Abstract
Objective
To assess the clinical evidence for bee venom acupuncture (BVA) for rheumatoid arthritis (RA).
Design
Systematic review of randomised controlled trials (RCTs).
Setting
We searched 14 databases up to March 2014 without a language restriction.
Participants
Patients with RA.
Intervention
BVA involved injecting purified, diluted BV into acupoints. We included trials on BVA used alone or in combination with a conventional therapy versus the conventional therapy alone.
Primary outcomes
Morning stiffness, pain and joint swelling
Secondary outcomes
Erythrocyte sedimentation rate (ESR), C reactive protein (CRP), rheumatoid factor, the number of joints affected by RA and adverse effects likely related to RA.
Results
A total of 304 potentially relevant studies were identified; only one RCT met our inclusion criteria. Compared with placebo, BVA may more effectively improve joint pain, swollen joint counts, tender joint counts, ESR and CRP but was not shown to improve morning stiffness.
Conclusions
There is low-quality evidence, based on one trial, that BVA can significantly reduce pain, morning stiffness, tender joint counts, swollen joint counts and improve the quality of life of patients with RA compared with placebo (normal saline injection) control. However, the number of trials, their quality and the total sample size were too low to draw firm conclusions.
Trial registration number
PROSPERO 2013: CRD42013005853.
Keywords: COMPLEMENTARY MEDICINE
Strengths and limitations of this study.
The strength of this systematic review is its extensive, unbiased search of various databases without a language restriction.
The trial screening and data extraction was conducted independently by two authors.
Use of the GRADE approach to assess confidence in estimates of effect.
We identified only one study, hence we could not draw strong conclusions.
Introduction
Description of the condition
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disorder that results in pain and stiffness, joint swelling, deformity of joints and the development of ankylosis. The complex, systemic nature of the disease makes RA treatment complex and involves a variety of approaches. The major aims of treatment are to relieve pain and swelling, reduce inflammation and joint damage, prevent disability and preserve or improve patients’ well-being and function.1
Untreated RA leads to joint destruction, functional limitation and severe disability,2 3 and has a significant impact on health-related quality of life (HRQoL).4 5
Description of the intervention
Bee venom (BV) therapy has been used since ancient times. Different forms of the therapy include the administration of live bee stings, injections of BV and BV acupuncture (BVA).6 BVA involves injecting purified and diluted BV into acupoints.7
How the intervention might work
BVA exhibits several pharmacological actions, including analgesic, anti-inflammatory, antiarthritic and anticancer effects through multiple mechanisms, such as activation of the central inhibitory and excitatory systems and modulation of the immune system.8 The analgesic effects of BVA have been reported in animal experiments9 10 and clinical settings.7 11 According to animal experiments, BV exhibits antiarthritic, anti-inflammatory and analgesic effects attributable to the suppression of cyclo-oxygenase-2 and phospholipase A2 expression and a decrease in the levels of tumour necrosis factor α, interleukin (IL)-1, IL-6, nitric oxide and oxygen-reactive species. It is also widely assumed that bioactive BV compounds, including enzymes (phospholipase A2), peptides (melittin, adolapin and apamin), and amines are associated with these actions.7 8 12–14 However, most therapeutic uses are not based on evidence.
One study was conducted to elucidate whether the synergistic antiarthritic effects produced by a combination of BV and conventional therapy enhances the therapeutic potency and minimises the adverse effects of methotrexate.15
Why this review is important
BV therapy or BVA has been used for reducing pain caused by inflammatory diseases such as osteoarthritis and RA in some Asian countries.11
However, there is no critically appraised evidence, such as a systematic review or meta-analysis, of the potential benefits and risks of BVA for RA. A comprehensive evaluation of the efficacy and safety of BVA for RA will inform the recommendation to patients to pursue BVA treatment.
Objectives
Although BVA for RA is used as an effective method for reducing RA-related symptoms and improving functioning, there is no critically appraised evidence regarding the safety and effectiveness of BVA for RA from a systematic review or meta-analysis.
We performed a systematic review to assess the safety and efficacy of BVA for the treatment of RA.
Materials and methods
The protocol of this SR is registered on PROSPERO 2013 (registration number: CRD42013005853) and published as a protocol.16
Data source
The following electronic databases were searched from the study's inception to March 2014: Medline, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), AMED and CINAHL. We also searched six Korean medical databases (OASIS, Korean Traditional Knowledge Portal, Korean Studies Information Service System, KoreaMed, Korean Medical Database and DBPIA) and three Chinese databases including CNKI (China Academic Journal, China Doctoral Dissertations and Master's Theses Full-text Database, China Proceedings of Conference Full Text Database and the Century Journal Project), Wanfang and VIP. Further, we conducted non-electronic searches of conference proceedings, our own files of articles and nine Korean traditional medical journals (Journal of Korean Medicine, The Journal of Korean Acupuncture and Moxibustion Society, Korean Journal of Acupuncture, Journal of Acupuncture and Meridian Studies, Journal of Pharmacopuncture, Journal of Oriental Rehabilitation Medicine, The Journal of Korea Chuna Manual Medicine for Spine and Nerves, Korean Journal of Oriental Physiology and Pathology and The Journal of Korean Oriental Internal Medicine). The strategy for searching the MEDLINE, EMBASE, Cochrane Library, and CINAHL database is presented in online supplement 1. Similar search strategies were applied for other databases.
Types of studies
All prospective, randomised controlled clinical trials (RCTs) were included if they were randomised studies of BV injections at acupoints as the sole treatment, or as an adjunct to other treatments if the control group received the same treatment as the BVA group. Trials comparing BVA with any type of control intervention were also included. We excluded trials of BV injections into parts of the body other than acupoints. Trials were also excluded if only immunological or biological parameters were assessed. Trials comparing two different types of BVA were also excluded. No language restrictions were imposed. Hard copies of all articles were obtained and read in full.
Types of participants
Patients suffering from RA were included.
Types of interventions
We included trials on BVA used alone or in combination with a conventional therapy versus the conventional therapy alone. BVA involved injecting purified, diluted BV into acupoints. Conventional therapies included medications such as non-steroidal anti-inflammatory drugs, steroids, disease-modifying antirheumatic drugs, immunosuppressants and TNF-α inhibitors.
Types of outcomes measured
Primary outcomes were symptoms (morning stiffness, pain and joint swelling) experienced. Secondary outcomes included erythrocyte sedimentation rate (ESR), C reactive protein (CRP), rheumatoid factor, the number of joints affected by RA and adverse effects likely related to RA.
Data extraction and quality assessment
Hard copies of all articles were obtained and read in full. Two authors (MJS and JHJ) performed the data extraction and quality assessment using a predefined data extraction form. The risk of bias was assessed using the assessment tool for risk of bias from the Cochrane Handbook V.5.1.0, which includes random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete outcome data, selective reporting and other sources of bias.17 Our review used ‘L’, ‘U’ and ‘H’ as results of the assessment; ‘L’ indicated a low risk of bias, ‘U’ indicated that the risk of bias was unclear and ‘H’ indicated a high risk of bias. Disagreements were resolved by a discussion between all of the authors. When disagreements on the selection were not resolved through discussions, the arbiter (MSL) made the final decision.
Data collection and synthesis
Data extraction and management
The data extraction and quality assessment were conducted by three authors (JAL, MJS and JHJ) using a predefined data extraction form. Any disagreement among the authors was resolved by a discussion between all of the authors. When the data were insufficient or ambiguous, MSL contacted the corresponding authors by email or telephone to request additional information or clarification. The data screening and selection process was performed independently by four authors and then was verified by a fifth author, JHJ, who is fluent in Chinese. We used GRADEpro software in the Cochrane Systematic Reviews to create a Summary of Findings table. When disagreements on the selections were not resolved through discussions, the arbiter (MSL) made the final decision.
Assessment of bias in the included studies
We independently assessed bias in the included studies according to criteria from the Cochrane Handbook, V.5.1.0, which includes random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete outcome data, selective reporting and other sources of bias.17 The quality of each trial was categorised into a low, unclear or high risk of bias, and the authors of the assessed trials were contacted for clarification as needed. We resolved any differences in opinion through discussion or consultation with a third author.
Data synthesis
The differences between the intervention and control groups were assessed. For the continuous data, we used mean differences (MDs) with 95% CIs to measure the treatment effects. We converted other forms of data into MDs. In the case of outcome variables with different scales, we used the standard mean difference (SMD) with 95% CIs. For dichotomous data, we presented the treatment effect as a relative risk (RR) with 95% CIs. We converted other binary data into an RR value.
All of the statistical analyses were conducted using Cochrane Collaboration's software programme, Review Manager (RevMan), V.5.2.7 for Windows (Copenhagen, The Nordic Cochrane Centre, the Cochrane Collaboration, 2012). For studies with insufficient information, we contacted the corresponding authors to acquire and verify data when possible. If appropriate, we pooled data across studies for a meta-analysis using fixed effects or random effects.
Unit of analysis issues
For cross-over trials, data from the first treatment period were used. For trials in which more than one control group was assessed, the primary analysis combined the data from each control group. Subgroup analyses of the control groups were performed. Each patient was counted only once in the analysis.
Addressing the missing data
Intention-to-treat analyses that included all of the randomised patients were performed. For patients with missing outcome data, a carry-forward of the last observed response was used. The individual patient data were sought from the original source or the published trial reports when the individual patient data were initially unavailable.
Assessment of heterogeneity
We used the random-effect or fixed-effect model for the meta-analysis according to the data analysis. The χ2 and I2 tests were used to evaluate the heterogeneity of the included studies and I2 >50 were considered to have high heterogeneity. If heterogeneity was observed, we conducted a subgroup analysis to explore the possible causes.18
Assessment of reporting biases
If a sufficient number of included studies (at least 10 trials) were available, we used funnel plots to detect reporting biases.19 However, funnel plot asymmetry was not the same as publication bias; therefore, we attempted to determine the possible reasons for the asymmetry, such as small-study effects, poor methodological quality and true heterogeneity in the included studies.19 20
Results
Study selection and description
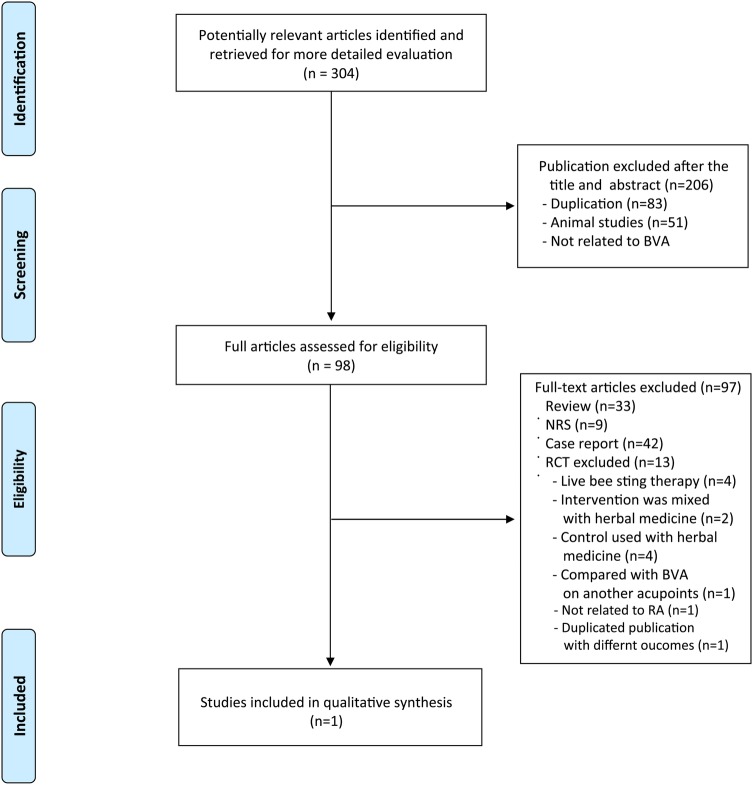
The search generated a total of 304 hits, of which only one met our inclusion criteria (figure 1). Thirteen RCTs were among the excluded articles for the following reasons: four RCTs, which were conducted in China, were excluded because the BVA was not made with purified, diluted BV but with live bee stings (see online supplements 2 and 3),21–24 four RCTs employed herbal medicine as coadministrator,25–28 two RCTs included herbal medicine as control treatment,29 30 one RCT compared two different acupoints,31 one RCT was not related to RA32 and one RCT was a duplicated publication.33 The key data from the eligible RCT are summarised in table 1. This trial was conducted in Korea.34
Figure 1.
Flow chart of trial selection process. BVA, bee venom acupuncture; CCT, case series trials; NRS: Non-RCT; RA, rheumatoid arthritis; RCT, randomised controlled trials.
Table 1.
Characteristics of included randomised controlled trials of bee venom acupuncture for rheumatoid arthritis
| Lee et al34 | |
|---|---|
| Methods | Design: prospective randomised controlled trial |
| Participants Country: South Korea Number of patients included (completed/randomised): A. 37/40 B. 32/40 Mean age (years) A. 49.2±9.6 B. 47.3±8.9 Duration of disease (years)(A) 9.2±7.0 (B) 7.3±4.6 Follow-up: 1 and 2 months | |
| Intervention | (A) BVA (ashi points, acupoints near the inflammation point, two times a week for 2 months) |
| Control | (B) Placebo (normal saline injection on ashi points, acupoints near the inflammation point, two times a week for 2 months) |
| Outcomes | Primary outcomes 1. Morning stiffness, MD, −0.70 (−2.00 to 0.60), p<0.05 2. HAQ, MD, 0.00 (−0.08 to 0.08), p<0.05 3. VAS-pain, MD, −18.10 (−23.71 to −12.49), p<0.05 Secondary outcomes 1. Tender joint count, MD, −1.30 (−1.91 to −0.69), p<0.0001p<0.05 Secondary outcomes 2. Swollen joint count, MD, −1.10 (−1.72 to −0.48), p=0.005 3. ESR, MD, 20.10 (−22.80 to −17.40), p<0.00001 4. CRP, MD, −1.90 (−2.86 to −0.94), p=0.0001 |
| Note | Treatment rationale: TKM theory, clinical experience Adverse effect: NR Funding: Korea Research Foundation Grant and Kyung Hee University Language: Korean Publication: full paper Withdrawal/dropouts: yes Intention-to-treat: no Author comment: these results suggest that bee venom therapy could be an effective method in the treatment of patients with RA |
| Risk of bias | ||
|---|---|---|
| Item | Authors’ judgement | Description |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but information not available |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) all outcomes | Low risk | Described as double blinding |
| Blinding of outcome assessment (detection bias), all outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias), all outcomes | High risk | Data from 11 participants were not included in the analysis |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but all expected outcomes reported |
| Other bias | Unclear risk | Small sample size |
BVA, bee venom acupuncture; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; HAQ, health assessment questionnaire; MD, mean differences; NR, not reported; RA, rheumatoid arthritis; TKM, traditional Korean medicine; VAS, visual analogue scale.
Risk of bias in the included studies
The RCT used34 has an uncertain risk of bias due to its random sequence generation, allocation concealment, outcome assessment blinding, selective reporting and other biases. This study used blinding of participants and personnel employing placebo as a comparison and to address incomplete outcome data.
Outcomes
The study tested the efficacy of BVA on morning stiffness, Health Assessment Questionnaire (HAQ) scores, pain, tender joint counts, swollen joint counts, ESR and CRP in patients with RA.34 Patients were randomised into two groups: one receiving BVA at ashi points and the other receiving normal saline injections at ashi points. After 2 months, the scores for morning stiffness, HAQ, pain on visual analogue scale, tender joint counts, swollen joint counts, ESR and CRP were significantly better in the BVA group than in the placebo control group.
Adverse events
This trial did not assess adverse events related to BVA used for RA.34
Discussion
Only one trial testing the effects of BVA for RA is currently available.34 There is low-quality evidence based on this one trial that BVA significantly reduces pain, morning stiffness, tender joint counts, swollen joint counts and improves the quality of life of patients with RA compared with placebo (normal saline injection) control patients (table 2). To date, however, the effects of BVA for RA have not been confirmed because of small sample sizes and high risks of bias.
Table 2.
Summary of findings
| Bee venom acupuncture for patients with rheumatoid arthritis | |||||
| Patient or population: patients with rheumatoid arthritis Settings: Korea Intervention: bee venom acupuncture vs normal saline injection as placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (studies) | Quality of the evidence (Grade) | Comments | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Control (normal saline injection) | Bee venom acupuncture | ||||
| Pain (VAS) |
16.9 WMD lower† (26.57 to 7.23 lower) |
69 (1 study) |
⊕⊕⊝⊝ low‡§ |
After 1 month −10.40 (−16.47 to −4.33) |
|
| Morning stiffness | 12.1 WMD higher† (11.61 to 12.59 higher) |
69 (1 study) |
⊕⊕⊝⊝ low‡§ |
After 1 month −0.30 (−1.01 to 0.41) |
|
| Swollen joint count | 0.9 WMD lower† (1.97 lower to 0.17 higher) |
69 (1 study) |
⊕⊕⊝⊝ low‡§ |
After 1 month 0.50 (−0.70 to −1.70) |
|
| Tender joint count | 0.9 WMD lower† (1.97 lower to 0.17 higher) |
69 (1 study) |
⊕⊕⊝⊝ low‡§ |
After 1 month 0.50 (−0.73 to −1.73) |
|
| Quality of life (HAQ) |
0.3 WMD higher† (0.08 to 0.52 higher) |
69 (1 study) |
⊕⊕⊝⊝ low‡§ |
After 1 month 0.20 (−0.06 to 0.46) |
|
| ESR | 19.4 WMD lower† (28.51 to 10.29 lower) |
69 (1 study) |
⊕⊕⊝⊝ low‡§ |
After 1 month −2.30 (−10.17 to 5.57) |
|
| CRP | 1.7 WMD lower† (2.6 to 0.8 lower) |
69 (1 study) |
⊕⊕⊝⊝ low‡§ |
After 1 month 1.40 (−8.27 to 5.47) |
|
GRADE Working Group grades of evidence.
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Very low quality: we are very uncertain about the estimate.
*The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
†After 2 months treatment.
‡Poorly reported paper (see ‘Risk of bias’ table).
§Small sample size.
CRP, C reactive protein; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; VAS, visual analogue scale; WMD, weight mean difference.
This systematic review has several limitations. First, although extensive efforts were made to retrieve all of the RCTs with no language and publication status limitations, only one study of BVA for RA qualified for our review. Second, the included RCT was conducted in East Asian countries, and studies from East Asian countries do not apply globally because of their lack of external validity. Third, Korean researchers tend to have positive results,35 but we could not minimise the results because of the lack of methodology. Fourth, despite the possibility of delayed-type hypersensitivity occurring, there was no prolonged follow-up.
The included RCT used saline injections at the same acupoints used in the BVA group for the placebo control treatment.34 The use of placebo is essential for differentiating non-specific from specific treatment effects. If we consider that the effects of BVA could come from stimulating acupoints with the immune-modulative effect of BV, it is necessary to implement further RCTs that use the appropriate placebo. This study has some potential caveats. One is that a normal saline injection at the same acupoints used in the experimental group could be an inappropriate placebo. BVA combines biochemical effects of the BV and mechanical effects from the needles. As a result, this placebo could invoke mechanical effects from the acupoint injection. The other is that there was no reporting of previous experiences with BVA. BVA has uncomfortable sensations such as swelling and burning during the treatment. Some participants who have previously experienced BVA treatment could know what they were treated with, thereby interrupting patient blinding. To use normal saline injections as a placebo, it is important to recruit patients who have not experienced BVA.
In the absence of a sufficient number of RCTs, other types of evidence might be helpful. There was one observational study that showed favourable effects of BVA for several symptoms of RA (see online supplement 4).36 However, this type of study, lacking in controls, was open to selection bias, which could lead to false-positive results.
Traditional BVA includes live bee sting acupuncture. It may be more commonly used when treating patients with RA in China. In considering traditional BVA, we found four additional RCTs that compared live bee sting acupuncture combined with conventional drugs with conventional treatments alone for the treatment of RA symptoms.21–24 Three RCTs21–23 showed favourable effects of BVA on at least one of the main outcomes including total improvement, morning stiffness, pain, joint pain or joint swelling, while one RCT failed to do so.24 These RCTs did not report serious adverse effects.
Both BVA (diluted or purified) and live bee stings can also cause diverse clinical responses depending on the amount of venom used and the frequency and duration of the treatment.37–39 The acute or delayed adverse reaction is an inflammatory reaction, such as anaphylaxis or urticarial.36–40 No studies were made comparing the occurrence of adverse events between traditional live bee sting acupuncture and BVA. Although trials are conducted safely, some problems remain in using BVA in clinical practice.
The injection parts may be one issue for the assessment. Although it is very common to inject on the painful point (ashi point) in patients with RA, we excluded studies using ashi points because of only assessing the evidence of efficacy of BV on acupoint. Even if we expand the inclusion criteria to these points, no further studies were found. However, many trials used acupoints with painful points. Further comparative studies are needed for finding the difference of effects of BVA on acupoints and painful points.
One could question the validity of the conclusion by pointing to the review method used (reviewing a small number of trials with many limitations). However, reasons for doing a systematic review would be to answer questions not posted by individual studies, to settle controversies arising from apparently conflicting studies, or to generate new hypotheses.41 A systematic review with a small number of trials can be done.
In conclusion, currently, very few trials have tested the effects of BVA in the management of RA. Collectively, the evidence is insufficient to suggest that BVA is an effective therapy for RA. Further studies should be of high quality, with a particular emphasis on designing adequate and appropriate control groups.
Supplementary Material
Footnotes
Contributors: MSL and JAL conceived and designed the review. JHJ and MJS extracted the data. MJS, JC and J-IK analysed the data. JAL, MJS, JC, J-IK and MSL wrote the paper. JHJ, MJS and JC searched and selected studies. JAL and MSL revised the paper. MSL monitored data collection.
Funding: JAL, JC, JHJ and MSL were supported by Korea Institute of Oriental Medicine (K14281, K14400). MJS was supported by the same institute (K14380).
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Han A, Robinson V, Judd M et al. . Tai chi for treating rheumatoid arthritis. Cochrane Database Syst Rev 2004;(3):CD004849. [DOI] [PubMed] [Google Scholar]
- 2.Odegard S, Finset A, Kvien TK et al. . Work disability in rheumatoid arthritis is predicted by physical and psychological health status: a 7-year study from the Oslo RA register. Scand J Rheumatol 2005;34:441–7. [DOI] [PubMed] [Google Scholar]
- 3.Yelin E. Work disability in rheumatic diseases. Curr Opin Rheumatol 2007;19:91–6. [DOI] [PubMed] [Google Scholar]
- 4.Kvien TK, Uhlig T. Quality of life in rheumatoid arthritis. Scand J Rheumatol 2005;34:333–41. [DOI] [PubMed] [Google Scholar]
- 5.Lubeck DP. Patient-reported outcomes and their role in the assessment of rheumatoid arthritis. Pharmacoeconomics 2004;22(2 Suppl 1):27–38. [DOI] [PubMed] [Google Scholar]
- 6.Munstedt K, Hackethal A, Schmidt K. Bee venom therapy, bee venom acupuncture of apipunture: what is the evidence behind the various health claims? Am Bee J 2005;145:665–8. [Google Scholar]
- 7.Lee MS, Pittler MH, Shin BC et al. . Bee venom acupuncture for musculoskeletal pain: a review. J Pain 2008;9:289–97. [DOI] [PubMed] [Google Scholar]
- 8.Son DJ, Lee JW, Lee YH et al. . Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol Ther 2007;115:246–70. [DOI] [PubMed] [Google Scholar]
- 9.Baek YH, Huh JE, Lee JD et al. . Antinociceptive effect and the mechanism of bee venom acupuncture (Apipuncture) on inflammatory pain in the rat model of collagen-induced arthritis: mediation by alpha2-adrenoceptors. Brain Res 2006;1073–1074:305–10. [DOI] [PubMed] [Google Scholar]
- 10.Chen HS, Qu F, He X et al. . The anti-nociceptive effect and the possible mechanism of acupoint stimulation caused by chemical irritants in the bee venom pain model Brain Res 2010;1355:61–9. [DOI] [PubMed] [Google Scholar]
- 11.Lee JD, Park HJ, Chae Y et al. . An overview of bee venom acupuncture in the treatment of arthritis. Evid Based Complement Alternat Med 2005;2:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim BS, Moon HJ, Li DX et al. . Effect of bee venom acupuncture on oxaliplatin-induced cold allodynia in rats. Evid Based Complement Alternat Med 2013;2013:369324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon DO, Park SY, Lee KJ et al. . Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. Int Immunopharmacol 2007;7:1092–101. [DOI] [PubMed] [Google Scholar]
- 14.Nah SS, Ha E, Mun SH et al. . Effects of melittin on the production of matrix metalloproteinase-1 and -3 in rheumatoid arthritic fibroblast-like synoviocytes. J Pharmacol Sci 2008;106:162–6. [DOI] [PubMed] [Google Scholar]
- 15.Darwish SF, El-Bakly WM, Arafa HM et al. . Targeting TNF-alpha and NF-kappaB activation by bee venom: role in suppressing adjuvant induced arthritis and methotrexate hepatotoxicity in rats. PLoS ONE 2013;8:e79284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JA, Son MJ, Choi J et al. . Bee venom acupuncture for rheumatoid arthritis: a systematic review protocol. BMJ Open 2014;4:e004602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Altman DG, Sterne JAC. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011) The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org [Google Scholar]
- 18.Deeks JJ, Higgins JPT, Altman DG. Chapter 9: analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011) The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org [Google Scholar]
- 19.Sterne JAC, Egger M, Moher D. Chapter 10: addressing reporting biases. In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011) The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M et al. . Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng M, Zhang WN. Clinical observation on 20 cases of bee needle therapy in the treatment of rheumatoid arthritis. Guiding J Tradit Chin Med Pharm 2011;17:71–3. [Google Scholar]
- 22.Liu XD, Zhang JL, Zheng HG et al. . Effect of bee-sting therapy on TNF-α and IL-1β in peripheral blood of rheumatoid arthritis patients. Chin Arch Tradit Chin Med 2008;26:996–7. [Google Scholar]
- 23.Zhang JL, Liu XD, Ye LH et al. . Clinical padomized comparison study of bee-sting therapy for knee synovitis caused by rheumatoid arthritis. Chin Arch Tradit Chin Med 2011;29:1904–6. [Google Scholar]
- 24.Zhou YF, Li WY. Effect of needle on hypothalamic-pituitary-adrenal axis of patient with rheumatoid arthritis. Nei Mongol J Tradit Chin Med 2012;26:1–3. [Google Scholar]
- 25.Zhu HJ, Huang SG, Tan N et al. . Clinical observation of apiotherapy combined with Chinese drug fumigation for rheumatoid arthritis. J Tradit Chin Med Univ Hunan 2010;30:70–2. [Google Scholar]
- 26.Zhou XM, Xie XL. Bee needle combined with nursing and effect of external application of Chinese medicine in the treatment of rheumatoid arthritis. Nurs Res Pract 2013;10:11–12. [Google Scholar]
- 27.Kuang HT, Lan HQ, Zhou K et al. . Clinical observation on combination of yangxuetongbi decoction and bee pricking for the treatment of 32 cases of atrophic arthritis. Hunan Guiding J Tradit Chin Med Pharmacol 2004;10:6–8. [Google Scholar]
- 28.Ji W, Zhang MJ, Ma YZ. Clinical observation of tripterygium forrestii and bee venom in treating rheumatoid arthritis. Zhongguo Zhong Xi Yi Jie He Za Zhi [Chin J Integrated Tradit West Med] 1993;13:743–4. [Google Scholar]
- 29.Cai J. Clinical observation on 42 cases of needle treatment of rheumatoid arthritis. Clin J Anhui Tradit Chin Med 1997;9:16–17. [Google Scholar]
- 30.Xu J, Pan ZG, Chen LL et al. . Clinical study on apistoxin injection direct current electric acupoint introduction for the treatment of Bi syndrome. J Bee 1999(2):3–5. [Google Scholar]
- 31.Li L, Yi R, Wang YM et al. . Clinical observation on bee-sting therapy with ashi points and with points of corresponding meridians in treating rheumatoid arthritis. Shanghai J Acu-Mox 2013;32:121–2. [Google Scholar]
- 32.Pertsulenko VA. Bee venom in the treatment of infectious non-specific (rheumatoid) arthritis. Sov Med 1961;25:94–101. [PubMed] [Google Scholar]
- 33.Li L, Yi R, Wang YM et al. . Clinical observation on bee-sting therapy with ashi points and with points of corresponding meridians in treating rheumatoid arthritis. Shanghai J Acu-Mox 2013;(2):121–2. [Google Scholar]
- 34.Lee SH, Hong SJ, Kim SY. Randomized controlled double blind study of bee venom therapy on rheumatoid arthritisis. J Kor Acu Mox Soc 2003;20:80–8. [Google Scholar]
- 35.Vickers A, Goyal N, Harland R et al. . Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials 1998;19:159–66. [DOI] [PubMed] [Google Scholar]
- 36.Hwang YJ, Lee GM, Hwang WJ et al. . Clinical research of bee-venom acupuncture effects on rheumatoid arthritis. J Korean Acupunct Mox Soc 2001;18:33–42. [Google Scholar]
- 37.Hwang YJ, Lee BC. Clinical study of anaphylaxis on bee-venom acupuncture. J Korean Acupunct Mox Soc 2000;17:149–59. [Google Scholar]
- 38.Jung JW, Jeon EJ, Kim JW et al. . A fatal case of intravascular coagulation after bee sting acupuncture. Allergy Asthma Immunol Res 2012;4:107–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YK, Jang YS, Jung JW et al. . Prevalence of bee venom allergy in children and adults living in rural area of Cheju Island. J Asthma Allergy Clin Immunol 1998;18:451–7. [Google Scholar]
- 40.Yao H. Bee needle therapy. J Shanxi Elderly 2000;(9):39. [Google Scholar]
- 41.Green S, Higgins JPT, Alderson P et al. . Chapter 1: introduction. In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011) The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.