Abstract
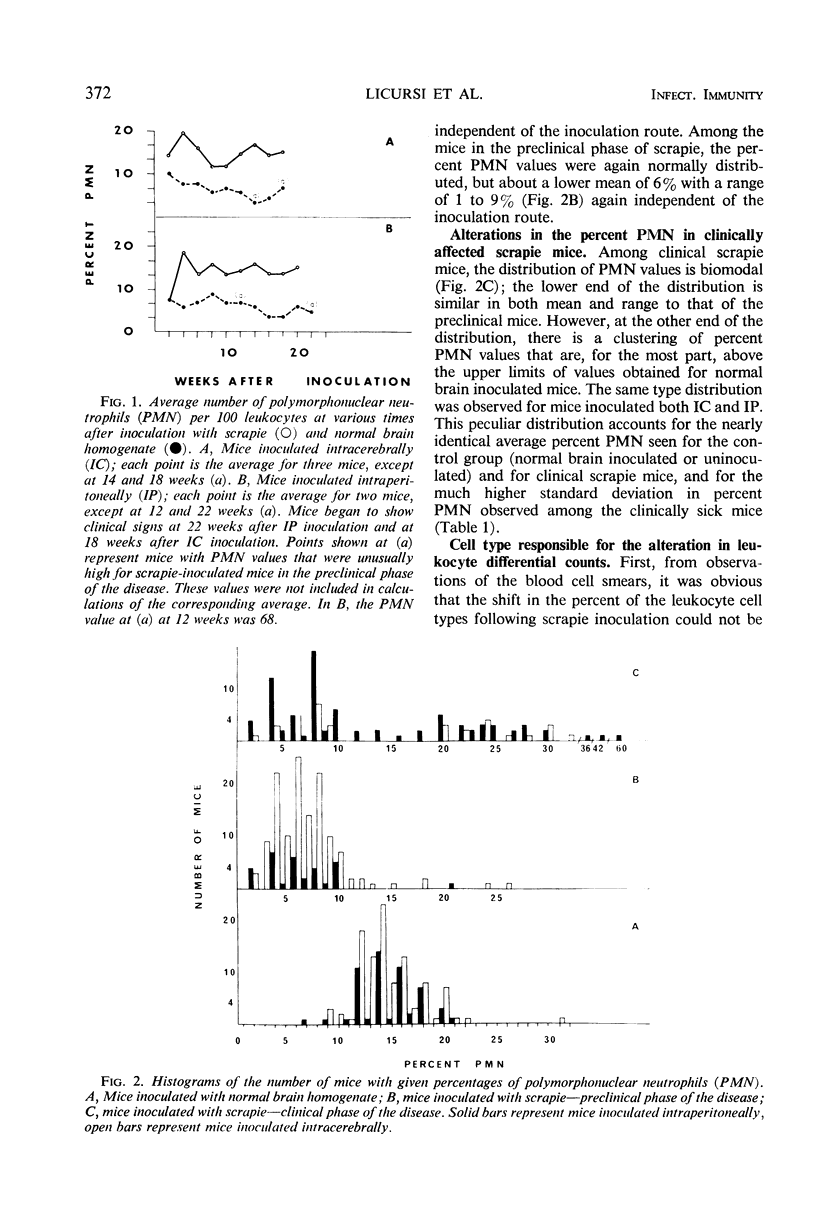
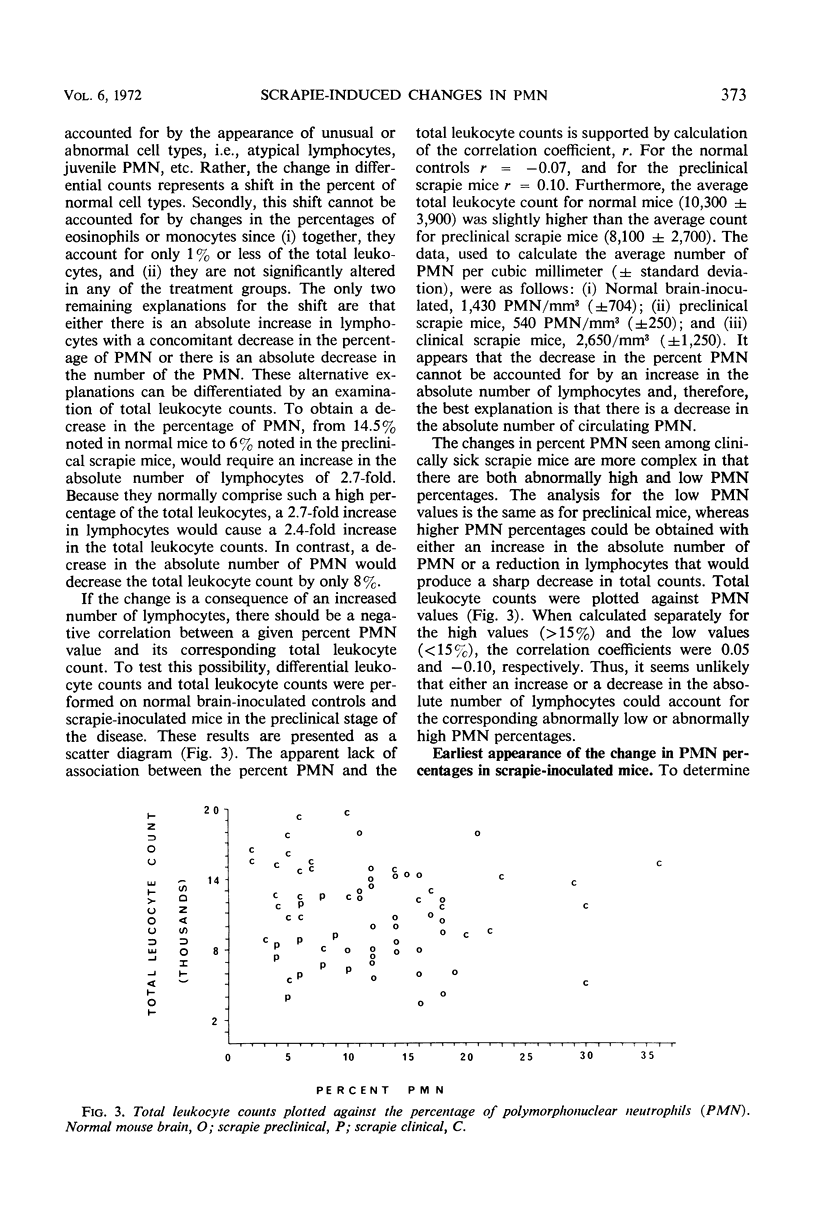
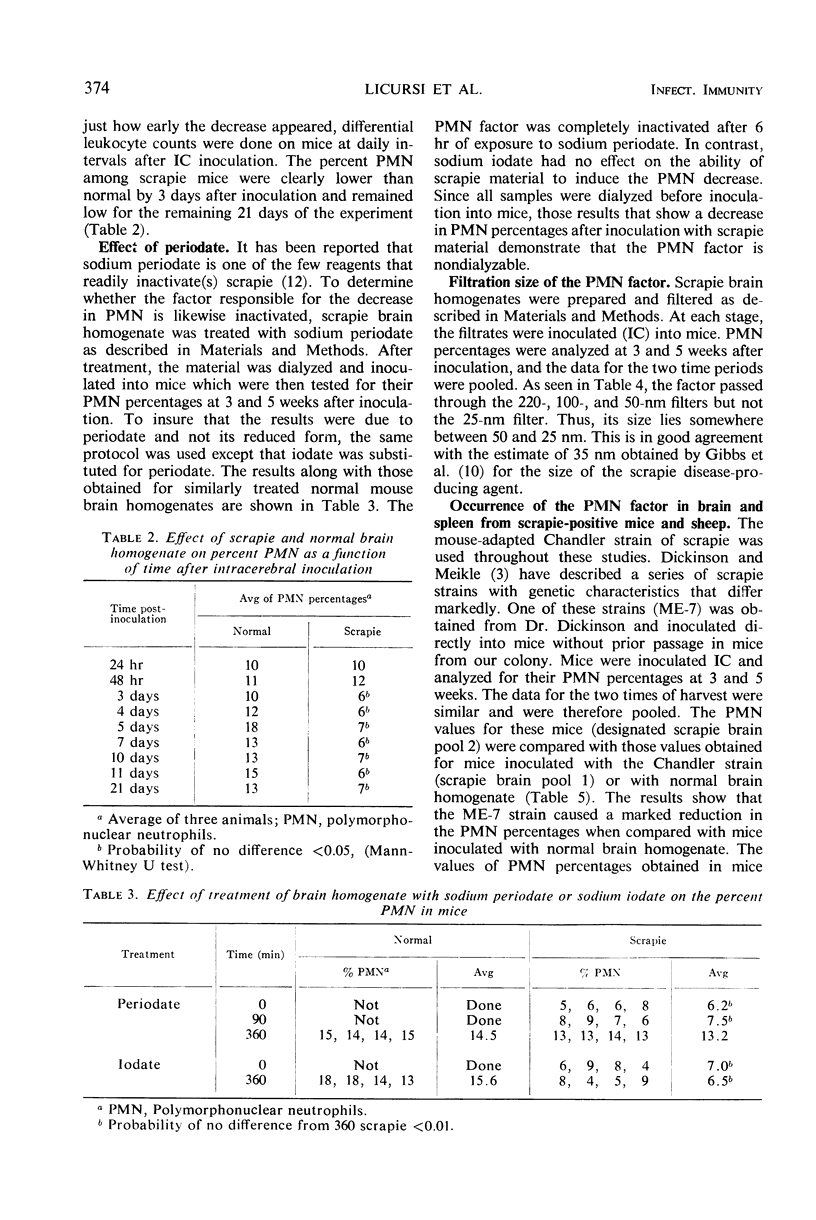
A decrease in the percentage of polymorphonuclear neutrophils (PMN) in the peripheral blood of mice appeared 3 days after intracerebral (IC) inoculation with scrapie mouse brain homogenate. Mice inoculated IC with normal mouse brain had PMN percentages similar to those found for uninoculated mice. This difference between normal and scrapie-inoculated mice continued throughout the preclinical phase of the disease. In the clinical phase of the disease, the percentage of PMN was either higher or lower than that found in normals. The factor causing the decrease in PMN percentages was found in the filtrates from 220-, 100-, and 50-nm filters, but not in the filtrates from a 25-nm filter. Sodium periodate treatment of the scrapie brain samples eliminated their ability to cause the decrease in PMN percentages, whereas sodium iodate had no effect. In addition to two genetically different scrapie mouse brain isolates, homogenates of mouse spleen, sheep brain, and sheep spleen from scrapie-affected animals caused a decrease in percent PMN, whereas the corresponding normal tissue homogenates did not.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANDLER R. L. Encephalopathy in mice produced by inoculation with scrapie brain material. Lancet. 1961 Jun 24;1(7191):1378–1379. doi: 10.1016/s0140-6736(61)92008-6. [DOI] [PubMed] [Google Scholar]
- Clarke M. C. The antibody response of scrapie-affected mice to immunisation with sheep red blood cells. Res Vet Sci. 1968 Nov;9(6):595–597. [PubMed] [Google Scholar]
- DUNN T. B. Normal and pathologic anatomy of the reticular tissue in laboratory mice, with a classification and discussion of neoplasms. J Natl Cancer Inst. 1954 Jun;14(6):1281–1433. [PubMed] [Google Scholar]
- Dickinson A. G., Meikle V. M. Host-genotype and agent effects in scrapie incubation: change in allelic interaction with different strains of agent. Mol Gen Genet. 1971;112(1):73–79. doi: 10.1007/BF00266934. [DOI] [PubMed] [Google Scholar]
- Eklund C. M., Kennedy R. C., Hadlow W. J. Pathogenesis of scrapie virus infection in the mouse. J Infect Dis. 1967 Feb;117(1):15–22. doi: 10.1093/infdis/117.1.15. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C., Gibbs C. J., Jr, Alpers M. Transmission and passage of experimenal "kuru" to chimpanzees. Science. 1967 Jan 13;155(3759):212–214. [PubMed] [Google Scholar]
- Gajdusek D. C., Gibbs C. J., Jr, Asher D. M., David E. Transmission of experimental kuru to the spider monkey (Ateles geoffreyi). Science. 1968 Nov 8;162(3854):693–694. doi: 10.1126/science.162.3854.693. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C., Gibbs C. J., Jr Transmission of two subacute spongiform encephalopathies of man (Kuru and Creutzfeldt-Jakob disease) to new world monkeys. Nature. 1971 Apr 30;230(5296):588–591. doi: 10.1038/230588a0. [DOI] [PubMed] [Google Scholar]
- Gardiner A. C., Marucci A. A. Immunological responsiveness of scrapie infected mice. J Comp Pathol. 1969 Apr;79(2):233–235. doi: 10.1016/0021-9975(69)90010-3. [DOI] [PubMed] [Google Scholar]
- Hourrigan J. L., Klingsporn A. L., McDaniel H. A., Riemenschneider M. N. Natural scrapie in a goat. J Am Vet Med Assoc. 1969 Mar 1;154(5):538–539. [PubMed] [Google Scholar]
- Hunter G. D., Gibbons R. A., Kimberlin R. H., Millson G. C. Further studies of the infectivity and stability of extracts and homogenates derived from scrapie affected mouse brains. J Comp Pathol. 1969 Jan;79(1):101–108. doi: 10.1016/0021-9975(69)90033-4. [DOI] [PubMed] [Google Scholar]
- Kimberlin R. H., Millson G. C., Mackenzie A. Biochemical and histopathological changes in the brains of mice inoculated with scrapie by the intraperitoneal route. J Comp Pathol. 1971 Oct;81(4):469–477. doi: 10.1016/0021-9975(71)90074-0. [DOI] [PubMed] [Google Scholar]
- MILLSON G. C., WEST L. C., DEW S. M. Biochemical and haematological observation on the blood and cerebrospinal fluid of clinically healthy and scrapie-affected goats. J Comp Pathol. 1960 Apr;70:194–198. doi: 10.1016/s0368-1742(60)80019-7. [DOI] [PubMed] [Google Scholar]
- May W. W. Creutzfeldt-Jakob disease. 1. Survey of the literature and clinical diagnosis. Acta Neurol Scand. 1968;44(1):1–32. doi: 10.1111/j.1600-0404.1968.tb07440.x. [DOI] [PubMed] [Google Scholar]
- Mould D. L., Dawson A. M., Rennie J. C. Very early replication of scrapie in lymphocytic tissue. Nature. 1970 Nov 21;228(5273):779–780. doi: 10.1038/228779a0. [DOI] [PubMed] [Google Scholar]
- Mould D. L., Dawson M., Slater J. S., Zlotnik I. Relationships between chemical changes and histological damage in the brains of scrapie affected mice. J Comp Pathol. 1967 Oct;77(4):393–403. doi: 10.1016/0021-9975(67)90024-2. [DOI] [PubMed] [Google Scholar]
- WILSON D. R., ANDERSON R. D., SMITH W. Studies in scrapie. J Comp Pathol. 1950 Oct;60(4):267–282. doi: 10.1016/s0368-1742(50)80025-5. [DOI] [PubMed] [Google Scholar]


