Abstract
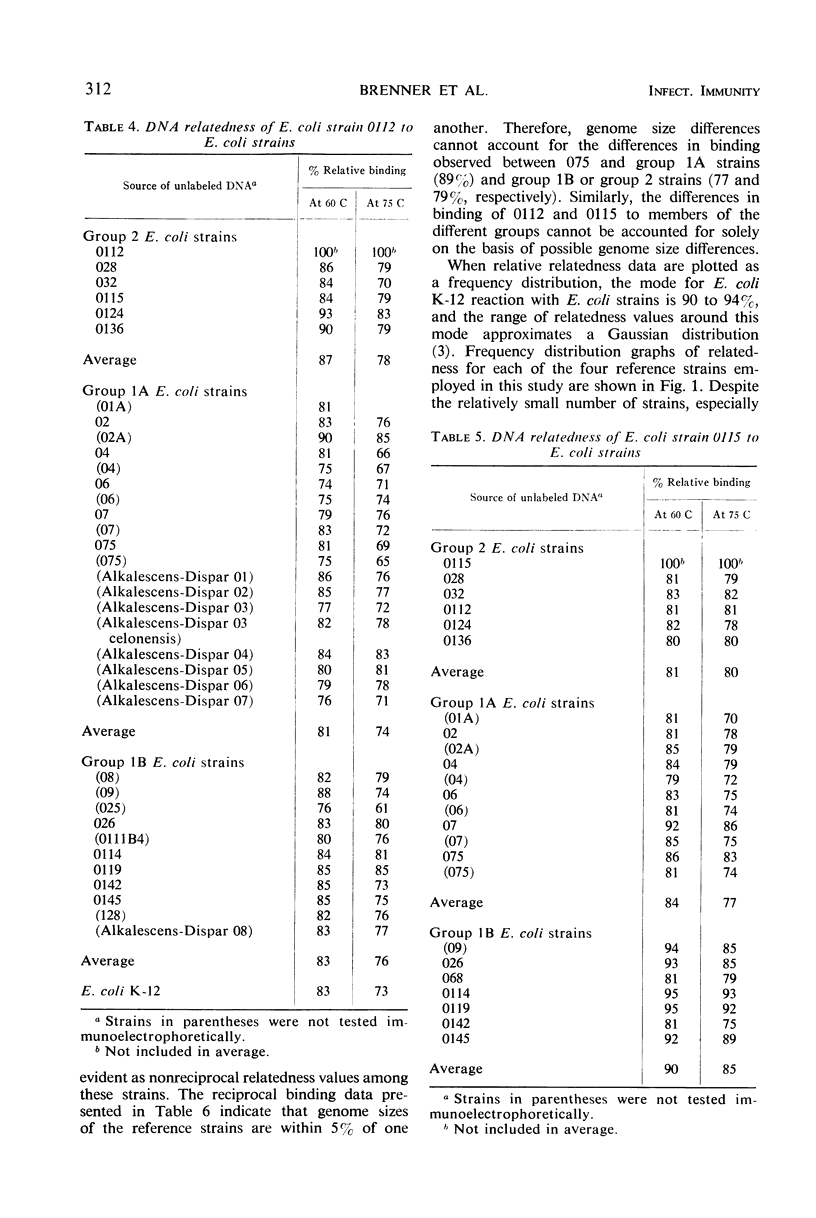
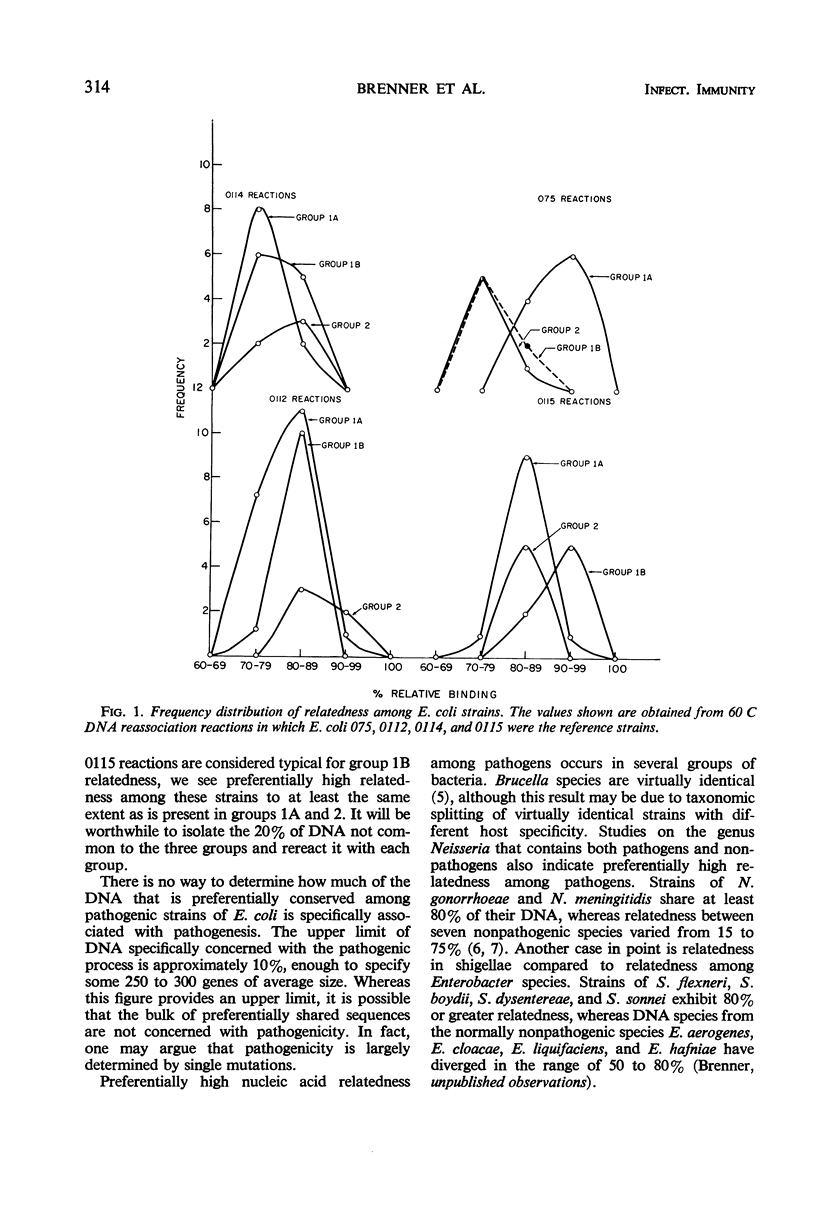
Escherichia coli strains that cause dysentery-like disease, parenteral infection, and infantile diarrhea form specific groups based on mobility of O and K antigens in immunoelectrophoresis. Members from each of these groups were assayed for gross nucleotide sequence relatedness. The method used was interspecific deoxyribonucleic acid (DNA) reassociation reactions carried out free in solution. Reassociated DNA was separated from unreacted DNA by passage through hydroxyapatite. DNA relatedness between these groups was approximately 80%. The groups containing those strains causing parenteral infection and those responsible for dysentery-like disease showed preferentially high intragroup DNA relatedness. The group containing strains responsible for infantile diarrhea did not show preferentially high intragroup DNA relatedness with the reference strain employed. These strains, however, did exhibit preferentially high DNA relatedness to a second reference strain.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner D. J., Fanning G. R., Johnson K. E., Citarella R. V., Falkow S. Polynucleotide sequence relationships among members of Enterobacteriaceae. J Bacteriol. 1969 May;98(2):637–650. doi: 10.1128/jb.98.2.637-650.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Rake A. V., Johnson K. E. Batch procedure for thermal elution of DNA from hydroxyapatite. Anal Biochem. 1969 Apr 4;28(1):447–459. doi: 10.1016/0003-2697(69)90199-7. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Skerman F. J., Falkow S. Polynucleotide sequence divergence among strains of Escherichia coli and closely related organisms. J Bacteriol. 1972 Mar;109(3):953–965. doi: 10.1128/jb.109.3.953-965.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis M., De Ley J., De Cleene M. The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem. 1970 Jan;12(1):143–153. doi: 10.1111/j.1432-1033.1970.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Hoyer B. H., McCullough N. B. Polynucleotide homologies of Brucella deoxyribonucleic acids. J Bacteriol. 1968 Feb;95(2):444–448. doi: 10.1128/jb.95.2.444-448.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T. Deoxyribonucleic acid homologies among species of the genus Neisseria. J Bacteriol. 1967 Oct;94(4):870–874. doi: 10.1128/jb.94.4.870-874.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T., Fanning G. R., Johnson K. E., Brenner D. J. Thermal stability of interspecies Neisseria DNA duplexes. J Gen Microbiol. 1969 Feb;55(2):201–208. doi: 10.1099/00221287-55-2-201. [DOI] [PubMed] [Google Scholar]
- Orskov F., Orskov I., Jann B., Jann K. Immunoelectrophoretic patterns of extracts from all Escherichia coli O and K antigen test strains: correlation with pathogenicity. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(2):142–152. doi: 10.1111/j.1699-0463.1971.tb02141.x. [DOI] [PubMed] [Google Scholar]


