Abstract
The blood-brain barrier (BBB) maintains the brain homeostasis and dynamically responds to events associated with systemic and/or rheological impairments (e.g., inflammation, ischemia) including the exposure to harmful xenobiotics. Thus, understanding the BBB physiology is crucial for the resolution of major central nervous system CNS) disorders challenging both health care providers and the pharmaceutical industry. These challenges include drug delivery to the brain, neurological disorders, toxicological studies, and biodefense. Studies aimed at advancing our understanding of CNS diseases and promoting the development of more effective therapeutics are primarily performed in laboratory animals. However, there are major hindering factors inherent to in vivo studies such as cost, limited throughput and translational significance to humans. These factors promoted the development of alternative in vitro strategies for studying the physiology and pathophysiology of the BBB in relation to brain disorders as well as screening tools to aid in the development of novel CNS drugs. Herein, we provide a detailed review including pros and cons of current and prospective technologies for modelling the BBB in vitro including ex situ, cell based and computational (in silico) models. A special section is dedicated to microfluidic systems including micro-BBB, BBB-on-a-chip, Neurovascular Unit-on-a-Chip and Synthetic Microvasculature Blood-Brain Barrier.
Keywords: Cell lines, Microfluidic, Drug Development, Pharmacokinetic, Pharmacodynamic, Permeability, Alternative, Endothelial, Tight Junction, Biotechnology, Translational
Introduction
Blood-brain barrier (BBB): Structure and Functions
At the brain microvessels level, the BBB acts as a highly dynamic and functional interface between the systemic circulation and the CNS. While maintaining a stable brain environment and protecting the CNS from potentially harmful chemicals or systemic fluctuations, the BBB strictly and accurately regulates transport of essential molecules and nutrients necessary for optimal neuronal function. The current notion of the BBB as evolved from past decades embracing the concept of a multifunctional (1) and dynamic vascular interface that responds to a large array of physiological and pathological cues such as acute brain injury (2), rheological disturbances (3), pro-inflammatory stimuli (4)), diabetes and hypercholesterolemia (5) etc. From a functional and structural point of view, the BBB consists of highly specialized vascular endothelial cells (EC) lining the brain micromicrovessels. Closely associated astrocytic end-feet processes (6) and pericytes (7) modulate endothelial cell differentiation and maintain BBB properties such as tight junction (TJ) – expression/regulation and vesicular trafficking (8). The intrinsically unique and utmost complex functional interaction between the BBB endothelium and the cellular milieu of the brain environment (including extracellular matrix components, astrocytes and neurons) led to the conceptualization of the term “neurovascular unit (NVU)” to define the close structural and functional relationships between brain and vascular cells (6, 9). More recently the “NVU concept” has evolved towards ‘extended NVU’ which encompasses other cell types, (such as microglia, myocytes and pericytes) as well as specialized cellular compartments (e.g., endothelial glycocalyx) (10, 11).
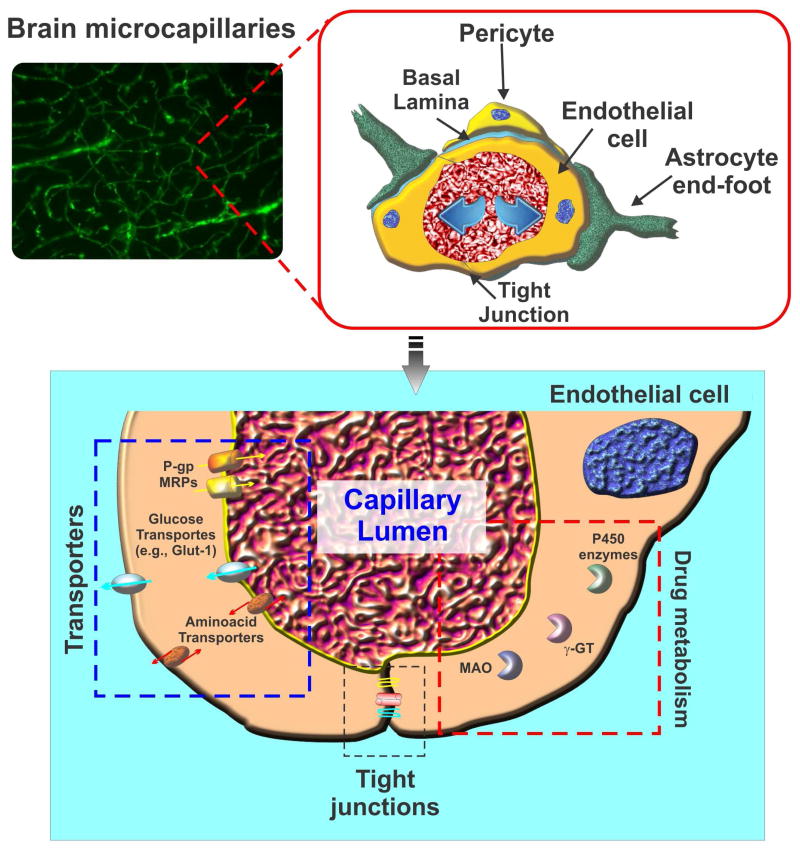
Unlike their peripheral counterparts, the BBB endothelial cells are characterized by limited pinocytosis, relative absence of fenestrations, and asymmetrical expression (lumen versus albumen) of trans-membrane transport and efflux systems regulating the traffic of substances between the blood and the brain parenchyma (12). Transmembrane inter-endothelial TJ proteins (e.g., occludin, claudins etc.) form homophilic binding with corresponding proteins on adjacent endothelial cells (although claudins can also form heterophilic trans-interactions with different claudins such as Cld3/Cld1, Cld5/Cld1 or Cld3/Cld5 to a lesser extent. (13)) and restrict the paracellular flux of ions and hydrophilic solutes between the endothelial cells (14). TJ appear at sites of contact between outer leaflets of plasma membrane of endothelial cells. These TJs form a genuine physical barrier to the paracellular diffusion of blood-borne substances and xenobiotics into the CNS. The impediment to ion movement results in high electrical resistance of the BBB in vivo, with readings ≥1800 Ω.cm2 (12). TJs also work as a “fence” that limits the free movement of lipids and proteins within the plasma membrane between the apical and the basal surface. Thus, water soluble nutrients and other biologically vital substances (such as, Amino acids, D-glucose, and mono-carboxylic acids (12)) are carried into the brain by specialized carrier-mediated transport systems (12) (see Fig. 1).
Figure 1. Schematic illustration of typical brain micromicrovessels.
The passage of substances across the BBB endothelium is controlled by a multimodal barrier system; 1) gating barrier (tight junctions) which prevent paracellular diffusion of polar molecules; 2) transport barrier which includes a number of active efflux systems (P-gp, MRPs, etc) with affinity for lipophilic substances; 3) metabolic/enzymatic barrier (cytochrome P450 enzymes, MAO, etc.) which catalyze the oxidation/metabolism of organic substrates including xenobiotic substances such as drugs and other potentially toxic chemicals.
Importantly, a range of ATP-binding cassette (ABC) family-related efflux transporters including P-glycoprotein (P-gp/ABCB1) (15), breast cancer resistance protein (BCRP/ ABCG2) (16) and multidrug resistance related protein 4 (MRP4) (17) are highly expressed at the BBB endothelium. Coupled with significant metabolic capabilities granted by specific cytochrome P450 (e.g., CYP3A4, NADPH-CYP P450 reductase) (18) and Phase II enzymes (e.g., UGT1A4) (19) which are also expressed at the BBB endothelial level, this complex machinery of drug efflux transporters and P450 enzymes ultimately protect the CNS from either water or lipid soluble harmful substances (15). However on the negative side, the same machinery plays a significant role in the onset of pharmaco-resistance. This is one of the key challenges hindering drug delivery to the brain for the treatment of major neurological disorders including epilepsy and brain tumors (20).
BBB involvement in neurological disorders
Numerous studies have shown that BBB impairment can be prodromal to the pathogenesis and/or progression of major neurological disorders such as epilepsy, multiple sclerosis (MS), Parkinson's, and Alzheimer's (21). Observed changes include alterations in BBB permeability (22, 23), caused by disruption and/or structural alteration of TJ proteins (24), which can be accompanied by degradation of the basement membrane through increased expression/activity of matrix metalloproteinases (MMPs) such as MMP-9 (25), extravasation of plasma proteins (26) and altered expression of drug transporters and ion channels on endothelial cells and glial cells (27). A heightened inflammatory response via secretion of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α), up-regulation of permeability promoter vascular adhesion molecules (E-selectin and VCAM-1) (28, 29), increased secretion of vascular endothelial growth factor / VEGF (30) and oxidative stress in the vasculature (31, 32) are all crucial factors associated with the loss of BBB function in this setting. Neurodegenerative disorders such as Alzheimer's disease (AD) and Parkinson's disease (PD) typically manifest with aging in correlation with compromised BBB functions (33). Classical amyloid beta (Aβ) accumulation is observed in brain tissue from Alzheimer's disease patients which further leads to neuronal damage and dementia. In recent reports, the endothelial expression of a receptor for advanced glycation end products (RAGE) and its interaction with circulating Aβ were shown to mediate Aβ influx, promoting BBB permeability through disruption of TJs (34, 35). Existing evidence indicates a decreased efflux of brain Aβ via BBB, thus leading to accumulation and increased half-life of this toxic peptide. Whether or not Aβ entry is the main cause or the secondary manifestation of BBB disruption in AD remains to be determined, however. The underlying etiology is not clear in PD, but PD is associated with P-gp damage and infiltration of neurotoxins (36).
Stroke is another major neurological disease, which directly involves BBB impairment. Using the middle cerebral artery occlusion (MCAO) animal model of stroke, studies have shown that BBB permeability during a stroke attack was observed to increase bimodally (rapid opening followed by a lag period and second phase of prolonged opening) (37). A striking disruption and/or redistribution of TJ proteins such as claudin-5, ZO-1, and occludin (38) is observed along with increased expression MMP-9 and inflammation (39). On the same line traumatic brain injuries (TBI) cause both immediate and delayed dysfunction of the BBB leading to inflammation (40) and the rapid activation of the coagulation cascade (41). This ultimately causes post-traumatic intravascular coagulation and a significant reduction in blood flow in the pericontusional brain tissue closely resembling a post ischemic injury.
On the other hand, MS is characterized by an inflammatory autoimmune response to the myelin sheath of the CNS, which leads to impairment of motor and sensory functions of MS patients. BBB dysfunction is clearly established as a key early event in MS progression (at least the relapsing-remitting inflammatory form) whereas BBB breakdown and enhanced permeability precedes and leads to infiltration of encephalitogenic T cells, monocytes and likely B cells into the brain. The role of the BBB is evident when therapeutic options to improve the BBB have proven to ameliorate MS disease progression (42, 43). Similarly in epilepsy, seizures show a typical pattern of BBB impairment (44, 45) where conditions involving BBB damage such as stroke increase the propensity to develop seizures (46). These collective findings indicate that the BBB is intricately involved in several neurological and/or neurovascular complications.
Ideal characteristics of an in vitro BBB model: What should we aim for?
Advancement in the BBB modeling field as observed in the last two decades was primarily dictated by three factors: 1) the necessity to dissect out and understand the multifaceted molecular mechanisms that regulate and maintain the brain homeostasis; 2) the need to support and facilitate the development of novel and more effective CNS drugs; 3) the requirement to consolidate in vivo studies to reduce cost and increase the translational relevance of experimental results. As a general scope, in vitro models aim at providing a highly controlled environment outside a living organism to assess the physiological and pathological responses to specific experimental stimuli which otherwise are difficult to reproduce, dissect out and/or characterize in vivo. However, mimicking the functional properties and physiological responses of the BBB is an extremely challenging task. An ideal in vitro BBB model should be able to accurately reproduce the complex vascular microenvironment found at the brain level taking into consideration all possible physiological conditions. From a functional and physiological standpoint, this ideal model should: 1) promote endothelial cell differentiation into a mature BBB phenotype demonstrating asymmetric distribution and expression (cell polarization) of relevant transporters (e.g., P-gp, MRP-2, etc.) (47), functional efflux mechanisms (e.g., P-gp (48)), and drug metabolizing enzymes (49); 2) promote the development of a highly stringent and selective barrier by enabling the expression of tight and adherent junctions between adjacent endothelial cells including the associated signaling pathways; 3) enable realistic cell to cell interactions with glia and pericytes as well as the exposure to circulating immune and inflammatory cells necessary for studying disease models; 4) enable endothelial exposure to “biologically and mechanically active factors” including shear stress (3), numerous growth factors and cytokines, which play a role in the modulation of BBB functions (12); 5) permit the reproduction of pathophysiological stimuli/insults including but not limited to, hypoperfusion, hypoxia, altered glycaemia, and exposure to xenobiotics) which can be prodromal to major CNS disorders (21) such as AD (50), MS (51), and epilepsy (52)). Finally the system should be easy to establish, allow for scale-up to high-throughput screening (HTS) capabilities, enable realisticallycomplex multi-cultures, offer a tightly regulated microenvironment and provide reproducible data at high turnover with contained costs (53).
Unfortunately, due to incomplete understanding of the molecular mechanisms regulating differentiation, maturation and maintenance of BBB properties as well as technological limitations to recreate a proper physiological environment in vitro, current models fall short of these expectations. Replicating the characteristics and physiological responses of the BBB in vitro continues to pose a major challenge to the field. Although still far from an ideal situation, recent advancement in the field of biotechnology and further understanding of BBB biology have enabled the development of various in vitro approaches designed to meet several of the requirements listed above to varying degrees. In this review we will provide a detailed coverage of these systems including an analysis of the pros and cons of each model.
Predictive non-cell based models
In silico models: Advantages and Limitations
Computer-assisted, structure-based drug design known as in silico modeling enables the biotech and pharmaceutical industry to predict the pharmacokinetic and pharmacodynamics properties of a drug in early phase drug discovery and development program. Without the investment of biological-based in vitro and in vivo models, data on specific biological phenomena are first analyzed. To begin, a computer model based around biological algorithms containing a number of well define molecular descriptors is generated to allow the researcher to perform in silico experiments to validate the accuracy of the model versus the original biological system, refine the model if necessary, and use the model to predict the behavior of the biological system under specific experimental conditions. Once an in silico model of a biological system has been validated, it now provides a cost effective and high throughput screening of the efficacy, bioavailability and potential toxicity for drugs targeting that specific biological system. In CNS drug discovery this translates into an invaluable tool that supports and facilitates the development of novel CNS therapeutics (54) while assessing potential brain toxicity of non-CNS drugs (55). An important BBB permeability parameter is logBB (defined as logarithm of a compound's brain to blood ratio under steady state conditions (56)). In the past, logBB was quantitatively related to a number of molecular descriptors like octanol/water partition coefficient (logP), molecular weight, molecular volume, dipolarity/polarizability, refraction index, hydrogen bond acceptor and donor number including the polar surface area (57). These molecular descriptors, in combination with different statistical methods, are used to build computational models which screen libraries of compounds (58); a technique which recently led to the discovery of promising new anticancer drugs (59) (60). In addition to the considerable advantages and benefits of in silico predictive models there are also limitations that need to be taken into consideration. Most of the current in silico models within CNS drug development programs lack molecular descriptors of important biological functions of the BBB such as active drug transport, drug metabolism, endothelial enzymatic activity, and drug–drug interactions which hinder their translational significance. However, advances in this rapidly evolving field including the use of multiple linear regression (MLR) models incorporating additional molecular descriptors (such as plasma protein binding ratio -PPBR and high affinity P-glycoprotein substrate probability - HAPSP) (61), in vitro/in silico-in vivo data extrapolation (IVIVE) (62) and the use of bayesian statistic models (63) are closing the gap (64). At this stage, in silico models cannot be considered as standalone tools since in vivo and in vitro studies are required to validate the results and/or refine the working hypotheses the original computational algorithm(s) were built upon (65) (see also Fig. 2).
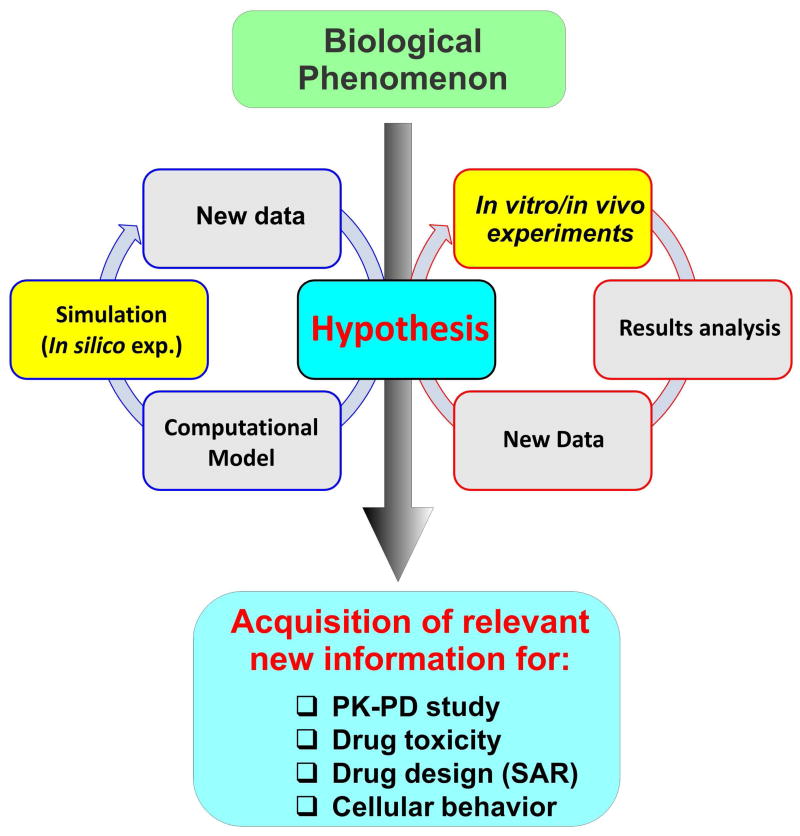
Figure 2. Stepwise development of a computational model from in vitro and in vivo data.
A model is first built up from data collected from in vitro and in vivo experiments. The model is then used to predict the behavior/response of a certain biological phenomenon and results are compared and validated against in vitro and in vivo results. The process can further cycle to refine the model (biological descriptors) and the underlying hypothesis.
Solid phase biological membrane mimetics
Immobilized artificial membrane (IAM) chromatography and parallel artificial membrane permeation assay (PAMPA) are physicochemical methods developed to enhance the throughput of permeability studies and membrane interaction of CNS drugs (66). IAM chromatography was developed at Purdue University by Charles Pidgeon to mimic the cell membranes' environment utilizing an inert chromatographic stationary phase (67). In this system amphiphilic cell membrane phospholipid analogs (e.g., phosphatidylcholine - PC, phosphatidylglycerol – PG, phosphatidic acid - PA, phosphatidylethanolamine - PE, and phosphatidylserine - PS) are immobilized on a rigid silica surface by covalent bonding (68). The resulting phospholipid layer reproduces the lipid outer sheet of a biological cell membrane. Biopartitioning potential of IAM chromatography has proved remarkably effective for rapid purification of functional membrane proteins (69) and predicts the phospholipophilicity and potential membrane permeability of small drugs (70). As an alternative approach to measure lipophilicity parameters, IAM retention can replace liposomes by facilitating membrane simulation for rapid permeability measurements and minimizing the difficulties associated with liposome-water partitioning (71). However, several drawbacks limit the translational reliability and accuracy of this method to assess drug permeability including the inability to mimic reproducible lateral membrane diffusion that occurs at cellular level in vivo, difficult and erratic extrapolation of solute diffusion across double layered biological membrane using single layered IAM and the lack of biological phenomena such as metabolism and active efflux of drugs normally observed at the BBB level (72).
Parallel Artificial Membrane Permeability Assays (PAMPA) have been successfully introduced into the pharmaceutical industry as in vitro tools to predict passive oral absorption in vivo. This in vitro model is designed as a sandwich-like configuration where a solid support (e.g. polycarbonate) imbued with lipid is placed between a donor and an acceptor compartment. The drug is administered in the donor compartment and allowed to pass through the membrane into the acceptor compartment where it is quantified by spectroscopic and chromatographic methods such as ultraviolet-visible spectroscy (UV VIS) or liquid chromatography-mass spectrometry (LC/MS).. Passive diffusion though a physiological membrane is one of the vital entry routes of drug absorption into the body and PAMPA. This passive permeability screen is considered a low-cost alternative to cellular models for the earliest ADME primary screening of novel drugs. PAMPA is unique in its versatility, automation and reproducibility which successfully mimics varied biological membranes of interest (73).
PAMPA models can effectively reproduce most of skin's features providing a quick, reliable, and cost-effective permeability model for predicting transdermal penetration of drugs (74). Furthermore, PAMPA systems have found successful use as predictive models for passive gastrointestinal absorption (75), BBB permeability (including efflux ratios) (76) and temperature dependence of permeability (77).
Recently PAMPA models with pH gradients have been developed to measure one way transport of drug compounds across membrane exposed to an acidic or neutral environment. For example, models have been developed to model specific physiological environments such as the gastric and intestinal cavities which exhibit pH ranges of 1.0-2.5 and 6.6-7.0 respectively (78). In addition, solubilizing or binding agents (affecting the compound ability to diffuse across the lipid membrane) can be added to the either compartment thus, allowing for a more accurate in vivo-like reproduction of the biological environment. Incorporating these two features double sink or DS-PAMPA has been developed, where a pH gradient is maintained between donor (pH 3-10) and acceptor (pH 7.4) compartments. A lipophilic scavenger is added to receiver compartment to mimic nonstatic equilibrium established in biological system by plasma protein and blood flow (73). Although this platform is best suited for studies on intestinal absorption DS-PAMPA systems with specialized membrane (20% w/v lecithin in n-dodecane) and surfactant in the acceptor compartment have been used by the researchers in several occasions for measuring the permeability properties of molecules across the BBB although with a modest ability to discriminate between BBB permeable versus impermeable compounds (79, 80). Better results were obtained with modified PAMPA systems using either a black lipid membrane (PAMPA BLM) or a porcine polar brain lipid membrane (PAMPA BBB) which provided a a better level of accuracy in terms of BBB permeability with promising result for their use in early stage of CNS drug discovery process (79). In summary, PAMPA assays are considered suitable low cost, HTS alternatives for early stage absorption screening of drugs. As with IAM platforms, PAMPA systems cannot reproduce metabolic transformation and/or phenomena of active extrusion to which a drug could be subjected during transcellular routing across physiologically active interfaces (such as the BBB). This is a major limiting factor of this model and may result in incorrect estimates of drug bioavailability in the targeted site. Combination of PAMPA study with efflux assay could further improve data reliability on BBB permeability of novel drug compounds.
Plasma membrane vesicles
The polar distribution of membrane transport proteins promotes a unidirectional movement of specific solutes across the BBB. Isolation of vesicles from these luminal and abluminal plasma membranes of brain capillary endothelial cells are used to characterize drug/substrate transport processes across BBB. These studies on drugs/substrates fulfill a number of purposes such as measuring their transport, determining initial rate of transport, distinguishing binding and transport, measuring kinetic constants and determining the distribution of transport activities (81-83). This method has the advantage of measuring the actual disposition of the substrate across the BBB membrane. However, it is difficult to perform direct transport measurements for certain compounds (medium-to-high passive permeability) which are not retained inside the vesicles (84). Further, it involves lot of cost as a number of large brains (usually bovine brains) are required to provide enough tissue for membrane isolation for a typical series of transport experiments besides concerns of change in membrane protein characteristics and regulation during isolation process (81).
Isolated brain microvessels
Functionally intact and purified cerebral microvessels can be isolated from animal as well as human brain tissue (generally from autopsy and tissue resections). These purified brain microvessels consist of vascular endothelium ensheathed by a basement membrane containing pericytes and offcuts of astrocytic endfeet. The purification procedure consists of a combination of mechanical homogenization, enzymatic dissociation, filtration, and density gradient centrifugation followed by column filtration (85). Visual inspection by light/fluorescence microscopy as well as scanning electron microscopy and transmission electron microscopy (85) for positive expression of typical BBB biomarkers (such us glucose transporter-1 (glut-1); transferrin receptor (OX-26); ZO-1, claudin-5 and 12, (86)) are concomitantly used to assess the purity of the preparation. Lack of typical smooth muscle cell biomarkers such as desmin and calponin (87, 88) in isolates is generally indicative of a preparation free of post-capillary fragment contaminants. Freshly isolated brain microvessels are metabolically active although a measurable amount of the original activity is lost during sample preparation, thus viability and integrity of the BBB is not guaranteed.
Isolated brain microvessels have been used to dissect out and characterize a variety of molecular signals and biochemical mechanisms regulating BBB functions under normal (89-91) or pathological conditions such as brain tumors (92). These include: a) mechanisms of transport across the BBB such as receptor mediated transcytosis (e.g., insulin, transferrin, leptin); bi) absorptive mediated transcytosis (e.g., albumin) and active transport systems for glucose and other essential nutrients (aminoacids); c) transport activity of P-gp in response to Vitamin D (93); d) modulatory mechanisms of blood-brain barrier P-gp transport activity (94); and e) transendothelial transport of fluorescent drugs by confocal microscopy (95). In addition, these brain microvessels once purified are a viable source of brain microvascular endothelial cells which can be isolated from these microvessel fragments allowing the preparation of a relatively pure BBB endothelial primary culture (96). Structural and functional characteristics of the BBB in vivo are maintained in isolated brain microvessels thus representing one of the major advantages inherent to this model. Brain microvessels can be isolated from patients affected by various CNS disorders post-mortem or from tissue resections following brain surgery providing unique specimens to study the pathophysiological cues and BBB involvement in relation to a specific brain disorder. The expression of multidrug resistance protein in drug refractory epileptic patients by the quantitative proteomic analysis of transporters has been characterized this way (97). These disease-specific isolated microvessels are an invaluable resource to study the pathogenic role of the BBB to the onset/progression of the disease and characterize the corresponding pharmacokinetic parameters for CNS drug delivery. The availability of a large number of detailed protocols to obtain purified brain microvessels (methods described elsewhere; (98, 99)) is also an advantage.
On the other hand, there are a number of disadvantages that may limit the use of isolated brain microvessels such as: 1) Difficulties associated with the process of isolating and purifying brain microvessels. This is a labor intensive procedure and the risk of contaminants in the preparation is high; 2) Metabolic deficiencies occurring throughout the isolation and preparation processes are significant side effects which can seriously hamper the viability and usability of the capillary endothelium; 3) The luminal surface of the microvessels is not so easily accessible, although confocal IF analysis of ABC-transporter activity has been recently reported (100).
The use of pial microvessels has also been considered. However, these vessels lack the proper phenotypic differentiation characteristics of the BBB such as vascular polarization of transport mechanisms, selective permeability to xenobiotics and endogenous substances, drug metabolism (18) etc., which significantly decrease their reliability and usability as BBB models.
In vitro cell-based BBB models
Cell-based in vitro BBB models emerged in the early 1990s as alternative and/or complementary research tools to aid and facilitate in vivo and human studies. In vitro cell based BBB models can be established from cell cultures originating from practically any viable source (human, animal or continuous cell lines). A major advantage of these platforms lie in their capacity to provide a highly controllable environment where cell cultures can be exposed to large sets of well-defined experimental paradigms under strict controlled conditions that are often difficult to reproduce in vivo while simplifying the work at hand by eliminating a large number of physiological variable present in vivo. Bearing a number of desirable advantages both for basic/translational studies and pharmaceutical industry (such as versatility, HTS capability, relative simplicity and flexibility), in vitro BBB models have consistently and rapidly progressed during the last decade evolving from “omni tools” to research devices with precise sets of characteristics, adapted to fill specific research niches and requirements (101). These sub-specialties can range from investigating basic physiologic and/or pathologic aspects of the BBB related to CNS disorders to testing or quantifying permeation mechanisms of therapeutics (102) as well as toxicity of xenobiotics (103). One remaining dilemma is whether cell lines or primary cultures are better suited for the scope of establishing these models.
Endothelial cells (ECs) can be isolated from fresh tissue to generate primary cultures (104). Tissue sources include fetal human brain specimens, autopsy or tissue resections from brain surgeries. This latter provide disease-specific endothelial cells which can be helpful to dissect out basic BBB pathogenic mechanisms and/or relevant pathological traits. Animals such as bovine, porcine (105) or rodents (usually rats) are also a major source of primary BBB ECs. When using primary cultures, cell isolation and purification play key roles in the establishment of BBB models and the formation of a highly stringent barrier (frequently characterized by a relatively high TEER comparable to in vivo values (106). TY08 (107), HMEC-1 (108-110), and HCMEC/D3 (111, 112) are among the limited number of immortalized human brain endothelial cell lines that have been established and seemingly used as BBB models in vitro. HCMEC/D3 is currently the most widely used brain microvascular endothelial cell line (113-117). It is important to note that from a practical stand point and cost effectiveness, the use of cell line is best suited for the development of BBB model geared toward the development of HTS platforms while primary endothelial cells may be more indicated for basic and translational studies since may maintain some phenotypic (like TJ integrity) and pathological properties (e.g., drug resistance (118, 119)) more efficiently than cell lines.
As an alternative to primary brain endothelial cells, non-brain vascular endothelium (such as umbilical vein; e.g., HUVEC), endothelial (either human or animal derived) and to a limited extent non-endothelial cell lines (e.g., Madin-Darby canine kidney/MDCK) (120) and human intestinal Caco-2 (121)) have been used. Although epithelial cells such Caco-2 and MDCK express TJs and represent good models for studies of passive diffusion across the BBB, they do not recapitulate the central role of transporters in the regulation of BBB selective permeability. Glial cells used in co-culture models can also be primary or cell lines such as C6 cells (122). Co-culture combinations can be syngeneic, where both endothelial glial cells originate from the same source (123) or can have different species origin (124).
Considerable effort has been spent to establish humanized BBB models; however one of the main limiting factors hindering the widespread use of humanized in vitro BBB systems is the difficulty to access human brain tissue. Until recently, renewable sources of human BBB endothelial cells have been derived from human pluripotent stem cells (hPSCs). These cells can be influenced to acquire endothelial BBB properties with the use of proper physiological cues including exposure to neuronal cells (125).
Static BBB models: from mono to multi cultures approaches on Transwell platforms
Currently considered the gold standard in the field of BBB modeling, Transwell® platforms were initially built upon a limited understanding of the processes involved in the development and maintenance of BBB properties in vivo. However, the intrinsic simplicity and affordability of the system coupled with a much desired potential for HTS capability in terms of drug permeability testing and binding affinity measurements (126) make it the most common and widely used in vitro BBB model today.
The Transwell® apparatus is a vertical side by side diffusion system through a microporous (semi-permeable) membrane separating two adjacent chambers (see Fig. 3). Traditionally the upper chamber generally functions as the luminal (vascular) side whiles the bottom one acts as the abluminal (parenchymal side) recipient. Fixed volume of each compartment allows for the simplified study of Michaelis-Menten kinetics of transport (126). The system allows for either top to bottom or ‘bottom to top’ (most usually referred to as B-to-A, with B for basal, A for apical) diffusion of drugs.
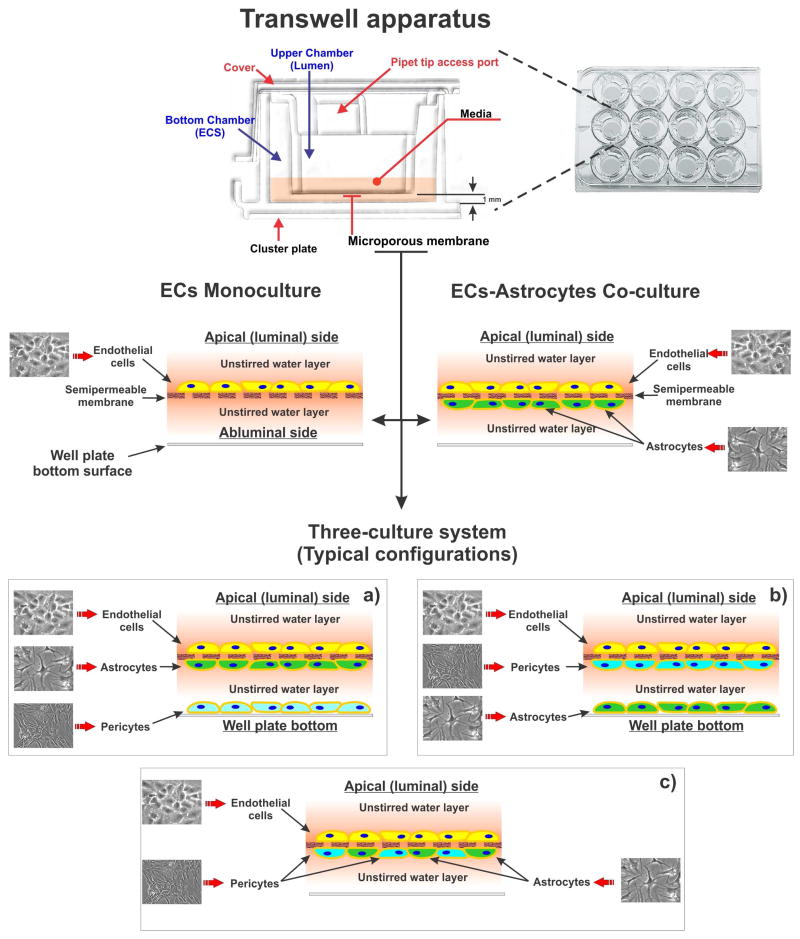
Figure 3. Schematic illustration of a Transwell apparatus.
The Transwell apparatus is a vertical top to bottom diffusion platform through a semipermeable microporous membrane. Depending on the pore size and requirements for free passage of nutrients and diffusible factors the membrane can be permissive to immune cell trafficking across the compartments. Note the cellular layout in monoculture (endothelial cells only) and co-culture (luminal endothelial cells which juxtapose perivascular/abluminal astrocytes) and typical three-culture configurations (a) to (c).
Endothelial cells (ECs) either from brain or peripheral vascular districts from various sources (human, bovine, rodent, porcine, non-human primate and cell lines) have been used to establish BBB models on Transwell® support with various degrees of success. ECs are grown to confluence on the vascular side (top surface) of the microporous support immersed in their respective growth media. Depending on the culture setup, glial cells, pericytes or other cells can be accommodated on the abluminal surface side in juxtaposition to the endothelial layer. The membrane interface, available in polypropylene, polycarbonate and polyethylene terephthalate / polyester – PET, polytetrafluoroethylene (PTFE) (some also available with collagen pre-coating) allows for the exchange of nutrients as well as promoting and/or differentiating factors between the two compartments. Based on the median pore size of the membrane, which range from 0.4 to 8μm Ø, cell movement across the luminal/abluminal interface can be restricted or partially restricted using the 0.4μm and 3.0μm sizes respectively allowing to study leukocytes migration across the BBB (127). Conveniently, each TW compartment is easily accessible for sampling or delivery of experimental agents, and some recent platform improvements include automatic sampling as well as compatible trans-endothelial electrical resistance (TEER) measurement systems. The latter measures the electrical impedance across the artificial BBB interface (microporous membrane plus cell layer/s) providing a rapid assessment of its integrity and relative barrier stringency. TEER measurement can then be correlated to the relative permeability of key paracellular markers (e.g., sucrose, dextrans, mannitol, lucifer yellow, fluorescin, etc.) quite accurately (124).
Initial BBB models on Transwell® support were developed based on a very simplistic reconstruction of the BBB using only ECs monocultures. Such models lacked the ability to reproduce a number of critical physiological factors (close interaction between ECs and perivascular glial (128) and/or pericytes (129, 130) as wells as intraluminal shear stress) necessary for the development and maintenance of true BBB properties in vitro (3). As a result, the endothelial cells were impaired by accelerated dedifferentiation, irregular patterns of cell adhesion and inability to form proper intercellular TJ. Ultimately, the net loss of BBB properties caused by non-physiological culture conditions paired with the so called “edge effect”, a response where endothelial cells alongside the edge of the membrane support cannot form a seal with the inner wall of the luminal chamber, can lead to artefactual paracellular diffusion.
In vivo and in vitro studies have now clearly shown that astrocyte interaction with the cerebral endothelium enhances BBB function. This includes expression and physiological distribution of TJ resulting in the formation of a tighten barrier (131), endothelial polarization (132, 133) and expression of specialized carrier systems including facilitative glucose transporter type 1 (GLUT-1), A-system amino acid carriers, γ-glutamyltransferase (γ-GTP), efflux transporters (Pg-p, MRPs, BCRP, etc.) and many others (131). Although the TEER value recorded in ECs-glia co-culture systems is typically higher than ECs monocultures (indicating the formation of a more stringent barrier) there are exceptions to this rule. A recently developed in vitro BBB model based solely on primary porcine brain endothelial cells (PBECs) (105) displayed many typical BBB features (including stable high TEER values ranging from 800 to 1300 Ω cm2 and remarkable expression of TJ and major efflux transport systems) in the absence of abluminal glia.
Despite very few exceptions in order for endothelial cells to exhibit more complex features (e.g., receptor-mediated transcytosis – RMT (134)) and maintain a sufficiently differentiated phenotype, co-culture with glial cells is highly recommended (135).
Triculture BBB models where endothelial cells, glia and pericytes are cultured in unison have been recently developed (136). Although these systems may show some additional BBB properties over standard endothelial-glia co-cultures, questions remain whether the reduced practicality in favor of added complexity proves necessary for mechanistic studies and permeability assays (101). Furthermore, in terms of physiological stimuli, all static systems, despite the complexity of the culture milieu, still exclude the equally important physiological and biomechanical cues provided by the physiological shear stress (SS) (3, 137) to which BBB endothelial cells are exposed in vivo. In recent years, this has led to the development of sophisticated, although potentially less practical, dynamic (flow-based) BBB models (118, 138, 139) which are also described herein.
Ussing chambers
Differently from Transwell apparati, Ussing chambers consists of two halves of the chamber clamped together having an epithelia sheet (mucosa or monolayer of epithelial cells grown on permeable supports) as the “barrier (140, 141). The epithelia are polar in nature, thus it is possible to isolate the apical side from the basolateral side. Each half chamber is filled with Ringer solution to remove any unwanted driving forces (chemical, electrical or mechanical). While Ussing chambers are widely used for studying intestinal permeability (142, 143) whereas movements of ions/drugs across the epithelium are measured using electrophysiological or radioactive methods; the platform has been rarely used to model BBB functions.
Role of shear stress in BBB endothelial physiology
It is now well accepted that exposure to “permissive” or “promoting” factors released by the surrounding microenvironment, cell-cell interaction with glial and possibly pericytes (12) (7) and exposure to physiological shear stress (SS) (3) are required for the brain vascular endothelium to properly differentiate and maintain a BBB phenotype. In this respect, SS not only modulates endothelial morphology, (cells appears larger in volume, and flat with abundance of microfilaments, clathrin coated pits, and endocytic vesicles, when compared to cultures grown under static conditions) (144), but also their function and physiological responses.
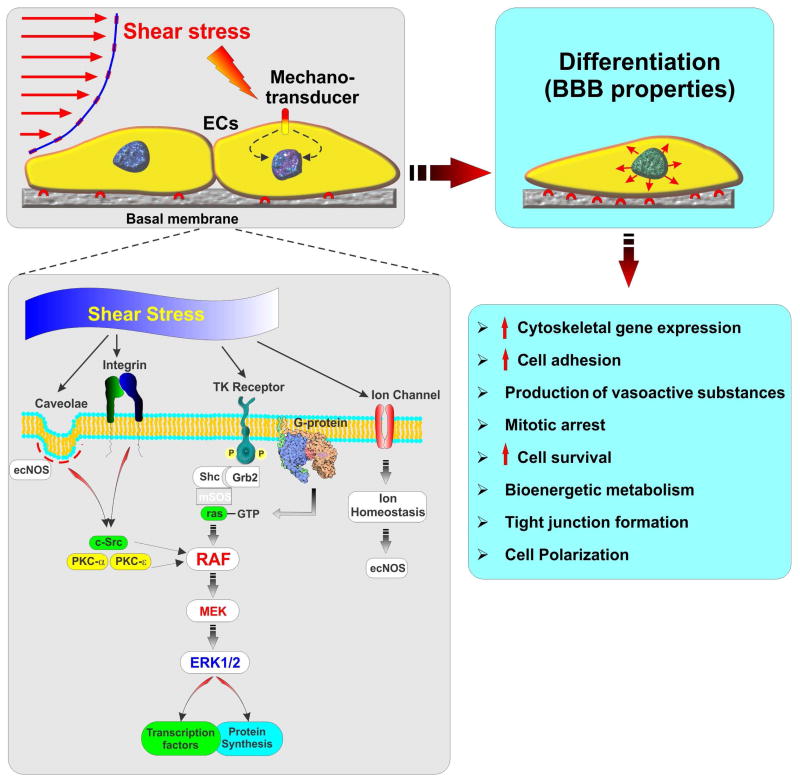
Vascular endothelial cells are supported with a wide range of mechanosensors such as ion channels, integrins, G proteins, and caveolae (145), which transduce physical stimuli generated by flow into biochemical signals leading to the activation of pleiotropic modulators of the cell physiology such as extracellular-signal-regulated kinases 1/2 - ERK1/2 (146). Cell differentiation, apoptosis, regulation of cell division and cell migration are some of the major end points of these biochemical pathways (3) (see also Fig. 4) which affect multiple endothelial functions. These include the production of vasoactive substances (147-149), improved cell adhesion (150), improved barrier tightness through expression of tight junctions (151, 152), cell survival, energy metabolism (3), and cell polarization (153).
Figure 4. Role of shear stress in modulating BBB endothelial physiology.
Shear stress is a major pleiotropic modulator of endothelial cell physiology regulating cell division, differentiation, migration, and apoptosis.
While cellular interaction issues can be addressed to various degrees under static culture conditions, exposing endothelial cells to SS required the development of a complete new set of platforms; the so called “Dynamic Models”.
Dynamic in vitro BBB models
The cone-plate apparatus (plate viscometer) described by Dewey and Bussolari was the first attempt to enable endothelial exposure to flow in vitro (154, 155). Within the system, SS is generated by a rotating cone which causes the media in the chamber to start spinning in the same direction, thus generating shear forces on the underlying endothelial monolayer seeded at the bottom of the chamber. The level of SS to which the cells are exposed depends on the angular velocity and the angle of the rotating cone.
Albeit a step forward in comparison to static monoculture this platform cannot reproduce the hemodynamic or the microenvironmental characteristics, such as cellular milieu and cross signaling, of the brain micromicrovessels. The importance of SS as a prodromal differentiating factor for the BBB endothelium has been well established and characterized (3, 153). Thus the need for vascular models enabling endothelial exposure to a larger and comprehensive array of physiological factors, both biological and mechanical such as SS, paved the way toward the development of more sophisticated in vitro culture systems.
Several in vitro BBB platforms enabling endothelial exposure to SS are currently available: 1) Parallel-Plate Flow Chambers; 2) the “Dynamic in vitro BBB model”/DIV-BBB; 3) Microfluidic systems; which is the newest and one of the most promising technologies introduced in the field thus far.
Parallel-Plate Flow Chamber
Use of parallel plate flow chambers (PPFC) have consistently increased in the past two decades as the platform of choice when studying the physiological and morphological response to a number cell types, like endothelial cells, stem cells, leukocytes etc., under quasi-physiological levels of fluid shear stress. Pertinent to the vascular research field, PPFC have recently been used to study the adhesion of metastatic tumor cells to the brain vascular endothelium and subsequent trans-endothelial migration (156), leukocytes-endothelial interactions (157), cellular chemotaxis (158) and more generically, SS modulation of endothelial morphology (cell alignment) (159) as well as endothelial function related to drug absorption (160),
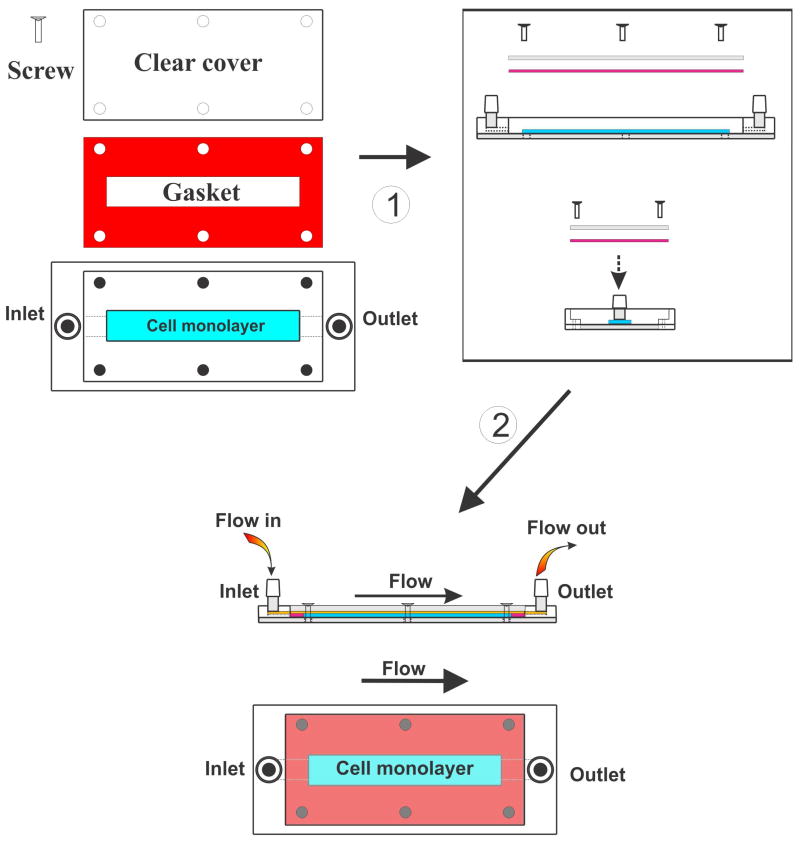
A typical PPFC consists of a clear polycarbonate distributor forming the upper portion of the platform, a silicon gasket of which the thickness determines the height of the flow path, and a glass coverslip, which can be coated with various adhesion factors (collagen, fibronectin, ECM, etc. – see Fig. 5). The distributor includes the inlet and outlet ports and a vacuum slot. The glass coverslip provides the culture support for the cells of interest and forms the lower side of the PPFC platform. Vacuum generated between the distributor and the coverslip positioned on the opposite side of the silicon gasket hold the parts together. The height of the channel is determined by the thickness of the gasket, which is uniform throughout the length of the PPFC platform. The culture medium flows unidirectional entering the chamber from one side and leaving from the opposite one. The resulting PPFC platform is transparent allowing for a variety of either transmitted or reflective light microscopy-based studies of cells including commonly-used immunocytochemistry techniques..
Figure 5. Schematic illustration of a typical parallel plate chamber platform.
Note the flow chamber assembly. Once setup is complete the system longitudinal study of cells cultured under flow (changes in cell morphology, adhesion etc.) can be performed by phase-contrast video microscopy.
The wall shear stress (τwall) is derived from Navier-Stokes equations describing the motion of fluid and the continuity equation for rectangular geometry:
Where Q is the volumetric flow rate, μ is the dynamic viscosity, and w and h are the width and height of the chamber, respectively. The shear stress exerted on the cells is assumed ≈ to the chamber wall shear stress.
In addition, the ratio of inertial viscous forces, known as the Reynolds Number (RN) can be calculated to determine whether flow within the PPFC chamber is either laminar (most desirable) or turbulent. Generally laminar flow is defined by RN <250 in biological in vitro systems mimicking the blood flow of the brain microvasculature (161). Flow properties can also be assessed by injecting a fluorescent dye upstream from the PPFC chamber making the flow streamlines visible. More recently a technique utilizing high-resolution, high-speed planar Micro-Particle Image Velocimetry (μ-PIV) has been developed (162). In this case fluorescent particles are injected upstream from the chamber and the emitted wavelength generated by exposure to a laser beam is then read and analyzed to determine the property of flow.
The major points of strength of the PPFC are: 1) the ability to reproduce physiological distinct wall shear-stress in the range from 0.01 up to 30 dyne/cm2; 2) inherent simplicity in the design and user operation; 3) contained dimensions which result in a relatively small number of cells required to setup the platform; 4) imaging-compatible design enabling the use of video microscopy for longitudinal studies in real time. Modified versions of the original design can accommodate an abluminal chamber separated by the one exposed to flow through a semi-porous membrane, similar to that of a Transwell® (138). This PPFC enables studying chemotaxis and leukocyte transmigration across an endothelial monolayer under flow conditions. However, one of the major limitations of this platform is its inability to accommodate perivascular glial or pericytes in juxtaposition to the vascular endothelium (contact based co-cultures). This limits the ability of the system to reproduce the structural and functional properties of the BBB in vivo.
Capillary-like 3D in vitro BBB models
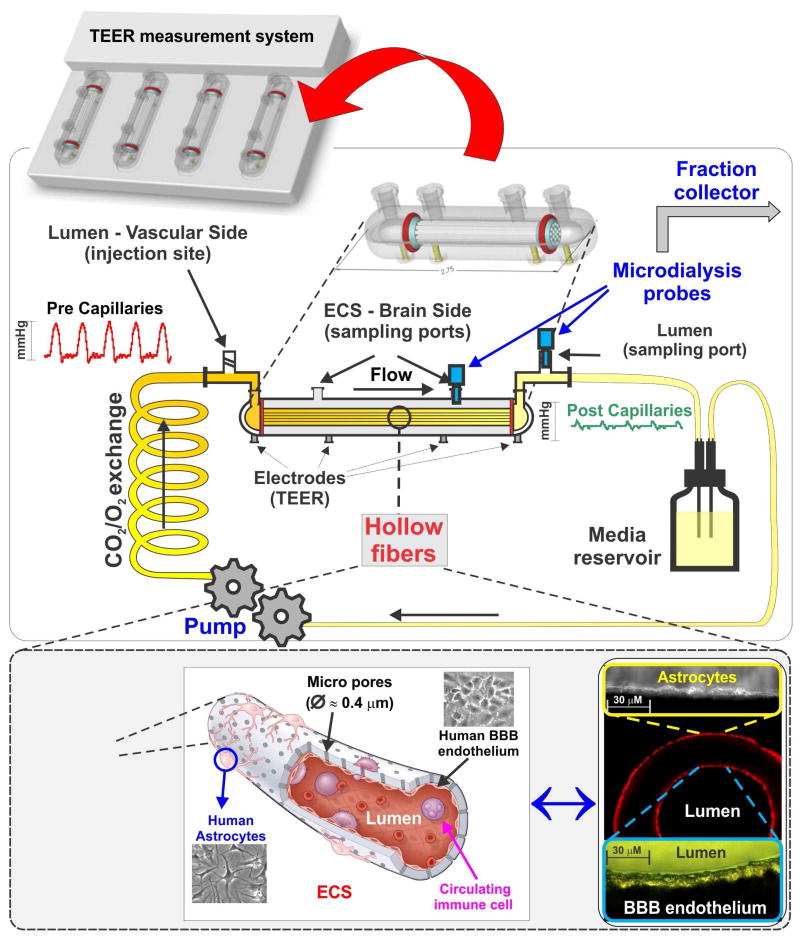
Artificial hollow fiber constructs made of thermoplastic polymers such as polysulfone, polypropylene, etc., which were initially used for the construction of bioreactors,provided the technology to enable modelling hollow organ-like structures including brain microvessels and other CNS vascular beds (163, 164). In this system, referred to as a “dynamic in vitro BBB model / DIV-BBB”, endothelial cells are seeded on the luminal surface of the artificial microvessels (lumen or vascular side) while glial cells are distributed on the outer surface of the same hollow fibers in juxtaposition to endothelial cells (ablumen or parenchymal side). The advantage of using hollow fibers is that the EC-glia co-culture can now be arranged on a 3D environment with a spatial and topographical distribution that resembles the anatomy of brain microvessels in vivo (165) (see Fig. 6) allowing solely the endothelium to be exposed to quasi-physiological intraluminal flow. The platform is supported with a servo-controlled (variable-speed) pumping mechanism that generates a pulsatile flow through the hollow fibers via pre and post gas permeable silicon tube connectors. Through these connectors the microporous pronectin-coated polypropylene microvessels are in continuity with a media reservoir. SS level is modulated by adjusting the flow rate across the capillary system and depends upon the viscosity of the medium and the inner diameter of the hollow fibers. SS levels comprised between 5 and 23 dynes/cm2 are considered comparable to those observed in vivo in the CNS vasculature (166).
Figure 6. Schematic illustration of the DIV-BBB model.
In this system BBB endothelial cells are cultured in the lumen of fibronectin-coated polypropylene microporous hollow fibers. Astrocytes are then seeded on the abluminal surface of the same fibers in juxtaposition to ECs. The bundle of hollow fibers is suspended inside a sealed chamber and in continuity with gas-permeable silicon tubing circulating media throughout the system. Access to the luminal (vascular) and abluminal (parenchymal) compartments is granted through inlet and outlet ports positioned on the opposite sides of the module and 2 additional ports positioned directly on top of the DIV-BBB. TEER is measured in real time through a set of electrodes embedded in the module's scaffold and in contact with either the luminal or the abluminal chambers. Note that this platform allows for recirculation of plasma cells, thus closely reproducing the vascular milieu observed in vivo. Note also the pressure waveforms changes across the system mimicking the rheological characteristics of pre- and post-microvessels segments.
In this platform, rheological parameters can be set to mimic different vascular districts including distal post-capillary venules when combined in modular sequence (167). Under these controlled hemodynamic conditions combined with exposure to glial cells, ECs acquire more stringent BBB properties than static platform. Such properties include low permeability to paracellular markers and high TEER (168), negligible extravasation of proteins, physiological/polarized distribution and expression of specialized transporters (169) and efflux systems (118). Further, cell viability and maintenance of BBB properties is retain for an extended period of time up to several months under optimal culture conditions. This enable longer term studies otherwise unfeasible in other BBB platforms. In addition, the DIV-BBB allows for circulation of blood cells thus facilitating the study of CNS disorders related to altered rheological conditions like stroke, ischemia/reperfusion injuries and inflammation (170).
To counterbalance the appealing advantages the DIV-BBB provides over conventional static systems there are several drawbacks to consider prior using this platform. Since the model relies on capillary-like tubes surrounded by a larger enclosure, no practical way exists to visualize the cells cultured on or within the artificial microvessels. Therefore, experiments need to be terminated and the microvessels forcefully removed (permanently damaging the BBB module) to carry out various types of visual/morphological examination. In addition to that the relative large diameter of the capillaries compared to brain microvessel is more representative of larger vascular bed like distal per and post capillary segments. Use of lipophilic material such as polypropylene in the manufacturing of the artificial vessel could hinder drug transport studies of lipophilic drugs. Furthermore, the scale of the device requires a large volume of reagents and high quantities of cells (on the magnitude of >106) for culture initiation. The DIV-BBB does not have HTS capabilities in its current format and the initial setup of the system requires a large amount of technical skills, time and resources which is more than conventional platforms like the Transwell need. From an experimental and user demand point of view, limited choice of hollow fiber typologies (e.g., materials and pore Ø) further reduce the flexibility and/or usability of this model.
CNS Drug Screening and the Need for more Advanced In Vitro Cerebrovascular Models
Current in vitro cerebrovascular models typically fall short of providing translational results at the required turnover rate and cost due to their lack of HTS capabilities, realistically complex multi-cultures and a proper microenvironment. Microfluidic systems have been developed to reproduce physiological cues necessary to generate an optimal culture microenvironment within an in vitro platform containing the ideal balance of high throughput screening capabilities with more physiologically relevant scale, dynamics and complexity. If these platforms are widely adopted, they could potentially address many of the current needs of the pharmaceutical industry, thus facilitating the streamlined production of novel and more effective CNS drugs at a contained cost.
Microfluidic platforms may improve translational relevance, accuracy and cost effectiveness of in vitro modeling
Microfluidic systems critically address some concerns of static and bulky systems to accurately mimic the spatial, mechanical, and physiological conditions found in vivo. Microfluidic-based platforms also enable low-cost screening advantages due to low volumes and higher throughput, provide high-resolution imaging, and open up opportunities to integrate measurement systems to dynamically monitor cerebrovascular interface integrity. The possibility of dynamically monitoring TEER or electric impedance, an important indicator of intercellular tight junction functionality in cerebrovascular models, provides further benefits of a platform designed around microfluidics (171). Microfluidics offer an improved alternative to the current Transwell® and dynamic cerebrovascular models that lack the apparent functionality, complexity and reproducibility required for a suitable, HTS-capable CNS drug screening model.
Current Microfluidic Models
Over the past several years, a variety of unique microfluidic-based cerebrovascular models have been developed to accomplish a shared goal; a more physiologically relevant model on a miniaturized scale. Though most platforms described are designed around a poly(dimethylsiloxide/glass interface incorporating a porous membrane at the intersection of two microfluidic channels, the models vary in complexity, functionality and targeted applications (Table 1). Follows are summaries of some of the pros and cons of each of the prevalent microfluidic models recently proposed.
Table 1. Summary of Microfluidic Cerebrovascular Models.
| Model Name | Cell Types Used | Max TEER Achieved (Q cm2) | Markers Examined | Functional Barrier | Flow: Shear Stress (dyne/cm2) |
|---|---|---|---|---|---|
| Microfluidic BBB (uBBB) | b.End3; C8D1A | 250 | ZO-1, GFAP | Recovery from histamine exposure | Y: 2.3×10-2 |
| BBB-on-a-Chip (BBB-Chip) | hCMEC/D3 | 120 | ZO-1 | TNF-a response | 5.8 |
| Neurovascular Unit-on-a-Chip (NVU-Chip) | RBE4 + Primary rat astrocytes, microglia and neurons | N/A | ZO-1, VWF, GFAP, MAP-2, OX-42 | TNF-a response | N/A |
| Synthetic Microvasculature BBB (SyM-BBB) | RBE4 | N/A | ZO-1 Claudin-1 P-gP | P-gp efflux | 4.4×10-2 |
Microfluidic Blood-Brain Barrier (μBBB)
Designed to mimic in vivo BBB traits such dynamic culture while improving HTS capabilities, ths μBBB is made up of a total of four PDMS layers encompassing two embedded electrode layers. The platform provides flow capability for both the luminal and abluminal channels as well as built in electrodes for ease-of-use and real-time TEER measurements (Fig. 7a) (172). When co-culturing mouse-derived endothelial and astrocyte cell lines (b.End3 and C8D1A respectively) under flow, TEER values often exceeded 250 Ω.cm2, compared to 25 Ω.cm2 in parallel static co-cultures, and permeability coefficients correlated well with examined compounds' Stokes Radii. Furthermore, the co-culture in the μBBB chip demonstrated a functional barrier by responding to and recovering from exposure to a 150 μM solution of histamine which is a known barrier disruptor (173). The μBBB provides an inexpensive and reproducible microfluidic platform capable of establishing a physiologically relevant cerebrovascular model using immortalized endothelium and astrocytes. The use of PDMS enables clear imaging and built in electrodes allow for real-time measurements of TEER. However, due to shear stress forces used for the initial μBBB tests (2.3 × 10-2 dyne/cm2) being significantly lower than that measured in brain microcapillaries in vivo (∼5-20 dyne/cm2), the platform's maximum flow capabilities have yet to be reported (166) (174). Additionally, published work only characterizes the platform using cells from mouse-derived cell lines; further work is needed to confirm the feasibility of culturing human-derived cells within the μBBB. In order to improve the relevance of such a model, these questions would need to be addressed.
Figure 7. Schematic illustrations of various microfluidic platforms.
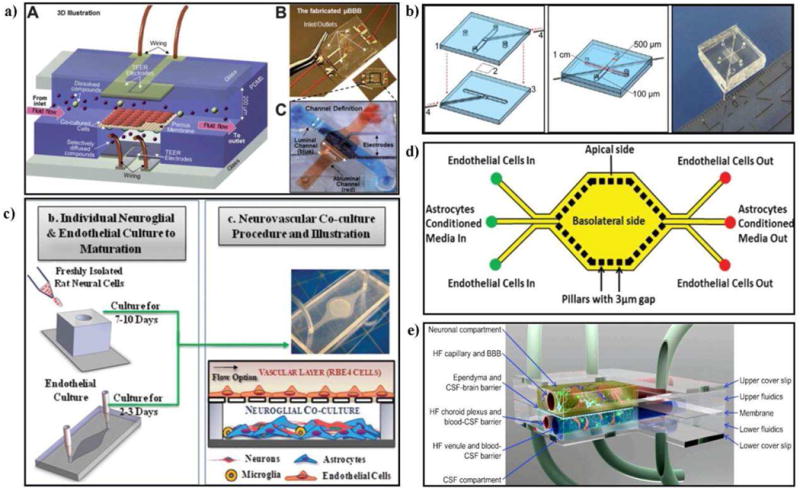
(A) μBBB design and device. It consists of two perpendicular microfluidic channels with a 10 mm2 culturing area and built-in electrodes for TEER measurements. Reproduced from (172) with permission of The Royal Society of Chemistry (RSC). (B) BBB-chip design and device. It consists of two cperpendicular microfluidic channels with a 0.25 mm2 culturing area and electrode insertion channels. Reproduced from (174) with permission from Springer US. (C) NVU-chip design, device and culture diagram. Modular chip made up of neural and vascular components including several cell types. (D) SyM-BBB design. Apical and basolateral chambers each with flow capabilities. 3um micro-gaps built into PDMS layer to act as pores allowing fluid interchange between chambers. Reproduced from (176) with permission from The Royal Society of Chemistry (RSC). (E) Proposed concept of advanced NVU model. Dynamic system consisting of central nervous system and cerebral spinal fluid compartments with associated fluid barriers. Reproduced from (175) with permission from BMC Central.
Blood-Brain Barrier-on-a-chip (BBB-chip)
The smallest BBB-based microfluidic platform to date, the BBB-chip is also made up of two PDMS layers each containing grooves to hold Pt electrodes which are separated by a 10 μm thick polycarbonate membrane containing 0.4 μm pores (174). When assembled, two channels (1 cm long × 500 μm wide × 100 μm high) each with flow capabilities are formed and run perpendicular to each other with a cross sectional area of 0.25 mm2 (Fig. 7b). The simplified design of a BBB-chip is an easy and straightforward platform for BBB modeling, providing optical clarity, flow-culture capabilities and accessible TEER measurements. Shear stress values examined were at magnitudes considered physiologically relevant (5.8 dynes/cm2), proving the device can be a solid foundation for future flow-based studies. However, only limited characterization data has been presented to provide a proof-of-concept for the BBB-chip; more complex studies such as examining barrier functionality/permeability and long-term (co)-culture response to shear stress will need to be conducted to truly validate the platform. Lastly, current data solely provides insight into the monoculture of a human-derived cell line, which is only the first step towards a more complete BBB model.
Neurovascular Unit-on-a-Chip (NVU-chip)
A more complex microfluidic platform, the Neurovascular Unit-on-a-Chip (NVU-chip), differs from the previously discussed platforms by its unique modular-based design allowing for complex multi-cultures (139). The chip is comprised of ‘neural’ (neurons, astrocytes and microglia) and ‘vascular’(endothelial cells) sides which are both initially seeded separately at different times and are then combined to form one NVU-chip (Fig. 7c). This platform permits the study of CNS neuron interactions with the cerebral spinal fluid (CSF). Further, the model incorporates a blood-surrogate supply, including a venous return system, circulating immune cells and the choroid plexus (175). To assess barrier tightness and function, permeability and biochemical modulation assays involving the exposure of the barrier to TNF-α were conducted. An approximate 2-fold Increase of fluorescently-labeled dextran permeability in the presence of TNF-α was observed confirming an active and responsive barrier. Additionally, TNF-α introduced to the vascular side stimulated microglia and astrocyte activation on the neural side shown by changes in microglia morphology and significant increase in glial fibrillary acidic protein (GFAP) expression respectively thus, providing evidence for neurovascular communication; a crucial factor in CNS drug screening. However, shear stress was not incorporated into initial experiments due to problems associated with leaky device bonds at high (physiological) pressures. The absence of shear stress cannot be ignored due to its reported and significant role in influencing BBB properties in vivo. Furthermore, the initial design of the NVU-chip limits culture times due to a lack of access to the neural side once the device is assembled and does not allow for TEER measurements to be taken. Though limiting, a majority of these negative caveats can be addressed through vast changes in device design. Despite these drawbacks, the NVU-chip provides a solid foundation on which future complex, multi-culture cerebrovascular models can be built upon.
Synthetic Microvasculature Blood-Brain Barrier (SyM-BBB)
The synthetic microvasculature blood-brain barrier model (SyM-BBB), consisting of adjacent “apical” and “basolateral” channels in the same horizontal plane, deviates slightly from the aforementioned microfluidic platforms in its design and concept (176). To create the channels connecting the flow-capable apical and basolateral sides of the device, 3 μm × 3 μm × 50 μm channels were casted into the PDMS frame. PDMS is a widely utilized elastomeric material that offers a great versatility for manufacturing culture substrates (either planar surfaces or micro-channels). However, at the micron scale, channel deformation effect can become important and must be quantified for predictable assay performance since it may lead to channel deformation (177). However, Depending upon the crosslinking level of the PDMS (curing conditions) the rigidity of the material can be increased to values > 4MPa thus, decreasing the amount of channel deformation under a given flow rate and consequently reducing unwanted alteration of the shear stress (177). Depending on the needs it is also possible to generate variable rigidity surfaces for mechanobiology measurements (178). These channels mimic the 3 μm pores commonly found on Transwell® membranes and allow for fluid interchange between channels (Fig. 7d). Results indicated that endothelium exposed to astrocyte-conditioned medium (ACM) formed tighter barriers by excluding greater levels of dextran from permeating into the basolateral channel compared to control samples (137). Cells seeded in the SyM-BBB also expressed significantly higher levels of ZO-1and P-glycoprotein when compared to static Transwell® cultures. Furthermore, P-gp efflux activity was confirmed by observing uninhibited function versus a significant drop in efflux function in the presence of verapamil, a known P-gp inhibitor (179). By design, such a platform enables continuous perfusion of media to and in between both apical and basolateral chambers as well as complete optical clarity. On the other hand, TEER measurements cannot be taken when working with the SyM-BBB due to design, and the channels connecting both chambers of the device are 50 μm in length which is far larger than the barriers between cells observed in vivo or when using Transwell®-based membranes. As with the other platforms, the SyM-BBB provides sufficient and novel BBB-modeling capabilities, but requires further characterization and inclusion of primary and mixed cell types.
Prospective development in BBB modeling
3D ECM matrices for BBB modeling
Three-dimensional extra cellular matrices-based scaffolds (3D ECM) allow for in vitro recapitulation of many physiological and structural aspects of the native or in situ cellular microenvironment. 3D ECM platforms have been widely used in drug discovery and solute transport studies (180), stem cell differentiation studies (181, 182), and studies of the patterns of migration and invasion of cancerous cells into surrounding tissues (183).
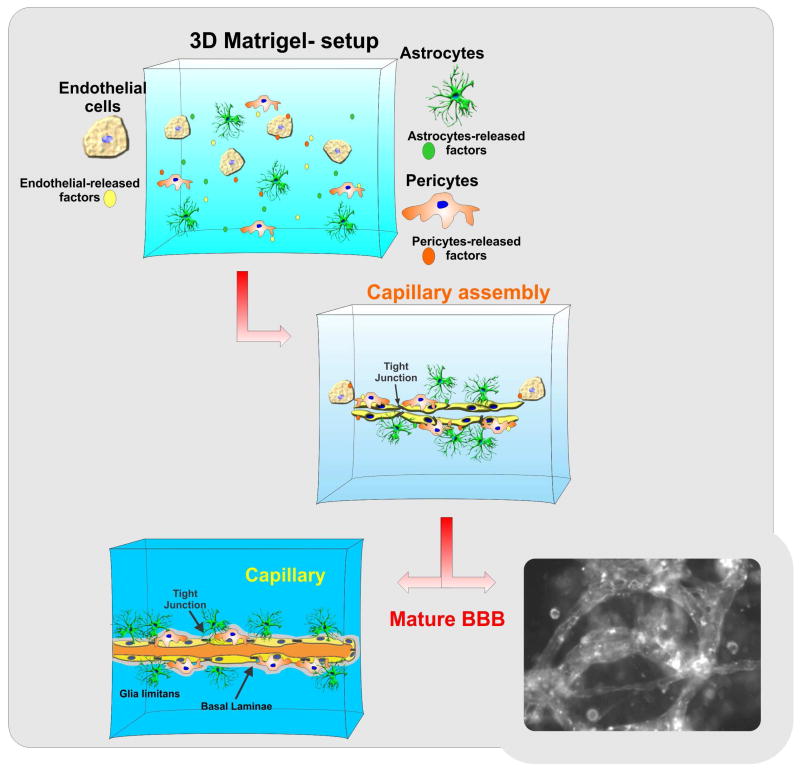
Use of 3D ECM in neurobiology and cerebrovascular research however, is still limited. One of the major benefits of using 3D ECM culture is the ability to reproduce the hierarchical biological makeup of brain micromicrovessels and/or more complex neurovascular units (184) which cannot be effectively reproduced in 2D culture environments. 3D ECM platforms are permissive for the formation of biochemical gradient of trophic factors and other biologically active molecules necessary for cell-cell communication and cross signaling. In vivo-like cell-cell and cell-matrix interactions (185) that closely resemble the physiological interactions observed in situ are also enabled (see also Fig. 8). 3D ECM models incorporating microfluidic systems have found direct application in cancer research to study the migration and penetration of metastatic tumor cells (186, 187) and dissect out the complex mechanisms of angiogenesis. Cellular dynamic changes in the 3D ECM microenvironments can be monitored in real time using high-resolution 3D imaging techniques based on confocal or multiphoton microscopy as well as optical coherence tomography. Recently a technique based on magnetic resonance imaging has been developed to assess cell movement and ECM interactions (188).
Figure 8. Schematic illustration of a 3D ECM BBB model.
Microcapillary like structures forms within the 3D matrix. This platform allows the reproduction of physiological-like interactions between cells as well as natural gradients of promoting factors for vasculogenesis/angiogenesis and/or cell migration.
On the negative side, developing an in vivo-like matrix architecture is quite a complex process not yet fully addressed. Cell-derived matrices can reproduce the physiological gaps found in the native ECM to accommodate the cells (189) and allow for cell migration. These reconstitute biological matrices may lack important ECM factors that can impair the architectural assembly and the associated matrix properties. Reconstituted ECM matrix from animal derived sources is subject to a significant variability between lots, decreasing reproducibility and introducing possible contaminants. Alternatively, synthetic materials (e.g., hydrogels) are available to create defined 3D microenvironment. Hydrogels such as Puramatrix™ are a peptide material composed of 99% water and 1% w/v standard amino acids which then self-assemble into a 3D hydrogel with nanometer-scale fibrous structures under physiological conditions. This material is biocompatible and devoid of animal-derived contaminants. However, identifying the biophysical and biochemical cues that need to be incorporated in the ECM scaffolds, including trophic factors, nutrients and other bioactive molecules, is of critical importance to achieve the desired cell growth and differentiation level. This task requires many steps of optimization and can negatively impact data reproducibility across different laboratories. Furthermore, nanofibrous scaffolds may not be strong enough to withstand the mechanical stimuli (such as shear stress) needed to activate downstream biological stimuli (190).
Conclusion and final remarks
The recently developed microfluidic platforms provide the early foundation necessary to develop a cerebrovascular model that emulates in vivo conditions while maintaining an accurate microenvironment and high-throughput capabilities. Though only a proposed concept at this time, currently in development is a novel neurovascular unit-on-a-chip that incorporates cerebral spinal fluid and blood-brain barrier compartments alongside a CNS compartment to further reproduce the dynamic biological interfaces encompassing the BBB and CNS interfaces. (Fig. 7e) (191). The chip is being designed to enable flow throughout each compartment allowing for shear stress and immune cell circulation as well as massive parallelization for increased screening capabilities. Such a complex chip may allow for more accurate in vitro investigation of key physiological phenomena including inflammatory response mechanisms and drug pharmacokinetics. Further progress is still required, however. Though cost and material-saving benefits are linked with them, the small volumes associated with microfluidic-based devices often are insufficient for applicable downstream drug analysis. Furthermore, PDMS, a common material used in all reported microfluidic BBB models, has been shown to allow significant diffusion of hydrophobic and fluorescent molecules leading to complications revolving around solute concentration-dependent studies (192). Unfortunately, even a near-perfect cerebrovascular in vitro model will not provide absolute insight into the true effects seen in vivo of a given drug because the BBB is only a single interface at which the drug interacts with the recipient.
In a human body, drugs interact with a number of organs and systems while simultaneously undergoing sophisticated processes involving absorption, distribution, metabolism and elimination (193). Largely due to the advances in microfluidic technologies, recent progress in ‘organ-on-a-chip’ technology has been made towards the development of improved in vitro models capable of reproducing key human physiological responses, including multi organ interaction, with a significantly higher level of complexity than current platforms (194). The ‘human-on-a-chip’ system was conceptualized revolving around linking a variety of individual organ models together in a precise manner and using it as a platform to observe a drug's physiological effects on the body as a whole in vitro or to reproduce and monitor physiological interactions (193, 195). Such a predictive apparatus could revolutionize drug development and screening by more accurately clarifying the therapeutic and pathological side effects of a drug before costly in vivo or clinical trials are done, ultimately increasing drug development efficiency and quality while reducing costs. Advancement of an improved cerebrovasculature model with affordable HTS capabilities that incorporates key cell-cell interactions, 3D tissue structure, and an accurate microenvironment is an important step towards improving the current and future of drug permeability, toxicity and efficiency screening efforts.
Acknowledgments
This work was supported by NIH/NIDA R01-DA029121-01A1 and Alternative Research Development Foundation (A.R.D.F.) to Dr. Luca Cucullo.
Footnotes
Conflict of interest: The authors declare that they have no competing interests.
References
- 1.Banks WA. Blood-brain barrier as a regulatory interface. Forum Nutr. 2010;63:102–110. doi: 10.1159/000264398. [DOI] [PubMed] [Google Scholar]
- 2.Nag S, Kapadia A, Stewart DJ. Review: molecular pathogenesis of blood-brain barrier breakdown in acute brain injury. Neuropathol Appl Neurobiol. 2011;37:3–23. doi: 10.1111/j.1365-2990.2010.01138.x. [DOI] [PubMed] [Google Scholar]
- 3.Cucullo L, Hossain M, Puvenna V, Marchi N, Janigro D. The role of shear stress in Blood-Brain Barrier endothelial physiology. BMC Neurosci. 2011;12:40. doi: 10.1186/1471-2202-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B. Review: leucocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol. 2011;37:24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 5.Acharya NK, Levin EC, Clifford PM, Han M, Tourtellotte R, Chamberlain D, Pollaro M, Coretti NJ, Kosciuk MC, Nagele EP, Demarshall C, Freeman T, Shi Y, Guan C, Macphee CH, Wilensky RL, Nagele RG. Diabetes and hypercholesterolemia increase blood-brain barrier permeability and brain amyloid deposition: beneficial effects of the LpPLA2 inhibitor darapladib. J Alzheimers Dis. 2013;35:179–198. doi: 10.3233/JAD-122254. [DOI] [PubMed] [Google Scholar]
- 6.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 7.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 8.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, Leybaert L, Molnar Z, O'Donnell ME, Povlishock JT, Saunders NR, Sharp F, Stanimirovic D, Watts RJ, Drewes LR. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12:169–182. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ElAli A, Theriault P, Rivest S. The role of pericytes in neurovascular unit remodeling in brain disorders. Int J Mol Sci. 2014;15:6453–6474. doi: 10.3390/ijms15046453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muoio V, Persson PB, Sendeski MM. The neurovascular unit - concept review. Acta Physiol (Oxf) 2014;210:790–798. doi: 10.1111/apha.12250. [DOI] [PubMed] [Google Scholar]
- 12.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Piontek J, Fritzsche S, Cording J, Richter S, Hartwig J, Walter M, Yu D, Turner JR, Gehring C, Rahn HP, Wolburg H, Blasig IE. Elucidating the principles of the molecular organization of heteropolymeric tight junction strands. Cell Mol Life Sci. 2011;68:3903–3918. doi: 10.1007/s00018-011-0680-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 15.Schinkel AH. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv Drug Deliv Rev. 1999;36:179–194. doi: 10.1016/s0169-409x(98)00085-4. [DOI] [PubMed] [Google Scholar]
- 16.Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE. ABCG2: a perspective. Adv Drug Deliv Rev. 2009;61:3–13. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts LM, Black DS, Raman C, Woodford K, Zhou M, Haggerty JE, Yan AT, Cwirla SE, Grindstaff KK. Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience. 2008;155:423–438. doi: 10.1016/j.neuroscience.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh C, Marchi N, Desai NK, Puvenna V, Hossain M, Gonzalez-Martinez J, Alexopoulos AV, Janigro D. Cellular localization and functional significance of CYP3A4 in the human epileptic brain. Epilepsia. 2011;52:562–571. doi: 10.1111/j.1528-1167.2010.02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh C, Hossain M, Puvenna V, Martinez-Gonzalez J, Alexopolous A, Janigro D, Marchi N. Expression and functional relevance of UGT1A4 in a cohort of human drug-resistant epileptic brains. Epilepsia. 2013;54:1562–1570. doi: 10.1111/epi.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6:591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 21.Daneman R. The blood-brain barrier in health and disease. Ann Neurol. 2012;72:648–672. doi: 10.1002/ana.23648. [DOI] [PubMed] [Google Scholar]
- 22.Tomkins O, Shelef I, Kaizerman I, Eliushin A, Afawi Z, Misk A, Gidon M, Cohen A, Zumsteg D, Friedman A. Blood-brain barrier disruption in post-traumatic epilepsy. J Neurol Neurosurg Psychiatry. 2008;79:774–777. doi: 10.1136/jnnp.2007.126425. [DOI] [PubMed] [Google Scholar]
- 23.Bennett J, Basivireddy J, Kollar A, Biron KE, Reickmann P, Jefferies WA, McQuaid S. Blood-brain barrier disruption and enhanced vascular permeability in the multiple sclerosis model EAE. J Neuroimmunol. 2010;229:180–191. doi: 10.1016/j.jneuroim.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Bednarczyk J, Lukasiuk K. Tight junctions in neurological diseases. Acta Neurobiol Exp (Wars) 2011;71:393–408. doi: 10.55782/ane-2011-1861. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8:205–216. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- 26.Poduslo JF, Curran GL, Wengenack TM, Malester B, Duff K. Permeability of proteins at the blood-brain barrier in the normal adult mouse and double transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2001;8:555–567. doi: 10.1006/nbdi.2001.0402. [DOI] [PubMed] [Google Scholar]
- 27.Kovacs R, Heinemann U, Steinhauser C. Mechanisms underlying blood-brain barrier dysfunction in brain pathology and epileptogenesis: role of astroglia. Epilepsia. 2012;53(Suppl 6):53–59. doi: 10.1111/j.1528-1167.2012.03703.x. [DOI] [PubMed] [Google Scholar]
- 28.Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 29.Waubant E. Biomarkers indicative of blood-brain barrier disruption in multiple sclerosis. Dis Markers. 2006;22:235–244. doi: 10.1155/2006/709869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su JJ, Osoegawa M, Matsuoka T, Minohara M, Tanaka M, Ishizu T, Mihara F, Taniwaki T, Kira J. Upregulation of vascular growth factors in multiple sclerosis: correlation with MRI findings. J Neurol Sci. 2006;243:21–30. doi: 10.1016/j.jns.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Manzanero S, Santro T, Arumugam TV. Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem Int. 2013;62:712–718. doi: 10.1016/j.neuint.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer's disease. Neurosci Lett. 2010;469:6–10. doi: 10.1016/j.neulet.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 33.Starr JM, Farrall AJ, Armitage P, McGurn B, Wardlaw J. Blood-brain barrier permeability in Alzheimer's disease: a case-control MRI study. Psychiatry Res. 2009;171:232–241. doi: 10.1016/j.pscychresns.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Carrano A, Hoozemans JJ, van der Vies SM, Rozemuller AJ, van HJ, de Vries HE. Amyloid Beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid Redox Signal. 2011;15:1167–1178. doi: 10.1089/ars.2011.3895. [DOI] [PubMed] [Google Scholar]
- 35.Kook SY, Hong HS, Moon M, Ha CM, Chang S, Mook-Jung I. Abeta(1)(-)(4)(2)-RAGE interaction disrupts tight junctions of the blood-brain barrier via Ca(2)(+)-calcineurin signaling. J Neurosci. 2012;32:8845–8854. doi: 10.1523/JNEUROSCI.6102-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartels AL. Blood-brain barrier P-glycoprotein function in neurodegenerative disease. Curr Pharm Des. 2011;17:2771–2777. doi: 10.2174/138161211797440122. [DOI] [PubMed] [Google Scholar]
- 37.Pillai DR, Dittmar MS, Baldaranov D, Heidemann RM, Henning EC, Schuierer G, Bogdahn U, Schlachetzki F. Cerebral ischemia-reperfusion injury in rats--a 3 T MRI study on biphasic blood-brain barrier opening and the dynamics of edema formation. J Cereb Blood Flow Metab. 2009;29:1846–1855. doi: 10.1038/jcbfm.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiao H, Wang Z, Liu Y, Wang P, Xue Y. Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult. J Mol Neurosci. 2011;44:130–139. doi: 10.1007/s12031-011-9496-4. [DOI] [PubMed] [Google Scholar]
- 39.Barr TL, Latour LL, Lee KY, Schaewe TJ, Luby M, Chang GS, El-Zammar Z, Alam S, Hallenbeck JM, Kidwell CS, Warach S. Blood-brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke. 2010;41:e123–e128. doi: 10.1161/STROKEAHA.109.570515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayer CL, Huber BR, Peskind E. Traumatic brain injury, neuroinflammation, and post-traumatic headaches. Headache. 2013;53:1523–1530. doi: 10.1111/head.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res. 2011;2:492–516. doi: 10.1007/s12975-011-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoepner R, Faissner S, Salmen A, Gold R, Chan A. Efficacy and Side Effects of Natalizumab Therapy in Patients with Multiple Sclerosis. J Cent Nerv Syst Dis. 2014;6:41–49. doi: 10.4137/JCNSD.S14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson KP. Natalizumab (Tysabri) treatment for relapsing multiple sclerosis. Neurologist. 2007;13:182–187. doi: 10.1097/01.nrl.0000263760.53418.5b. [DOI] [PubMed] [Google Scholar]
- 44.Gurses C, Orhan N, Ahishali B, Yilmaz CU, Kemikler G, Elmas I, Cevik A, Kucuk M, Arican N, Kaya M. Topiramate reduces blood-brain barrier disruption and inhibits seizure activity in hyperthermia-induced seizures in rats with cortical dysplasia. Brain Res. 2013;1494:91–100. doi: 10.1016/j.brainres.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 45.Li YJ, Wang ZH, Zhang B, Zhe X, Wang MJ, Shi ST, Bai J, Lin T, Guo CJ, Zhang SJ, Kong XL, Zuo X, Zhao H. Disruption of the blood-brain barrier after generalized tonic-clonic seizures correlates with cerebrospinal fluid MMP-9 levels. J Neuroinflammation. 2013;10:80. doi: 10.1186/1742-2094-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamidou B, Aboa-Eboule C, Durier J, Jacquin A, Lemesle-Martin M, Giroud M, Bejot Y. Prognostic value of early epileptic seizures on mortality and functional disability in acute stroke: the Dijon Stroke Registry (1985-2010) J Neurol. 2013;260:1043–1051. doi: 10.1007/s00415-012-6756-3. [DOI] [PubMed] [Google Scholar]
- 47.Miller DS, Nobmann SN, Gutmann H, Toeroek M, Drewe J, Fricker G. Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol. 2000;58:1357–1367. doi: 10.1124/mol.58.6.1357. [DOI] [PubMed] [Google Scholar]
- 48.Shen S, Zhang W. ABC transporters and drug efflux at the blood-brain barrier. Rev Neurosci. 2010;21:29–53. doi: 10.1515/revneuro.2010.21.1.29. [DOI] [PubMed] [Google Scholar]
- 49.Ghosh C, Puvenna V, Gonzalez-Martinez J, Janigro D, Marchi N. Blood-Brain Barrier P450 Enzymes and Multidrug Transporters in Drug Resistance: A Synergistic Role in Neurological Diseases. Curr Drug Metab. 2011 doi: 10.2174/138920011798357051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simka M. Blood brain barrier compromise with endothelial inflammation may lead to autoimmune loss of myelin during multiple sclerosis. Curr Neurovasc Res. 2009;6:132–139. doi: 10.2174/156720209788185605. [DOI] [PubMed] [Google Scholar]
- 52.Marchi N, Granata T, Ghosh C, Janigro D. Blood-brain barrier dysfunction and epilepsy: pathophysiologic role and therapeutic approaches. Epilepsia. 2012;53:1877–1886. doi: 10.1111/j.1528-1167.2012.03637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alavijeh MS, Chishty M, Qaiser MZ, Palmer AM. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. Neurorx. 2005;2:554–571. doi: 10.1602/neurorx.2.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark DE. In silico prediction of blood-brain barrier permeation. Drug Discov Today. 2003;8:927–933. doi: 10.1016/s1359-6446(03)02827-7. [DOI] [PubMed] [Google Scholar]
- 55.Zhang EY, Phelps MA, Cheng C, Ekins S, Swaan PW. Modeling of active transport systems. Adv Drug Deliv Rev. 2002;54:329–354. doi: 10.1016/s0169-409x(02)00007-8. [DOI] [PubMed] [Google Scholar]
- 56.Sippl W. Development of biologically active compounds by combining 3D QSAR and structure-based design methods. J Comput Aided Mol Des. 2002;16:825–830. doi: 10.1023/a:1023888813526. [DOI] [PubMed] [Google Scholar]
- 57.Wu ZY, Pan J, Yuan Y, Hui AL, Yang Y, Zhou A. Comparison of prediction models for blood brain barrier permeability and analysis of the molecular descriptors. Pharmazie. 2012;67:628–634. [PubMed] [Google Scholar]
- 58.Narayanan R, Gunturi SB. In silico ADME modelling: prediction models for blood-brain barrier permeation using a systematic variable selection method. Bioorg Med Chem. 2005;13:3017–3028. doi: 10.1016/j.bmc.2005.01.061. [DOI] [PubMed] [Google Scholar]
- 59.Rohrig UF, Awad L, Grosdidier A, Larrieu P, Stroobant V, Colau D, Cerundolo V, Simpson AJ, Vogel P, Van den Eynde BJ, Zoete V, Michielin O. Rational design of indoleamine 2,3-dioxygenase inhibitors. J Med Chem. 2010;53:1172–1189. doi: 10.1021/jm9014718. [DOI] [PubMed] [Google Scholar]
- 60.Dolusic E, Larrieu P, Blanc S, Sapunaric F, Norberg B, Moineaux L, Colette D, Stroobant V, Pilotte L, Colau D, Ferain T, Fraser G, Galleni M, Frere JM, Masereel B, Van den EB, Wouters J, Frederick R. Indol-2-yl ethanones as novel indoleamine 2,3-dioxygenase (IDO) inhibitors. Bioorg Med Chem. 2011;19:1550–1561. doi: 10.1016/j.bmc.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 61.Wu ZY, Pan J, Yuan Y, Hui AL, Yang Y, Zhou A. Comparison of prediction models for blood brain barrier permeability and analysis of the molecular descriptors. Pharmazie. 2012;67:628–634. [PubMed] [Google Scholar]
- 62.Malmborg J, Ploeger BA. Predicting human exposure of active drug after oral prodrug administration, using a joined in vitro/in silico-in vivo extrapolation and physiologically-based pharmacokinetic modeling approach. J Pharmacol Toxicol Methods. 2013;67:203–213. doi: 10.1016/j.vascn.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Martins IF, Teixeira AL, Pinheiro L, Falcao AO. A Bayesian approach to in silico blood-brain barrier penetration modeling. J Chem Inf Model. 2012;52:1686–1697. doi: 10.1021/ci300124c. [DOI] [PubMed] [Google Scholar]
- 64.Fortuna A, Alves G, Soares-da-Silva P, Falcao A. Pharmacokinetics, brain distribution and plasma protein binding of carbamazepine and nine derivatives: new set of data for predictive in silico ADME models. Epilepsy Res. 2013;107:37–50. doi: 10.1016/j.eplepsyres.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 65.Ecker GF, Noe CR. In silico prediction models for blood-brain barrier permeation. Curr Med Chem. 2004;11:1617–1628. doi: 10.2174/0929867043365071. [DOI] [PubMed] [Google Scholar]
- 66.Hidalgo IJ. Assessing the absorption of new pharmaceuticals. Curr Top Med Chem. 2001;1:385–401. doi: 10.2174/1568026013395010. [DOI] [PubMed] [Google Scholar]
- 67.Pidgeon C, Ong S, Liu H, Qiu X, Pidgeon M, Dantzig AH, Munroe J, Hornback WJ, Kasher JS, Glunz L. IAM chromatography: an in vitro screen for predicting drug membrane permeability. J Med Chem. 1995;38:590–594. doi: 10.1021/jm00004a004. [DOI] [PubMed] [Google Scholar]
- 68.Ong S, Liu H, Pidgeon C. Immobilized-artificial-membrane chromatography: measurements of membrane partition coefficient and predicting drug membrane permeability. J Chromatogr A. 1996;728:113–128. doi: 10.1016/0021-9673(95)00837-3. [DOI] [PubMed] [Google Scholar]
- 69.Pidgeon C, Stevens J, Otto S, Jefcoate C, Marcus C. Immobilized artificial membrane chromatography: rapid purification of functional membrane proteins. Anal Biochem. 1991;194:163–173. doi: 10.1016/0003-2697(91)90164-o. [DOI] [PubMed] [Google Scholar]
- 70.Ong S, Liu H, Pidgeon C. Immobilized-artificial-membrane chromatography: measurements of membrane partition coefficient and predicting drug membrane permeability. J Chromatogr A. 1996;728:113–128. doi: 10.1016/0021-9673(95)00837-3. [DOI] [PubMed] [Google Scholar]
- 71.Giaginis C, Tsantili-Kakoulidou A. Alternative measures of lipophilicity: from octanol-water partitioning to IAM retention. J Pharm Sci. 2008;97:2984–3004. doi: 10.1002/jps.21244. [DOI] [PubMed] [Google Scholar]
- 72.Chaves C, Shawahna R, Jacob A, Scherrmann JM, Decleves X. Human ABC Transporters at blood-CNS Interfaces as Determinants of CNS Drug Penetration. Curr Pharm Des. 2013 doi: 10.2174/13816128113199990466. [DOI] [PubMed] [Google Scholar]
- 73.Avdeef A. The rise of PAMPA. Expert Opin Drug Metab Toxicol. 2005;1:325–342. doi: 10.1517/17425255.1.2.325. [DOI] [PubMed] [Google Scholar]
- 74.Sinko B, Garrigues TM, Balogh GT, Nagy ZK, Tsinman O, Avdeef A, Takacs-Novak K. Skin-PAMPA: a new method for fast prediction of skin penetration. Eur J Pharm Sci. 2012;45:698–707. doi: 10.1016/j.ejps.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 75.Nishimuta H, Sato K, Yabuki M, Komuro S. Prediction of the intestinal first-pass metabolism of CYP3A and UGT substrates in humans from in vitro data. Drug Metab Pharmacokinet. 2011;26:592–601. doi: 10.2133/dmpk.DMPK-11-RG-034. [DOI] [PubMed] [Google Scholar]
- 76.Mensch J, LJ L, Sanderson W, Melis A, Mackie C, Verreck G, Brewster ME, Augustijns P. Application of PAMPA-models to predict BBB permeability including efflux ratio, plasma protein binding and physicochemical parameters. Int J Pharm. 2010 doi: 10.1016/j.ijpharm.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 77.Vizseralek G, Balogh T, Takacs-Novak K, Sinko B. PAMPA study of the temperature effect on permeability. Eur J Pharm Sci. 2014;53:45–49. doi: 10.1016/j.ejps.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 78.Balogh GT, Muller J, Konczol A. pH-gradient PAMPA-based in vitro model assay for drug-induced phospholipidosis in early stage of drug discovery. Eur J Pharm Sci. 2013;49:81–89. doi: 10.1016/j.ejps.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 79.Mensch J, Melis A, Mackie C, Verreck G, Brewster ME, Augustijns P. Evaluation of various PAMPA models to identify the most discriminating method for the prediction of BBB permeability. Eur J Pharm Biopharm. 2010;74:495–502. doi: 10.1016/j.ejpb.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 80.Avdeef A, Tsinman O. PAMPA--a drug absorption in vitro model 13. Chemical selectivity due to membrane hydrogen bonding: in combo comparisons of HDM-, DOPC-, and DS-PAMPA models. Eur J Pharm Sci. 2006;28:43–50. doi: 10.1016/j.ejps.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 81.Nag Sukriti. The Blood -Brain Barrier : Biology and Research Protocols. Humana Press; 2003. pp. 233–247. [Google Scholar]
- 82.Sanchez del Pino MM, Hawkins RA, Peterson DR. Neutral amino acid transport by the blood-brain barrier. Membrane vesicle studies. J Biol Chem. 1992;267:25951–25957. [PubMed] [Google Scholar]
- 83.Lee WJ, Peterson DR, Sukowski EJ, Hawkins RA. Glucose transport by isolated plasma membranes of the bovine blood-brain barrier. Am J Physiol. 1997;272:C1552–C1557. doi: 10.1152/ajpcell.1997.272.5.C1552. [DOI] [PubMed] [Google Scholar]
- 84.Glavinas H, Mehn D, Jani M, Oosterhuis B, Heredi-Szabo K, Krajcsi P. Utilization of membrane vesicle preparations to study drug-ABC transporter interactions. Expert Opin Drug Metab Toxicol. 2008;4:721–732. doi: 10.1517/17425255.4.6.721. [DOI] [PubMed] [Google Scholar]
- 85.Silbergeld DL, Ali-Osman F. Isolation and characterization of microvessels from normal brain and brain tumors. J Neurooncol. 1991;11:49–55. doi: 10.1007/BF00166997. [DOI] [PubMed] [Google Scholar]
- 86.Orte C, Lawrenson JG, Finn TM, Reid AR, Allt G. A comparison of blood-brain barrier and blood-nerve barrier endothelial cell markers. Anat Embryol (Berl) 1999;199:509–517. doi: 10.1007/s004290050248. [DOI] [PubMed] [Google Scholar]
- 87.Bandopadhyay R, Orte C, Lawrenson JG, Reid AR, De SS, Allt G. Contractile proteins in pericytes at the blood-brain and blood-retinal barriers. J Neurocytol. 2001;30:35–44. doi: 10.1023/a:1011965307612. [DOI] [PubMed] [Google Scholar]
- 88.Duband JL, Gimona M, Scatena M, Sartore S, Small JV. Calponin and SM 22 as differentiation markers of smooth muscle: spatiotemporal distribution during avian embryonic development. Differentiation. 1993;55:1–11. doi: 10.1111/j.1432-0436.1993.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 89.Collado MP, Latorre E, Fernandez I, Aragones MD, Catalan RE. Brain microvessel endothelin type A receptors are coupled to ceramide production. Biochem Biophys Res Commun. 2003;306:282–285. doi: 10.1016/s0006-291x(03)00927-6. [DOI] [PubMed] [Google Scholar]
- 90.Catalan RE, Martinez AM, Aragones MD, Hernandez F, Diaz G. Endothelin stimulates protein phosphorylation in blood-brain barrier. Biochem Biophys Res Commun. 1996;219:366–369. doi: 10.1006/bbrc.1996.0239. [DOI] [PubMed] [Google Scholar]
- 91.Catalan RE, Martinez AM, Aragones MD, Garde E, Diaz G. Platelet-activating factor stimulates protein kinase C translocation in cerebral microvessels. Biochem Biophys Res Commun. 1993;192:446–451. doi: 10.1006/bbrc.1993.1435. [DOI] [PubMed] [Google Scholar]
- 92.Guerin C, Laterra J, Hruban RH, Brem H, Drewes LR, Goldstein GW. The glucose transporter and blood-brain barrier of human brain tumors. Ann Neurol. 1990;28:758–765. doi: 10.1002/ana.410280606. [DOI] [PubMed] [Google Scholar]
- 93.Durk MR, Chan GN, Campos CR, Peart JC, Chow EC, Lee E, Cannon RE, Bendayan R, Miller DS, Pang KS. 1alpha,25-Dihydroxyvitamin D3-liganded vitamin D receptor increases expression and transport activity of P-glycoprotein in isolated rat brain capillaries and human and rat brain microvessel endothelial cells. J Neurochem. 2012;123:944–953. doi: 10.1111/jnc.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hawkins BT, Sykes DB, Miller DS. Rapid, reversible modulation of blood-brain barrier P-glycoprotein transport activity by vascular endothelial growth factor. J Neurosci. 2010;30:1417–1425. doi: 10.1523/JNEUROSCI.5103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miller DS, Graeff C, Droulle L, Fricker S, Fricker G. Xenobiotic efflux pumps in isolated fish brain capillaries. Am J Physiol Regul Integr Comp Physiol. 2002;282:R191–R198. doi: 10.1152/ajpregu.00305.2001. [DOI] [PubMed] [Google Scholar]
- 96.Bernas MJ, Cardoso FL, Daley SK, Weinand ME, Campos AR, Ferreira AJ, Hoying JB, Witte MH, Brites D, Persidsky Y, Ramirez SH, Brito MA. Establishment of primary cultures of human brain microvascular endothelial cells to provide an in vitro cellular model of the blood-brain barrier. Nat Protoc. 2010;5:1265–1272. doi: 10.1038/nprot.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shawahna R, Uchida Y, Decleves X, Ohtsuki S, Yousif S, Dauchy S, Jacob A, Chassoux F, Daumas-Duport C, Couraud PO, Terasaki T, Scherrmann JM. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol Pharm. 2011;8:1332–1341. doi: 10.1021/mp200129p. [DOI] [PubMed] [Google Scholar]
- 98.Pardridge WM, Yang J, Eisenberg J, Tourtellotte WW. Isolation of intact capillaries and capillary plasma membranes from frozen human brain. J Neurosci Res. 1987;18:352–357. doi: 10.1002/jnr.490180213. [DOI] [PubMed] [Google Scholar]
- 99.Lenhard T, Hulsermann U, Martinez-Torres F, Fricker G, Meyding-Lamade U. A simple method to quickly and simultaneously purify and enrich intact rat brain microcapillaries and endothelial and glial cells for ex vivo studies and cell culture. Brain Res. 2013;1519:9–18. doi: 10.1016/j.brainres.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 100.Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS. Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci U S A. 2012;109:15930–15935. doi: 10.1073/pnas.1203534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ogunshola OO. In vitro modeling of the blood-brain barrier: simplicity versus complexity. Curr Pharm Des. 2011;17:2755–2761. doi: 10.2174/138161211797440159. [DOI] [PubMed] [Google Scholar]
- 102.Teow HM, Zhou Z, Najlah M, Yusof SR, Abbott NJ, D'Emanuele A. Delivery of paclitaxel across cellular barriers using a dendrimer-based nanocarrier. Int J Pharm. 2013;441:701–711. doi: 10.1016/j.ijpharm.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 103.Toimela T, Maenpaa H, Mannerstrom M, Tahti H. Development of an in vitro blood-brain barrier model-cytotoxicity of mercury and aluminum. Toxicol Appl Pharmacol. 2004;195:73–82. doi: 10.1016/j.taap.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 104.van Beijnum JR, Rousch M, Castermans K, van der Linden E, Griffioen AW. Isolation of endothelial cells from fresh tissues. Nat Protoc. 2008;3:1085–1091. doi: 10.1038/nprot.2008.71. [DOI] [PubMed] [Google Scholar]
- 105.Patabendige A, Skinner RA, Abbott NJ. Establishment of a simplified in vitro porcine blood-brain barrier model with high transendothelial electrical resistance. Brain Res. 2013;1521:1–15. doi: 10.1016/j.brainres.2012.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abbott NJ, Dolman DE, Drndarski S, Fredriksson SM. An improved in vitro blood-brain barrier model: rat brain endothelial cells co-cultured with astrocytes. Methods Mol Biol. 2012;814:415–430. doi: 10.1007/978-1-61779-452-0_28. [DOI] [PubMed] [Google Scholar]
- 107.Sano Y, Shimizu F, Abe M, Maeda T, Kashiwamura Y, Ohtsuki S, Terasaki T, Obinata M, Kajiwara K, Fujii M, Suzuki M, Kanda T. Establishment of a new conditionally immortalized human brain microvascular endothelial cell line retaining an in vivo blood-brain barrier function. J Cell Physiol. 2010;225:519–528. doi: 10.1002/jcp.22232. [DOI] [PubMed] [Google Scholar]
- 108.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 109.Demeule M, Bertrand Y, Michaud-Levesque J, Jodoin J, Rolland Y, Gabathuler R, Beliveau R. Regulation of plasminogen activation: a role for melanotransferrin (p97) in cell migration. Blood. 2003;102:1723–1731. doi: 10.1182/blood-2003-01-0166. [DOI] [PubMed] [Google Scholar]
- 110.Prato M, D'Alessandro S, Van den Steen PE, Opdenakker G, Arese P, Taramelli D, Basilico N. Natural haemozoin modulates matrix metalloproteinases and induces morphological changes in human microvascular endothelium. Cell Microbiol. 2011;13:1275–1285. doi: 10.1111/j.1462-5822.2011.01620.x. [DOI] [PubMed] [Google Scholar]
- 111.Weksler B, Romero IA, Couraud PO. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS. 2013;10:16. doi: 10.1186/2045-8118-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cucullo L, Couraud PO, Weksler B, Romero IA, Hossain M, Rapp E, Janigro D. Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J Cereb Blood Flow Metab. 2008;28:312–328. doi: 10.1038/sj.jcbfm.9600525. [DOI] [PubMed] [Google Scholar]
- 113.Qosa H, Abuasal BS, Romero IA, Weksler B, Couraud PO, Keller JN, Kaddoumi A. Differences in amyloid-beta clearance across mouse and human blood-brain barrier models: Kinetic analysis and mechanistic modeling. Neuropharmacology. 2014;79:668–678. doi: 10.1016/j.neuropharm.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vu K, Weksler B, Romero I, Couraud PO, Gelli A. Immortalized human brain endothelial cell line HCMEC/D3 as a model of the blood-brain barrier facilitates in vitro studies of central nervous system infection by Cryptococcus neoformans. Eukaryot Cell. 2009;8:1803–1807. doi: 10.1128/EC.00240-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Prato M, D'Alessandro S, Van den Steen PE, Opdenakker G, Arese P, Taramelli D, Basilico N. Natural haemozoin modulates matrix metalloproteinases and induces morphological changes in human microvascular endothelium. Cell Microbiol. 2011;13:1275–1285. doi: 10.1111/j.1462-5822.2011.01620.x. [DOI] [PubMed] [Google Scholar]
- 116.Naik P, Fofaria N, Prasad S, Sajja RK, Weksler B, Couraud PO, Romero IA, Cucullo L. Oxidative and pro-inflammatory impact of regular and denicotinized cigarettes on blood brain barrier endothelial cells: is smoking reduced or nicotine-free products really safe? BMC Neurosci. 2014;15:51. doi: 10.1186/1471-2202-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sajja RK, Prasad S, Cucullo L. Impact of altered glycaemia on blood-brain barrier endothelium: an in vitro study using the hCMEC/D3 cell line. Fluids Barriers CNS. 2014;11:8. doi: 10.1186/2045-8118-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cucullo L, Hossain M, Rapp E, Manders T, Marchi N, Janigro D. Development of a humanized in vitro blood-brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia. 2007;48:505–516. doi: 10.1111/j.1528-1167.2006.00960.x. [DOI] [PubMed] [Google Scholar]
- 119.Ghosh C, Gonzalez-Martinez J, Hossain M, Cucullo L, Fazio V, Janigro D, Marchi N. Pattern of P450 expression at the human blood-brain barrier: roles of epileptic condition and laminar flow. Epilepsia. 2010;51:1408–1417. doi: 10.1111/j.1528-1167.2009.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xie J, Lei C, Hu Y, Gay GK, Bin Jamali NH, Wang CH. Nanoparticulate formulations for paclitaxel delivery across MDCK cell monolayer. Curr Pharm Des. 2010;16:2331–2340. doi: 10.2174/138161210791920432. [DOI] [PubMed] [Google Scholar]
- 121.Lundquist S, Renftel M, Brillault J, Fenart L, Cecchelli R, Dehouck MP. Prediction of drug transport through the blood-brain barrier in vivo: a comparison between two in vitro cell models. Pharm Res. 2002;19:976–981. doi: 10.1023/a:1016462205267. [DOI] [PubMed] [Google Scholar]
- 122.Neuhaus W, Lauer R, Oelzant S, Fringeli UP, Ecker GF, Noe CR. A novel flow based hollow-fiber blood-brain barrier in vitro model with immortalised cell line PBMEC/C1-2. J Biotechnol. 2006;125:127–141. doi: 10.1016/j.jbiotec.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 123.Hutamekalin P, Farkas AE, Orbok A, Wilhelm I, Nagyoszi P, Veszelka S, Deli MA, Buzas K, Hunyadi-Gulyas E, Medzihradszky KF, Meksuriyen D, Krizbai IA. Effect of nicotine and polyaromtic hydrocarbons on cerebral endothelial cells. Cell Biol Int. 2008;32:198–209. doi: 10.1016/j.cellbi.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 124.Santaguida S, Janigro D, Hossain M, Oby E, Rapp E, Cucullo L. Side by side comparison between dynamic versus static models of blood-brain barrier in vitro: a permeability study. Brain Res. 2006;1109:1–13. doi: 10.1016/j.brainres.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 125.Lippmann ES, Azarin SM, Kay JE, Nessler RA, Wilson HK, Al-Ahmad A, Palecek SP, Shusta EV. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol. 2012;30:783–791. doi: 10.1038/nbt.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Berezowski V, Landry C, Lundquist S, Dehouck L, Cecchelli R, Dehouck MP, Fenart L. Transport screening of drug cocktails through an in vitro blood-brain barrier: is it a good strategy for increasing the throughput of the discovery pipeline? Pharm Res. 2004;21:756–760. doi: 10.1023/b:pham.0000026424.78528.11. [DOI] [PubMed] [Google Scholar]
- 127.Man S, Ubogu EE, Williams KA, Tucky B, Callahan MK, Ransohoff RM. Human brain microvascular endothelial cells and umbilical vein endothelial cells differentially facilitate leukocyte recruitment and utilize chemokines for T cell migration. Clin Dev Immunol. 2008;2008:384982. doi: 10.1155/2008/384982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.DeBault LE, Cancilla PA. Some properties of isolated endothelial cells in culture. Adv Exp Med Biol. 1980;131:69–78. doi: 10.1007/978-1-4684-3752-2_6. [DOI] [PubMed] [Google Scholar]
- 129.Al AA, Taboada CB, Gassmann M, Ogunshola OO. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. J Cereb Blood Flow Metab. 2011;31:693–705. doi: 10.1038/jcbfm.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lai CH, Kuo KH. The critical component to establish in vitro BBB model: Pericyte. Brain Res Brain Res Rev. 2005;50:258–265. doi: 10.1016/j.brainresrev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 131.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Beck DW, Vinters HV, Hart MN, Cancilla PA. Glial cells influence polarity of the blood-brain barrier. J Neuropathol Exp Neurol. 1984;43:219–224. doi: 10.1097/00005072-198405000-00001. [DOI] [PubMed] [Google Scholar]
- 133.Hori S, Ohtsuki S, Tachikawa M, Kimura N, Kondo T, Watanabe M, Nakashima E, Terasaki T. Functional expression of rat ABCG2 on the luminal side of brain capillaries and its enhancement by astrocyte-derived soluble factor(s) J Neurochem. 2004;90:526–536. doi: 10.1111/j.1471-4159.2004.02537.x. [DOI] [PubMed] [Google Scholar]
- 134.Candela P, Gosselet F, Miller F, Buee-Scherrer V, Torpier G, Cecchelli R, Fenart L. Physiological pathway for low-density lipoproteins across the blood-brain barrier: transcytosis through brain capillary endothelial cells in vitro. Endothelium. 2008;15:254–264. doi: 10.1080/10623320802487759. [DOI] [PubMed] [Google Scholar]
- 135.Cecchelli R, Berezowski V, Lundquist S, Culot M, Renftel M, Dehouck MP, Fenart L. Modelling of the blood-brain barrier in drug discovery and development. Nat Rev Drug Discov. 2007;6:650–661. doi: 10.1038/nrd2368. [DOI] [PubMed] [Google Scholar]
- 136.Hatherell K, Couraud PO, Romero IA, Weksler B, Pilkington GJ. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Methods. 2011;199:223–229. doi: 10.1016/j.jneumeth.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 137.Siddharthan V, Kim YV, Liu S, Kim KS. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 2007;1147:39–50. doi: 10.1016/j.brainres.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Man S, Tucky B, Cotleur A, Drazba J, Takeshita Y, Ransohoff RM. CXCL12-induced monocyte-endothelial interactions promote lymphocyte transmigration across an in vitro blood-brain barrier. Sci Transl Med. 2012;4:119ra14. doi: 10.1126/scitranslmed.3003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Achyuta AK, Conway AJ, Crouse RB, Bannister EC, Lee RN, Katnik CP, Behensky AA, Cuevas J, Sundaram SS. A modular approach to create a neurovascular unit-on-a-chip. Lab Chip. 2013;13:542–553. doi: 10.1039/c2lc41033h. [DOI] [PubMed] [Google Scholar]
- 140.Rohe I, Ruhnke I, Knorr F, Mader A, Boroojeni FG, Lowe R, Zentek J. Effects of grinding method, particle size, and physical form of the diet on gastrointestinal morphology and jejunal glucose transport in laying hens. Poult Sci. 2014 doi: 10.3382/ps.2013-03783. [DOI] [PubMed] [Google Scholar]
- 141.Clarke LL. A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1151–G1166. doi: 10.1152/ajpgi.90649.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Clarke LL. A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1151–G1166. doi: 10.1152/ajpgi.90649.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rohe I, Ruhnke I, Knorr F, Mader A, Boroojeni FG, Lowe R, Zentek J. Effects of grinding method, particle size, and physical form of the diet on gastrointestinal morphology and jejunal glucose transport in laying hens. Poult Sci. 2014 doi: 10.3382/ps.2013-03783. [DOI] [PubMed] [Google Scholar]
- 144.Ballermann BJ, Ott MJ. Adhesion and differentiation of endothelial cells by exposure to chronic shear stress: a vascular graft model. Blood Purif. 1995;13:125–134. doi: 10.1159/000170195. [DOI] [PubMed] [Google Scholar]
- 145.Ando J, Yamamoto K. Vascular mechanobiology: endothelial cell responses to fluid shear stress. Circ J. 2009;73:1983–1992. doi: 10.1253/circj.cj-09-0583. [DOI] [PubMed] [Google Scholar]
- 146.Chretien ML, Zhang M, Jackson MR, Kapus A, Langille BL. Mechanotransduction by endothelial cells is locally generated, direction-dependent, and ligand-specific. J Cell Physiol. 2010;224:352–361. doi: 10.1002/jcp.22125. [DOI] [PubMed] [Google Scholar]
- 147.Grabowski EF, Jaffe EA, Weksler BB. Prostacyclin production by cultured endothelial cell monolayers exposed to step increases in shear stress. J Lab Clin Med. 1985;105:36–43. [PubMed] [Google Scholar]
- 148.Moore JP, Weber M, Searles CD. Laminar shear stress modulates phosphorylation and localization of RNA polymerase II on the endothelial nitric oxide synthase gene. Arterioscler Thromb Vasc Biol. 2010;30:561–567. doi: 10.1161/ATVBAHA.109.199554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991;17:187–193. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- 150.Ott MJ, Ballermann BJ. Shear stress-conditioned, endothelial cell-seeded vascular grafts: improved cell adherence in response to in vitro shear stress. Surgery. 1995;117:334–339. doi: 10.1016/s0039-6060(05)80210-7. [DOI] [PubMed] [Google Scholar]
- 151.Walsh TG, Murphy RP, Fitzpatrick P, Rochfort KD, Guinan AF, Murphy A, Cummins PM. Stabilization of Brain Microvascular Endothelial Barrier Function by Shear Stress Involves VE-cadherin Signaling Leading to Modulation of pTyr-Occludin Levels. J Cell Physiol. 2011 doi: 10.1002/jcp.22655. [DOI] [PubMed] [Google Scholar]
- 152.Colgan OC, Ferguson G, Collins NT, Murphy RP, Meade G, Cahill PA, Cummins PM. Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am J Physiol Heart Circ Physiol. 2007;292:H3190–H3197. doi: 10.1152/ajpheart.01177.2006. [DOI] [PubMed] [Google Scholar]
- 153.Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res. 2010;87:320–330. doi: 10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981;103:177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 155.Bussolari SR, Dewey CF, Jr, Gimbrone MA., Jr Apparatus for subjecting living cells to fluid shear stress. Rev Sci Instrum. 1982;53:1851–1854. doi: 10.1063/1.1136909. [DOI] [PubMed] [Google Scholar]
- 156.Zhao F, Li L, Guan L, Yang H, Wu C, Liu Y. Roles for GP IIb/IIIa and alphavbeta3 integrins in MDA-MB-231 cell invasion and shear flow-induced cancer cell mechanotransduction. Cancer Lett. 2014;344:62–73. doi: 10.1016/j.canlet.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 157.Wiese G, Barthel SR, Dimitroff CJ. Analysis of physiologic E-selectin-mediated leukocyte rolling on microvascular endothelium. J Vis Exp. 2009 doi: 10.3791/1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mellado M, Martinez A, Rodriguez-Frade JM. Drug testing in cellular chemotaxis assays. Curr Protoc Pharmacol. 2008;Chapter 12(Unit) doi: 10.1002/0471141755.ph1211s41. [DOI] [PubMed] [Google Scholar]
- 159.Kemeny SF, Figueroa DS, Clyne AM. Hypo- and hyperglycemia impair endothelial cell actin alignment and nitric oxide synthase activation in response to shear stress. PLoS One. 2013;8:e66176. doi: 10.1371/journal.pone.0066176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu Y, Wang B, Deng J, Liu Z, Zhu L. A potential model for drug screening by simulating the effect of shear stress in vivo on endothelium. Biorheology. 2013;50:33–43. doi: 10.3233/BIR-130624. [DOI] [PubMed] [Google Scholar]
- 161.Mikhal J, Geurts BJ. Development and application of a volume penalization immersed boundary method for the computation of blood flow and shear stresses in cerebral vessels and aneurysms. J Math Biol. 2013;67:1847–1875. doi: 10.1007/s00285-012-0627-5. [DOI] [PubMed] [Google Scholar]
- 162.Lu L, Sick V. High-speed particle image velocimetry near surfaces. J Vis Exp. 2013 doi: 10.3791/50559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stanness KA, Guatteo E, Janigro D. A dynamic model of the blood-brain barrier “in vitro”. Neurotoxicology. 1996;17:481–496. [PubMed] [Google Scholar]
- 164.Cucullo L, Hossain M, Tierney W, Janigro D. A new dynamic in vitro modular capillaries-venules modular system: cerebrovascular physiology in a box. BMC Neurosci. 2013;14:18. doi: 10.1186/1471-2202-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cucullo L, McAllister MS, Kight K, Krizanac-Bengez L, Marroni M, Mayberg MR, Stanness KA, Janigro D. A new dynamic in vitro model for the multidimensional study of astrocyte-endothelial cell interactions at the blood-brain barrier. Brain Res. 2002;951:243–254. doi: 10.1016/s0006-8993(02)03167-0. [DOI] [PubMed] [Google Scholar]
- 166.Koutsiaris AG, Tachmitzi SV, Batis N, Kotoula MG, Karabatsas CH, Tsironi E, Chatzoulis DZ. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology. 2007;44:375–386. [PubMed] [Google Scholar]
- 167.Cucullo L, Marchi N, Hossain M, Janigro D. A dynamic in vitro BBB model for the study of immune cell trafficking into the central nervous system. J Cereb Blood Flow Metab. 2011;31:767–777. doi: 10.1038/jcbfm.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cucullo L, Couraud PO, Weksler B, Romero IA, Hossain M, Rapp E, Janigro D. Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J Cereb Blood Flow Metab. 2008;28:312–328. doi: 10.1038/sj.jcbfm.9600525. [DOI] [PubMed] [Google Scholar]
- 169.McAllister MS, Krizanac-Bengez L, Macchia F, Naftalin RJ, Pedley KC, Mayberg MR, Marroni M, Leaman S, Stanness KA, Janigro D. Mechanisms of glucose transport at the blood-brain barrier: an in vitro study. Brain Res. 2001;904:20–30. doi: 10.1016/s0006-8993(01)02418-0. [DOI] [PubMed] [Google Scholar]
- 170.Krizanac-Bengez L, Mayberg MR, Cunningham E, Hossain M, Ponnampalam S, Parkinson FE, Janigro D. Loss of shear stress induces leukocyte-mediated cytokine release and blood-brain barrier failure in dynamic in vitro blood-brain barrier model. J Cell Physiol. 2005 doi: 10.1002/jcp.20429. [DOI] [PubMed] [Google Scholar]
- 171.Benson K, Cramer S, Galla HJ. Impedance-based cell monitoring: barrier properties and beyond. Fluids Barriers CNS. 2013;10:5. doi: 10.1186/2045-8118-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Booth R, Kim H. Characterization of a microfluidic in vitro model of the blood-brain barrier (muBBB) Lab Chip. 2012;12:1784–1792. doi: 10.1039/c2lc40094d. [DOI] [PubMed] [Google Scholar]
- 173.Stamatovic SM, Keep RF, Andjelkovic AV. Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Curr Neuropharmacol. 2008;6:179–192. doi: 10.2174/157015908785777210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Griep LM, Wolbers F, de WB, ter Braak PM, Weksler BB, Romero IA, Couraud PO, Vermes I, van der Meer AD, van den Berg A. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed Microdevices. 2013;15:145–150. doi: 10.1007/s10544-012-9699-7. [DOI] [PubMed] [Google Scholar]
- 175.Alcendor DJ, Block FE, Iii, Cliffel DE, Daniels J, Ellacott KL, Goodwin CR, Hofmeister LH, Li D, Markov DA, May JC, McCawley LJ, McLaughlin B, McLean JA, Niswender KD, Pensabene V, Seale KT, Sherrod SD, Sung HJ, Tabb DL, Webb DJ, Wikswo JP. Neurovascular unit on a chip: implications for translational applications. Stem Cell Res Ther. 2013;4(Suppl 1):S18. doi: 10.1186/scrt379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Prabhakarpandian B, Shen MC, Nichols JB, Mills IR, Sidoryk-Wegrzynowicz M, Aschner M, Pant K. SyM-BBB: a microfluidic Blood Brain Barrier model. Lab Chip. 2013;13:1093–1101. doi: 10.1039/c2lc41208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gervais T, El-Ali J, Gunther A, Jensen KF. Flow-induced deformation of shallow microfluidic channels. Lab Chip. 2006;6:500–507. doi: 10.1039/b513524a. [DOI] [PubMed] [Google Scholar]
- 178.Palchesko RN, Zhang L, Sun Y, Feinberg AW. Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve. PLoS One. 2012;7:e51499. doi: 10.1371/journal.pone.0051499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bansal T, Mishra G, Jaggi M, Khar RK, Talegaonkar S. Effect of P-glycoprotein inhibitor, verapamil, on oral bioavailability and pharmacokinetics of irinotecan in rats. Eur J Pharm Sci. 2009;36:580–590. doi: 10.1016/j.ejps.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 180.Elliott NT, Yuan F. A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J Pharm Sci. 2011;100:59–74. doi: 10.1002/jps.22257. [DOI] [PubMed] [Google Scholar]
- 181.Yim EK, Leong KW. Proliferation and differentiation of human embryonic germ cell derivatives in bioactive polymeric fibrous scaffold. J Biomater Sci Polym Ed. 2005;16:1193–1217. doi: 10.1163/156856205774269485. [DOI] [PubMed] [Google Scholar]
- 182.Tanaka H, Murphy CL, Murphy C, Kimura M, Kawai S, Polak JM. Chondrogenic differentiation of murine embryonic stem cells: effects of culture conditions and dexamethasone. J Cell Biochem. 2004;93:454–462. doi: 10.1002/jcb.20171. [DOI] [PubMed] [Google Scholar]
- 183.Gill BJ, West JL. Modeling the tumor extracellular matrix: Tissue engineering tools repurposed towards new frontiers in cancer biology. J Biomech. 2013 doi: 10.1016/j.jbiomech.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 184.Wolburg H, Noell S, Mack A, Wolburg-Buchholz K, Fallier-Becker P. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 2009;335:75–96. doi: 10.1007/s00441-008-0658-9. [DOI] [PubMed] [Google Scholar]
- 185.Weber LM, Hayda KN, Anseth KS. Cell-matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue Eng Part A. 2008;14:1959–1968. doi: 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Toh YC, Zhang C, Zhang J, Khong YM, Chang S, Samper VD, van Noort D, Hutmacher DW, Yu H. A novel 3D mammalian cell perfusion-culture system in microfluidic channels. Lab Chip. 2007;7:302–309. doi: 10.1039/b614872g. [DOI] [PubMed] [Google Scholar]
- 187.Bersini S, Jeon JS, Dubini G, Arrigoni C, Chung S, Charest JL, Moretti M, Kamm RD. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. 2014;35:2454–2461. doi: 10.1016/j.biomaterials.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen Y, Dodd SJ, Tangrea MA, Emmert-Buck MR, Koretsky AP. Measuring collective cell movement and extracellular matrix interactions using magnetic resonance imaging. Sci Rep. 2013;3:1879. doi: 10.1038/srep01879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17:524–532. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 190.Gieni RS, Hendzel MJ. Mechanotransduction from the ECM to the genome: are the pieces now in place? J Cell Biochem. 2008;104:1964–1987. doi: 10.1002/jcb.21364. [DOI] [PubMed] [Google Scholar]
- 191.Alcendor Donald J, Block Frank E, III, Cliffel David E, Daniels John Scott, Ellacott Kate LJ, Goodwin Cody R, Hofmeister Lucas H, Li Deyu, Markov Dmitry A, May Jody C, McCawley Lisa J, McLaughlin BethAnn, McLean John A, Niswender Kevin D, Pensabene Virginia, Seale Kevin T, Sherrod Stacy D, Sung Hak-Joon, Tabb David L, Webb Donna J, Wikswo John P. Neurovascular unit on a chip: implications for translational applications. 2013 doi: 10.1186/scrt379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang JD, Douville NJ, Takayama S, ElSayed M. Quantitative analysis of molecular absorption into PDMS microfluidic channels. Ann Biomed Eng. 2012;40:1862–1873. doi: 10.1007/s10439-012-0562-z. [DOI] [PubMed] [Google Scholar]
- 193.Luni C, Serena E, Elvassore N. Human-on-chip for therapy development and fundamental science. Curr Opin Biotechnol. 2014;25C:45–50. doi: 10.1016/j.copbio.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 194.Esch MB, King TL, Shuler ML. The role of body-on-a-chip devices in drug and toxicity studies. Annu Rev Biomed Eng. 2011;13:55–72. doi: 10.1146/annurev-bioeng-071910-124629. [DOI] [PubMed] [Google Scholar]
- 195.Kim D, Wu X, Young AT, Haynes CL. Microfluidics-Based in Vivo Mimetic Systems for the Study of Cellular Biology. Acc Chem Res. 2014 doi: 10.1021/ar4002608. [DOI] [PMC free article] [PubMed] [Google Scholar]