Abstract
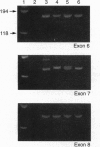
Ceruloplasmin is an abundant alpha 2-serum glycoprotein that contains 95% of the copper found in the plasma of vertebrate species. We report here on the identification of a genetic defect in the ceruloplasmin gene in a patient previously noted to have a total absence of circulating serum ceruloplasmin in association with late-onset retinal and basal ganglia degeneration. In this patient T2 (transverse relaxation time)-weighted magnetic resonance imaging of the brain revealed basal ganglia densities consistent with iron deposition, and liver biopsy confirmed the presence of excess iron. Although Southern blot analysis of the patient's DNA was normal, PCR amplification of 18 of the 19 exons composing the human ceruloplasmin gene revealed a distinct size difference in exon 7. DNA sequence analysis of this exon revealed a 5-bp insertion at amino acid 410, resulting in a frame-shift mutation and a truncated open reading frame. The validity of this mutation was confirmed by analysis of DNA from the patient's daughter, which revealed heterozygosity for this same 5-bp insertion. The presence of this mutation in conjunction with the clinical and pathologic findings demonstrates an essential role for ceruloplasmin in human biology and identifies aceruloplasminemia as an autosomal recessive disorder of iron metabolism. These findings support previous studies that identified ceruloplasmin as a ferroxidase and are remarkably consistent with recent studies on the essential role of a homologous copper oxidase in iron metabolism in yeast. The clinical and laboratory findings suggest that additional patients with movement disorders and nonclassical Wilson disease should be examined for ceruloplasmin gene mutations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askwith C., Eide D., Van Ho A., Bernard P. S., Li L., Davis-Kaplan S., Sipe D. M., Kaplan J. The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell. 1994 Jan 28;76(2):403–410. doi: 10.1016/0092-8674(94)90346-8. [DOI] [PubMed] [Google Scholar]
- Bull P. C., Thomas G. R., Rommens J. M., Forbes J. R., Cox D. W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993 Dec;5(4):327–337. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- CARTWRIGHT G. E., MARKOWITZ H., SHIELDS G. S., WINTROBE M. M. Studies on copper metabolism. XXIX. A critical analysis of serum copper and ceruloplasmin concentrations in normal subjects, patients with Wilson's disease and relatives of patients with Wilson's disease. Am J Med. 1960 Apr;28:555–563. doi: 10.1016/0002-9343(60)90150-9. [DOI] [PubMed] [Google Scholar]
- Calabrese L., Carbonaro M., Musci G. Presence of coupled trinuclear copper cluster in mammalian ceruloplasmin is essential for efficient electron transfer to oxygen. J Biol Chem. 1989 Apr 15;264(11):6183–6187. [PubMed] [Google Scholar]
- Church W. R., Jernigan R. L., Toole J., Hewick R. M., Knopf J., Knutson G. J., Nesheim M. E., Mann K. G., Fass D. N. Coagulation factors V and VIII and ceruloplasmin constitute a family of structurally related proteins. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6934–6937. doi: 10.1073/pnas.81.22.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D. W. Factors influencing serum ceruloplasmin levels in normal individuals. J Lab Clin Med. 1966 Dec;68(6):893–904. [PubMed] [Google Scholar]
- Dancis A., Yuan D. S., Haile D., Askwith C., Eide D., Moehle C., Kaplan J., Klausner R. D. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell. 1994 Jan 28;76(2):393–402. doi: 10.1016/0092-8674(94)90345-x. [DOI] [PubMed] [Google Scholar]
- Dexter D. T., Wells F. R., Lees A. J., Agid F., Agid Y., Jenner P., Marsden C. D. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease. J Neurochem. 1989 Jun;52(6):1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- Edwards C. Q., Williams D. M., Cartwright G. E. Hereditary hypoceruloplasminemia. Clin Genet. 1979 Apr;15(4):311–316. doi: 10.1111/j.1399-0004.1979.tb01740.x. [DOI] [PubMed] [Google Scholar]
- Fleming R. E., Gitlin J. D. Primary structure of rat ceruloplasmin and analysis of tissue-specific gene expression during development. J Biol Chem. 1990 May 5;265(13):7701–7707. [PubMed] [Google Scholar]
- Gitlin J. D., Schroeder J. J., Lee-Ambrose L. M., Cousins R. J. Mechanisms of caeruloplasmin biosynthesis in normal and copper-deficient rats. Biochem J. 1992 Mar 15;282(Pt 3):835–839. doi: 10.1042/bj2820835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin J. D. Transcriptional regulation of ceruloplasmin gene expression during inflammation. J Biol Chem. 1988 May 5;263(13):6281–6287. [PubMed] [Google Scholar]
- Hackett B. P., Gitlin J. D. Cell-specific expression of a Clara cell secretory protein-human growth hormone gene in the bronchiolar epithelium of transgenic mice. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9079–9083. doi: 10.1073/pnas.89.19.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A., Wada Y., Suzuki T., Shimizu A. Studies on familial hypotransferrinemia: unique clinical course and molecular pathology. Am J Hum Genet. 1993 Jul;53(1):201–213. [PMC free article] [PubMed] [Google Scholar]
- Koschinsky M. L., Funk W. D., van Oost B. A., MacGillivray R. T. Complete cDNA sequence of human preceruloplasmin. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5086–5090. doi: 10.1073/pnas.83.14.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. R., Nacht S., Lukens J. N., Cartwright G. E. Iron metabolism in copper-deficient swine. J Clin Invest. 1968 Sep;47(9):2058–2069. doi: 10.1172/JCI105891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J. F., Grimes A. Isolation of a human ceruloplasmin cDNA clone that includes the N-terminal leader sequence. FEBS Lett. 1986 Jul 28;203(2):185–190. doi: 10.1016/0014-5793(86)80739-6. [DOI] [PubMed] [Google Scholar]
- Miyajima H., Nishimura Y., Mizoguchi K., Sakamoto M., Shimizu T., Honda N. Familial apoceruloplasmin deficiency associated with blepharospasm and retinal degeneration. Neurology. 1987 May;37(5):761–767. doi: 10.1212/wnl.37.5.761. [DOI] [PubMed] [Google Scholar]
- Osaki S., Johnson D. A., Frieden E. The mobilization of iron from the perfused mammalian liver by a serum copper enzyme, ferroxidase I. J Biol Chem. 1971 May 10;246(9):3018–3023. [PubMed] [Google Scholar]
- Osaki S., Johnson D. A., Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966 Jun 25;241(12):2746–2751. [PubMed] [Google Scholar]
- Roeser H. P., Lee G. R., Nacht S., Cartwright G. E. The role of ceruloplasmin in iron metabolism. J Clin Invest. 1970 Dec;49(12):2408–2417. doi: 10.1172/JCI106460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEINBERG I. H., GITLIN D. Deficiency of ceruloplasmin in patients with hepatolenticular degeneration (Wilson's disease). Science. 1952 Oct 31;116(3018):484–485. doi: 10.1126/science.116.3018.484. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Gitlin J. D. Mechanisms of copper incorporation during the biosynthesis of human ceruloplasmin. J Biol Chem. 1991 Mar 15;266(8):5128–5134. [PubMed] [Google Scholar]
- Takahashi N., Ortel T. L., Putnam F. W. Single-chain structure of human ceruloplasmin: the complete amino acid sequence of the whole molecule. Proc Natl Acad Sci U S A. 1984 Jan;81(2):390–394. doi: 10.1073/pnas.81.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R. E., Petrukhin K., Chernov I., Pellequer J. L., Wasco W., Ross B., Romano D. M., Parano E., Pavone L., Brzustowicz L. M. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993 Dec;5(4):344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Heiny M. E., Gitlin J. D. Isolation and characterization of a human liver cDNA as a candidate gene for Wilson disease. Biochem Biophys Res Commun. 1993 Nov 30;197(1):271–277. doi: 10.1006/bbrc.1993.2471. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Heiny M. E., Shimizu N., Aoki T., Gitlin J. D. Expression of the Wilson disease gene is deficient in the Long-Evans Cinnamon rat. Biochem J. 1994 Jul 1;301(Pt 1):1–4. doi: 10.1042/bj3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Naylor S. L., Lum J. B., Cutshaw S., McCombs J. L., Naberhaus K. H., McGill J. R., Adrian G. S., Moore C. M., Barnett D. R. Characterization, mapping, and expression of the human ceruloplasmin gene. Proc Natl Acad Sci U S A. 1986 May;83(10):3257–3261. doi: 10.1073/pnas.83.10.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]