. 2009;87:249–260. doi: 10.1007/s00109-008-0423-2. [DOI] [PubMed] [Google Scholar]
- 223.Sun M, Ouzounian M, de Couto G, Chen M, Yan R, Fukuoka M, Li G, Moon M, Liu Y, Gramolini A, Wells GJ, Liu PP. Cathepsin-L ameliorates cardiac hypertrophy through activation of the autophagy-lysosomal dependent protein processing pathways. Journal of the American Heart Association. 2013;2:e000191. doi: 10.1161/JAHA.113.000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hua Y, Xu X, Shi GP, Chicco AJ, Ren J, Nair S. Cathepsin K knockout alleviates pressure overload-induced cardiac hypertrophy. Hypertension. 2013;61:1184–1192. doi: 10.1161/HYPERTENSIONAHA.111.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Imanishi T, Han DK, Hofstra L, Hano T, Nishio I, Liles WC, Gown AM, Schwartz SM. Apoptosis of vascular smooth muscle cells is induced by Fas ligand derived from monocytes/macrophage. Atherosclerosis. 2002;161:143–151. doi: 10.1016/s0021-9150(01)00631-1. [DOI] [PubMed] [Google Scholar]
- 226.Yao PM, Tabas I. Free cholesterol loading of macrophages induces apoptosis involving the fas pathway. J Biol Chem. 2000;275:23807–23813. doi: 10.1074/jbc.M002087200. [DOI] [PubMed] [Google Scholar]
- 227.Perlman H, Pagliari LJ, Georganas C, Mano T, Walsh K, Pope RM. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. The Journal of experimental medicine. 1999;190:1679–1688. doi: 10.1084/jem.190.11.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Nhan TQ, Liles WC, Schwartz SM. Role of caspases in death and survival of the plaque macrophage. Arterioscler Thromb Vasc Biol. 2005;25:895–903. doi: 10.1161/01.ATV.0000159519.07181.33. [DOI] [PubMed] [Google Scholar]
- 229.Nhan TQ, Liles WC, Chait A, Fallon JT, Schwartz SM. The p17 cleaved form of caspase-3 is present within viable macrophages in vitro and in atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2003;23:1276–1282. doi: 10.1161/01.ATV.0000078602.54433.07. [DOI] [PubMed] [Google Scholar]
- 230.Salvayre R, Auge N, Benoist H, Negre-Salvayre A. Oxidized low-density lipoprotein-induced apoptosis. Biochim Biophys Acta. 2002;1585:213–221. doi: 10.1016/s1388-1981(02)00343-8. [DOI] [PubMed] [Google Scholar]
- 231.Geng YJ. Biologic effect and molecular regulation of vascular apoptosis in atherosclerosis. Current atherosclerosis reports. 2001;3:234–242. doi: 10.1007/s11883-001-0066-z. [DOI] [PubMed] [Google Scholar]
- 232.Nakagami H, Kaneda Y, Ogihara T, Morishita R. Endothelial dysfunction in hyperglycemia as a trigger of atherosclerosis. Current diabetes reviews. 2005;1:59–63. doi: 10.2174/1573399052952550. [DOI] [PubMed] [Google Scholar]
- 233.Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta. 2002;1585:202–212. doi: 10.1016/s1388-1981(02)00342-6. [DOI] [PubMed] [Google Scholar]
- 234.Yokoyama M, Yagyu H, Hu Y, Seo T, Hirata K, Homma S, Goldberg IJ. Apolipoprotein B production reduces lipotoxic cardiomyopathy: studies in heart-specific lipoprotein lipase transgenic mouse. J Biol Chem. 2004;279:4204–4211. doi: 10.1074/jbc.M311995200. [DOI] [PubMed] [Google Scholar]
- 235.Li SY, Liu Y, Sigmon VK, McCort A, Ren J. High-fat diet enhances visceral advanced glycation end products, nuclear O-Glc-Nac modification, p38 mitogen-activated protein kinase activation and apoptosis. Diabetes, obesity & metabolism. 2005;7:448–454. doi: 10.1111/j.1463-1326.2004.00387.x. [DOI] [PubMed] [Google Scholar]
- 236.Barouch LA, Gao D, Chen L, Miller KL, Xu W, Phan AC, Kittleson MM, Minhas KM, Berkowitz DE, Wei C, Hare JM. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 2006;98:119–124. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 237.Lee SD, Tzang BS, Kuo WW, Lin YM, Yang AL, Chen SH, Tsai FJ, Wu FL, Lu MC, Huang CY. Cardiac fas receptor-dependent apoptotic pathway in obese Zucker rats. Obesity. 2007;15:2407–2415. doi: 10.1038/oby.2007.286. [DOI] [PubMed] [Google Scholar]
- 238.Lu MC, Tzang BS, Kuo WW, Wu FL, Chen YS, Tsai CH, Huang CY, Lee SD. More activated cardiac mitochondrial-dependent apoptotic pathway in obese Zucker rats. Obesity. 2007;15:2634–2642. doi: 10.1038/oby.2007.315. [DOI] [PubMed] [Google Scholar]
- 239.Peterson JM, Bryner RW, Sindler A, Frisbee JC, Alway SE. Mitochondrial apoptotic signaling is elevated in cardiac but not skeletal muscle in the obese Zucker rat and is reduced with aerobic exercise. Journal of applied physiology. 2008;105:1934–1943. doi: 10.1152/japplphysiol.00037.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wang HT, Liu CF, Tsai TH, Chen YL, Chang HW, Tsai CY, Leu S, Zhen YY, Chai HT, Chung SY, Chua S, Yen CH, Yip HK. Effect of obesity reduction on preservation of heart function and attenuation of left ventricular remodeling, oxidative stress and inflammation in obese mice. Journal of translational medicine. 2012;10:145. doi: 10.1186/1479-5876-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Panchal SK, Poudyal H, Arumugam TV, Brown L. Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats. The Journal of nutrition. 2011;141:1062–1069. doi: 10.3945/jn.111.137877. [DOI] [PubMed] [Google Scholar]
- 242.Ravassa S, Fortuno MA, Gonzalez A, Lopez B, Zalba G, Fortuno A, Diez J. Mechanisms of increased susceptibility to angiotensin II-induced apoptosis in ventricular cardiomyocytes of spontaneously hypertensive rats. Hypertension. 2000;36:1065–1071. doi: 10.1161/01.hyp.36.6.1065. [DOI] [PubMed] [Google Scholar]
- 243.Rodriguez-Feo JA, Fortes J, Aceituno E, Farre J, Ayala R, Castilla C, Rico L, Gonzalez-Fernandez F, Garcia-Duran M, Casado S, Lopez-Farre A. Doxazosin modifies Bcl-2 and Bax protein expression in the left ventricle of spontaneously hypertensive rats. Journal of hypertension. 2000;18:307–315. doi: 10.1097/00004872-200018030-00011. [DOI] [PubMed] [Google Scholar]
- 244.Der Sarkissian S, Marchand EL, Duguay D, Hamet P, deBlois D. Reversal of interstitial fibroblast hyperplasia via apoptosis in hypertensive rat heart with valsartan or enalapril. Cardiovasc Res. 2003;57:775–783. doi: 10.1016/s0008-6363(02)00789-7. [DOI] [PubMed] [Google Scholar]
- 245.Lee YI, Cho JY, Kim MH, Kim KB, Lee DJ, Lee KS. Effects of exercise training on pathological cardiac hypertrophy related gene expression and apoptosis. European journal of applied physiology. 2006;97:216–224. doi: 10.1007/s00421-006-0161-5. [DOI] [PubMed] [Google Scholar]
- 246.Gonzalez A, Ravassa S, Loperena I, Lopez B, Beaumont J, Querejeta R, Larman M, Diez J. Association of depressed cardiac gp130-mediated antiapoptotic pathways with stimulated cardiomyocyte apoptosis in hypertensive patients with heart failure. Journal of hypertension. 2007;25:2148–2157. doi: 10.1097/HJH.0b013e32828626e2. [DOI] [PubMed] [Google Scholar]
- 247.Isodono K, Takahashi T, Imoto H, Nakanishi N, Ogata T, Asada S, Adachi A, Ueyama T, Oh H, Matsubara H. PARM-1 is an endoplasmic reticulum molecule involved in endoplasmic reticulum stress-induced apoptosis in rat cardiac myocytes. PloS one. 2010;5:e9746. doi: 10.1371/journal.pone.0009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Silvello D, Narvaes LB, Albuquerque LC, Forgiarini LF, Meurer L, Martinelli NC, Andrades ME, Clausell N, Santos KG, Rohde LE. Serum levels and polymorphisms of matrix metalloproteinases (MMPs) in carotid artery atherosclerosis: higher MMP-9 levels are associated with plaque vulnerability. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2014;19:49–55. doi: 10.3109/1354750X.2013.866165. [DOI] [PubMed] [Google Scholar]
- 249.Shirakabe A, Asai K, Hata N, Yokoyama S, Shinada T, Kobayashi N, Mizuno K. Clinical significance of matrix metalloproteinase (MMP)-2 in patients with acute heart failure. International heart journal. 2010;51:404–410. doi: 10.1536/ihj.51.404. [DOI] [PubMed] [Google Scholar]
- 250.Opstad TB, Pettersen AA, Arnesen H, Seljeflot I. The co-existence of the IL-18+183 A/G and MMP-9 -1562 C/T polymorphisms is associated with clinical events in coronary artery disease patients. PloS one. 2013;8:e74498. doi: 10.1371/journal.pone.0074498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Xu X, Wang L, Xu C, Zhang P, Yong F, Liu H, Wang J, Shi Y. Variations in matrix metalloproteinase-1 -3, and -9 genes and the risk of acute coronary syndrome and coronary artery disease in the Chinese Han population. Coronary artery disease. 2013;24:259–265. doi: 10.1097/MCA.0b013e32835ea3af. [DOI] [PubMed] [Google Scholar]
- 252.Shi GP, Sukhova GK, Grubb A, Ducharme A, Rhode LH, Lee RT, Ridker PM, Libby P, Chapman HA. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest. 1999;104:1191–1197. doi: 10.1172/JCI7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Manzano-Fernandez S, Januzzi JL, Jr, Boronat-Garcia M, Bonaque-Gonzalez JC, Truong QA, Pastor-Perez FJ, Munoz-Esparza C, Pastor P, Albaladejo-Oton MD, Casas T, Valdes M, Pascual-Figal DA. beta-trace protein and cystatin C as predictors of long-term outcomes in patients with acute heart failure. J Am Coll Cardiol. 2011;57:849–858. doi: 10.1016/j.jacc.2010.08.644. [DOI] [PubMed] [Google Scholar]
- 254.Patel PC, Ayers CR, Murphy SA, Peshock R, Khera A, de Lemos JA, Balko JA, Gupta S, Mammen PP, Drazner MH, Markham DW. Association of cystatin C with left ventricular structure and function: the Dallas Heart Study. Circulation. Heart failure. 2009;2:98–104. doi: 10.1161/CIRCHEARTFAILURE.108.807271. [DOI] [PubMed] [Google Scholar]
- 255.Ankersmit HJ, Weber T, Auer J, Roth G, Brunner M, Kvas E, Moser B, Spreitzer S, Lassnig E, Maurer E, Hartl P, Wolner E, Boltz-Nitulescu G, Eber B. Increased serum concentrations of soluble CD95/Fas and caspase 1/ICE in patients with acute angina. Heart. 2004;90:151–154. doi: 10.1136/hrt.2003.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Singh KP, Jaffe AS, Liang BT. The clinical impact of circulating caspase-3 p17 level: a potential new biomarker for myocardial injury and cardiovascular disease. Future cardiology. 2011;7:443–445. doi: 10.2217/fca.11.29. [DOI] [PubMed] [Google Scholar]
- 257.Fingleton B. Matrix metalloproteinases as valid clinical targets. Current pharmaceutical design. 2007;13:333–346. doi: 10.2174/138161207779313551. [DOI] [PubMed] [Google Scholar]
- 258.Schulze CJ, Castro MM, Kandasamy AD, Cena J, Bryden C, Wang SH, Koshal A, Tsuyuki RT, Finegan BA, Schulz R. Doxycycline reduces cardiac matrix metalloproteinase-2 activity but does not ameliorate myocardial dysfunction during reperfusion in coronary artery bypass patients undergoing cardiopulmonary bypass. Critical care medicine. 2013;41:2512–2520. doi: 10.1097/CCM.0b013e318292373c. [DOI] [PubMed] [Google Scholar]
- 259.Duong le T. Therapeutic inhibition of cathepsin K-reducing bone resorption while maintaining bone formation. BoneKEy reports. 2012;1:67. doi: 10.1038/bonekey.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Fischer U, Schulze-Osthoff K. Apoptosis-based therapies and drug targets. Cell Death Differ. 2005;12(Suppl 1):942–961. doi: 10.1038/sj.cdd.4401556. [DOI] [PubMed] [Google Scholar]