Abstract
The field vole (Microtus agrestis) is a known maintenance host of Mycobacterium microti. Previous studies have shown that infected animals develop tuberculosis. However, the disease is also known in cats and is sporadically reported from humans and other mammalian species. We examined trapped field voles from an endemic area, using a range of diagnostic approaches. These confirmed that a combination of gross and histological examination with culture is most appropriate to identify the true prevalence of the disease, which was shown to be more than 13% at times when older animals that have previously been shown to be more likely to develop the disease dominate the population. The thorough pathological examination of diseased animals showed that voles generally develop systemic disease with most frequent involvement of spleen and liver, followed by skin, lymph nodes, and lungs. The morphology of the lesions was consistent with active disease, and their distribution suggested skin wounds or oral and/or aerogenic infection as the main portal of entry. The demonstration of mycobacteria in open skin lesions, airways, and salivary glands indicated bacterial shedding from the skin and with sputum and saliva. This suggests not only the environment but also direct contact and devouring as likely sources of infection.
Keywords: field vole (Microtus agrestis), Mycobacterium microti, pathogenesis, transmission, tuberculosis
Tuberculosis in field voles (Microtis agrestis, Linnaeus 1761) was first reported in 1937.48 The causative agent, initially named Mycobacterium tuberculosis subsp muris, is now called Mycobacterium microti and is a member of the M. tuberculosis complex.45,46 Differentiation of M. microti from other members of the M. tuberculosis complex is difficult on the basis of biochemical properties but is readily possible by genotypic methods.7,21,44,46 Based on phylogenetic analyses, M. microti could now be classified as a vole-adapted ecotype of the M. tuberculosis complex.35
The field vole has been identified as the most likely maintenance host of M. microti in Great Britain.5,7,36 However, natural infection has also been observed in other small rodents.7,49 Sporadic human cases have been reported throughout Europe, and a range of other mammalian species, including cats, alpacas, llamas, badgers, pigs, cattle, horses, dogs, and ferrets, have been diagnosed with tuberculosis due to M. microti; among these, cats are most frequently affected.24,31,36 Molecular typing of a large number of M. microti strains has identified a geographically localized genotypic diversity, similar to that seen for Mycobacterium bovis in cattle.36
A total of 27 human cases of M. microti tuberculosis have been reported, both in immunocompetent and immunosuppressed patients in Western Europe (including Great Britain, the Netherlands, Germany, and France), and in the vast majority (19/27) as pulmonary tuberculosis.31 M. microti has in the past also been used in antituberculosis vaccine trials.4,28
Vole tuberculosis is also of ecological interest due to its adverse effect on the condition and possibly also the survival of field voles in endemic areas.5 In the wild, the disease does not affect juvenile voles and has an increasing prevalence with age.5 Skin lesions, which have been used to diagnose the disease in host individuals, only occur in advanced stages and are therefore most prevalent in older animals.5,36,48,49 So far, however, more detailed studies on the pathological features and the pathogenesis of vole tuberculosis are scarce and focused mainly on the hallmark lesions in the skin, which can confirm the disease but have been shown to underestimate its actual prevalence.5,8,28,32,35 Its role in the dynamics of natural populations cannot be evaluated with confidence until its prevalence can be determined accurately and major routes of transmission identified. Therefore, we have undertaken a thorough pathological examination of the type, distribution, and composition of M. microti–induced tuberculosis in wild field voles from which we draw conclusions on the possible natural routes of transmission to voles, and from these to other species, such as cats.
Materials and Methods
Field Study and Animals
The study was performed on wild field voles in Kielder forest, a 600-km2 coniferous plantation forest in Northumberland, at the border between England and Scotland, United Kingdom. Field voles in Kielder forest had previously been identified as an endemically M. microti–infected population, with an 8% prevalence of tuberculosis, based on the presence of characteristic skin lesions.7,48 Trapping was performed biannually, in spring (March), before the breeding season, when only old, over-wintered animals (6–8 months old) are present, and in autumn (September), during and after the breeding season (dominance of 2- to 3-month-old voles), between September 2001 and March 2003 (4 surveys) and between March 2005 and March 2007 (5 surveys).5 Field voles were trapped in grassy clear-cuts within a coniferous forest. Field voles in Kielder exhibit cyclic dynamics, and hence a wide range of population densities were sampled throughout the study period.26 All animals were euthanized by exposure to isofluorane and subsequent dislocation of the cervical vertebrae. They were necropsied and thoroughly examined to identify any gross tuberculosis lesions. From the first 3 surveys, gross lesions were collected for histological confirmation of the disease, and culture was performed on lymph nodes (pooled superficial cervical, axillary, and brachial lymph nodes) of voles from the survey in March 2002 and lymph nodes (as above and mesenteric lymph nodes) and lung of animals from the survey in September 2002. From all animals trapped in March 2003, bacterial cultures were undertaken from lungs and lymph nodes, and organs (lungs, lymph nodes, spleens, and liver) were examined histologically in addition to and regardless of the presence of any gross lesions. In addition, from animals trapped in March 2006, lungs, spleens, and livers were examined histologically and cultured independent of the presence of gross lesions. From all other animals in the 5 surveys from March 2005 to March 2007, organs/tissues with gross tuberculosis lesions were examined by histology and bacterial culture. Polymerase chain reaction (PCR) was employed on cultures and/or gross lesions to confirm M. microti infection.5,27 In a proportion of cases, the mycobacterial species was confirmed by spoligotyping and IS6110 RFLP typing of positive cultures as part of another project.5,21,44
Case Selection and Tissue Processing
For a thorough assessment of the frequency and distribution of tuberculosis lesions, all voles from the survey in March 2003 (n = 327) were used. From these animals, sections of lungs, spleen, liver, kidneys, small intestine, and lymph nodes (see above) as well as all gross lesions indicative of tuberculosis were examined histologically. Gross lesions were classified as “external lesions” when obvious skin lesions with crust formation were observed, whereas “internal lesions” comprised those that were only obvious after skinning (mainly in lymph nodes) and any gross lesions within internal organs. In addition, gross lesions consistent with tuberculosis from all surveys were examined histologically.
Tissues were fixed in 10% nonbuffered formalin, either fresh (surveys in 2001–2003 and in March 2006) or after freezing and subsequent thawing (surveys in 2005, September 2006, and March 2007), and routinely paraffin embedded. The sections 3-5 μm were prepared and stained with hematoxylineosin (HE), the Ziehl-Neelsen (ZN) stain for the demonstration of acid-fast bacilli (AFB), and, in selected cases, the van Kossa stain for the demonstration of calcium salt deposition (mineralization). Additional sections were used for immunohistological examinations.
Immunohistology
For the identification of inflammatory cells within the tuberculous lesions, antibodies known to cross-react in many mammalian species were tested in the spleens of field voles and applied to sections of tuberculous lesions to demonstrate T cells (rabbit anti–human CD3; Dako, Glostrup, Denmark), B cells (rat anti–human CD79a; Dako), and neutrophils as well as macrophages, including epithelioid cells and multinucleate giant cells (rabbit anti–swine lysozyme; Dako). The peroxidase anti-peroxidase (PAP) and the avidin biotin peroxidase complex (ABC) method were applied according to previously published protocols.19,23
Consecutive sections, incubated with normal rabbit serum or a nonreacting mouse monoclonal antibody, respectively, were used as negative controls for polyclonal and monoclonal antibodies. A canine lymph node served as a positive control for all antigens, and the mesenteric lymph node section included from all voles served to confirm cross-reactivity with vole leukocytes.
Transmission Electron Microscopy
Sections of grossly identified lung lesions that were subsequently confirmed as tuberculous lesions by histology on adjacent tissue sections from a vole trapped in March 2006 were routinely processed for transmission electron microscopy (TEM). Briefly, tissue blocks (2 mm3) were prepared and fixed in 4% paraformaldehyde (pH 7.4) with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer at 4°C, then washed in 0.1 M sodium cacodylate buffer and secondarily fixed in 1% osmium tetroxide(aq) for 90 minutes, followed by routine resin embedding and the preparation of semi-thin sections (0.5 μm) for the identification of areas of interest, and the ultrastructural examination of subsequently prepared ultrathin sections (60 nm).
Results
Comparison of Diagnostic Methods
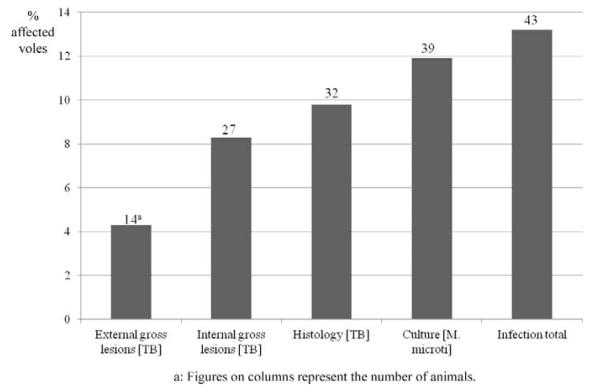
For a comparison of the sensitivity of the different methods (assessment of external and internal gross lesions, histology for the identification of tuberculous lesions, mycobacterial culture) for the detection/assessment of tuberculosis and mycobacterial infection, all 327 animals collected in March 2003 were examined (Fig. 1). Based on the presence of external gross lesions (prior to dissection; all confirmed by histology), a disease prevalence of 4.3% (n = 14) was identified. Internal gross lesions were observed in about twice as many cases (8.9%, n = 27), whereas the histological examination identified tuberculous lesions in 9.8% (n = 32) of the voles. Four of the latter animals (12.5%) were negative in the culture. On the other hand, M. microti was isolated from 11.9% (n = 39) of animals; 9 of these (23.1%) did not exhibit any gross and histological alterations indicative of the disease. Altogether, 13.2% (43/327) of animals were identified as infected with M. microti, by histopathology and/or culture.
Figure 1.
Prevalence of tuberculosis/Mycobacterium microti infection in 1 survey (survey 4; n = 327) assessed by different detection methods (external and internal gross lesions, histologically confirmed tuberculosis (TB) lesions, mycobacterial culture). “Infection total” represents the results of histology and culture.
Gross Tuberculosis Lesions
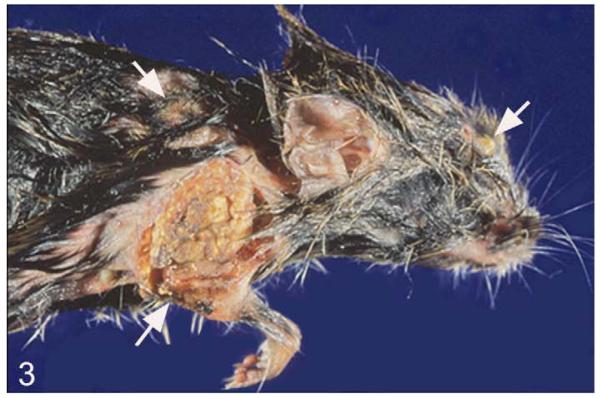
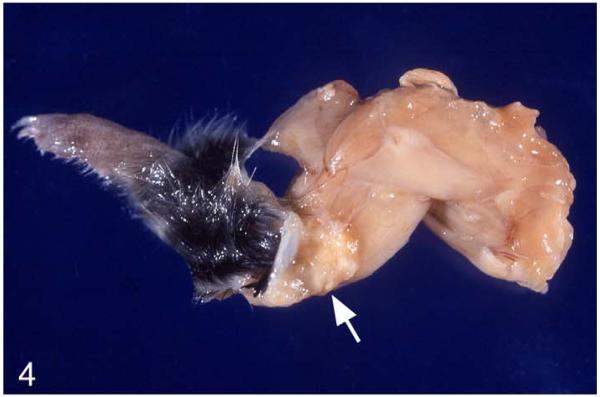
External lesions were represented by (multi)focal, nodular skin areas with superficial ulceration and crust formation, often between the shoulder blades (March 2003: n = 10 [31.3% of voles with histologically confirmed tuberculosis]; Fig. 2a), but also in the neck midline (Fig. 2a,b), on 1 shoulder, and, less frequently, on the head, back, abdomen, chest, and rump. Lesions often extended into the subcutaneous adipose tissue (Fig. 2b). In severe cases, large areas of the skin at any site of the body were affected (Fig. 3). Occasionally, skin lesions were inconspicuous and externally obvious only as a subcutaneous nodule (Fig. 4).
Figure 2.
Gross lesions of Mycobacterium microti tuberculosis in a field vole. (a) Skin scab in dorsal midline, between shoulder blades (arrow). (b) After partial skinning of the animal in (a), the inflammatory process underlying the external skin lesion is obvious as an area of hyperemia in the adipose tissue cushion between the shoulder blades (arrow). A further granulomatous lesion is present in the adipose tissue in the neck midline (arrowhead).
Figure 3.
M. microti tuberculosis in a field vole, represented by severe, multifocal skin lesions with superficial ulceration and crust formation (arrows).
Figure 4.
M. microti tuberculosis in a field vole. Foreleg with extensive multifocal, coalescing granulomatous cellulitis (arrow), involving the axillary lymph node.
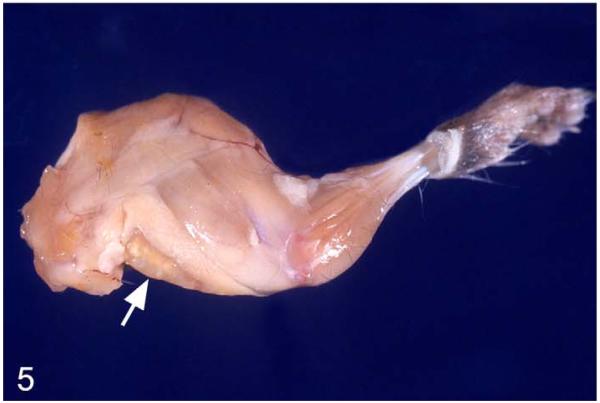
Internal lesions were represented by granulomas in the adipose tissue between the shoulder blades (consistent with the pyramid-shaped so-called anterior subcutaneous fat compartment described in mice) or in the neck midline (Fig. 2b), with (multiple) granulomas within enlarged lymph nodes (mainly axillary/brachial and superficial cervical lymph nodes; Fig. 5) often extending into the adjacent (adipose) tissue, enlargement of mediastinal lymph nodes, and granulomas within lungs (Fig. 6). Other internal organs did not exhibit any gross changes, apart from 1 case each with obvious granulomas in the mesenteric lymph nodes and the kidneys as well as the adrenal glands.
Figure 5.
M. microti tuberculosis in a field vole. Foreleg with multifocal, granulomatous lymphadenitis (brachial lymph node; arrow).
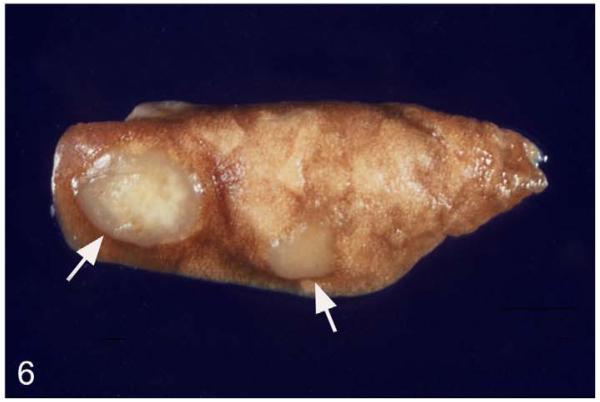
Figure 6.
M. microti tuberculosis in a field vole. Lung, left main lobe. Multifocal granulomatous pneumonia, represented by well-demarcated, elevated whitish nodules with central mineralization (arrows).
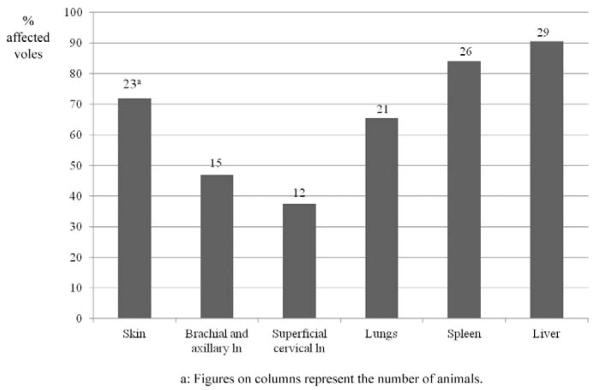
Distribution of Lesions, Based on Histological Examination
In the 32 diseased animals collected in March 2003, the histological examination identified tuberculous lesions in skin/subcutis, lymph nodes, salivary glands, lungs, liver, and spleen (Fig. 7). Interestingly, liver (90.6%; n = 29) and spleen (83.9%; n = 26) were most frequently affected, confirming that all but 2 animals had developed systemic disease. Among other tissues, the skin and subcutis were most frequently affected (71.9%; n = 23), almost exclusively at the cranial part of the body and mainly between the shoulder blades or at 1 shoulder, and less frequently at the neck, head, or thorax. In all but 1 animal in which lesions were found only in the subcutis between the shoulder blades and the axillary lymph nodes, skin involvement was seen in association with overt systemic disease (ie, lesions in spleen, liver, and lung) and in the majority of animals (19/22), also with involvement of the local lymph nodes (superficial cervical and brachial/axillary), suggesting that the skin in areas where bite wounds occur most frequently might have been the site of the primary lesion. The brachial/axillary lymph nodes were affected in 46.9% of the cases. In 2 animals, 1 brachial lymph node was the only affected tissue, and in a third vole, the only other lesion was in the subcutis between the shoulder blades. This suggests that the disease was confined to these lymph nodes (and their tributary skin area) and possibly at an early stage at the time of death of these voles. All other animals with lesions in the brachial/axillary lymph nodes showed systemic disease, as confirmed by the presence of liver and spleen lesions. The lack of further lesion sites in 4 of these voles (12.5%) suggests systemic spread of bacteria from an initial lymph node lesion, whereas the presence of lesions in tributary skin areas in the remaining 8 animals (25%) again indicates infection via the skin. Similarly, lesions in the superficial cervical lymph nodes (observed in 37.5% of voles with confirmed tuberculosis) were seen in the majority seen (9/12) with skin lesions at the head, neck, or shoulder. In the 3 animals that did not exhibit skin lesions, involvement of this lymph node might be the consequence of oral uptake of bacteria. The lung exhibited lesions in 65.6% of affected animals, always together with the mediastinal lymph nodes and other tissues, that is, spleen and liver as well as skin and draining lymph nodes (13/21), skin (6/21), or superficial cervical or brachial/axillary lymph nodes (2/12).
Figure 7.
Organ distribution of histologically confirmed tuberculosis lesions in affected animals (n = 32; survey 4). “Skin” refers to external lesions and those involving and/or restricted to the subcutis. Brachial and axillary lymph nodes are counted together, since they could not be discerned when extensive granulomatous lesions that extended into the surrounding adipose tissue were present. Mediastinal lymph nodes are not mentioned separately, since they were involved when lesions were observed in the lungs.
Morphological Features and Cellular Composition of Tuberculosis Lesions
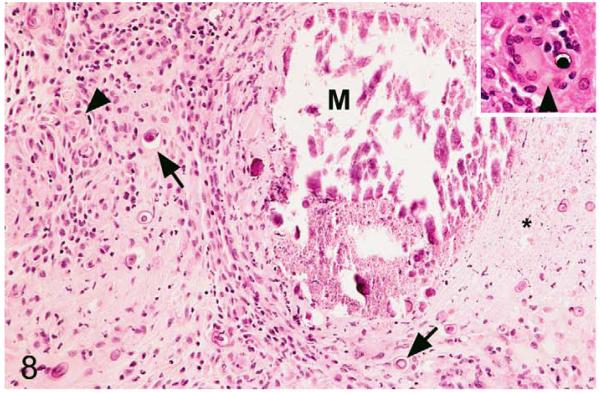
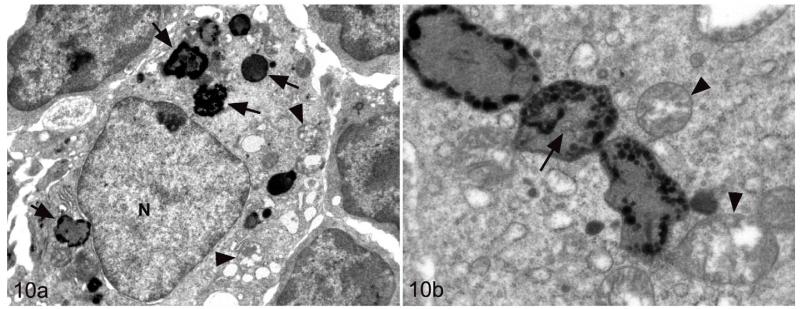
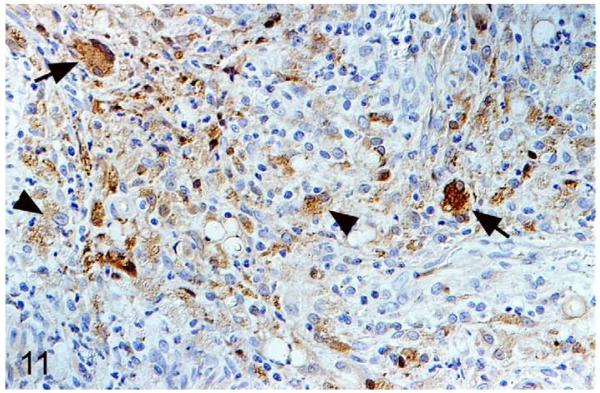
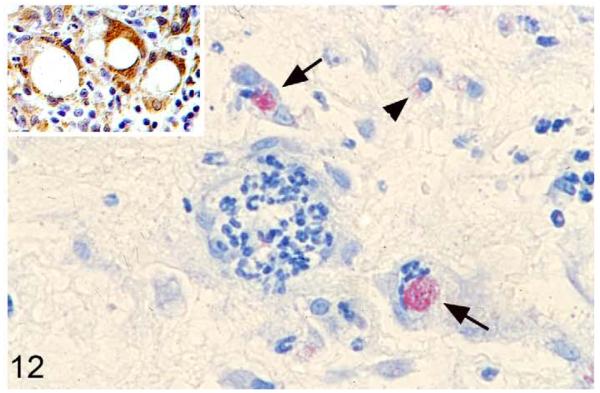
Tuberculosis lesions generally exhibited similar morphological features. They represented nondemarcated focal granulomatous infiltrates without obvious organization or evidence of peripheral fibroblast proliferation, neovascularization, or lymphatic follicle formation (Figs. 8, 9). Larger infiltrates showed central or multifocal caseous necrosis (Fig. 8) that often exhibited large irregular areas of dystrophic calcification (Fig. 8), as confirmed by the von Kossa stain. The latter also occurred as numerous, variably sized, often concentric, laminated mineralization bodies that were cell free in the infiltrate and in the periphery of the large necrotic areas (Figs. 8, 9). Mineralization was also seen intracellularly as small laminated pearls within scattered macrophages (Fig. 8) and appeared to develop within mitochondria, as the ultrastructural examination showed (Fig. 10a,b). Apart from macrophages and epithelioid cells that were the dominant cells in the infiltrate, variable numbers of multinucleated giant cells (MGCs) and numerous neutrophils were generally observed (Fig. 9). All these cells exhibited weak to intense lysozyme expression (Figs. 11, 12), which confirmed their activation.22 Lymphocytes were generally numerous in the infiltrates, both disseminated and as variably sized aggregates mainly in the periphery. They were in the majority T cells, whereas B cells dominated in the focal aggregates. In most lesions, AFB were numerous. They were found within macrophages, epithelioid cells, and MGCs, where they often appeared to aggregate within large cytoplasmic vacuoles (phagosomes) and were evident as large cell-free clumps within necrotic areas (remnants of bacilli-laden, necrotic cells; Fig. 12).
Figure 8.
Mycobacterium microti tuberculous lesion in a field vole. Histological features of tuberculous skin lesion. Granulomatous infiltrate with extensive focal caseous necrosis (highlighted by asterisk). Multifocal mineralization is seen, within the necrotic area (M), between infiltrating inflammatory cells (arrows) and intracellularly, within individual macrophages (arrowhead; see also inset). Hematoxylin and eosin (HE) stain.
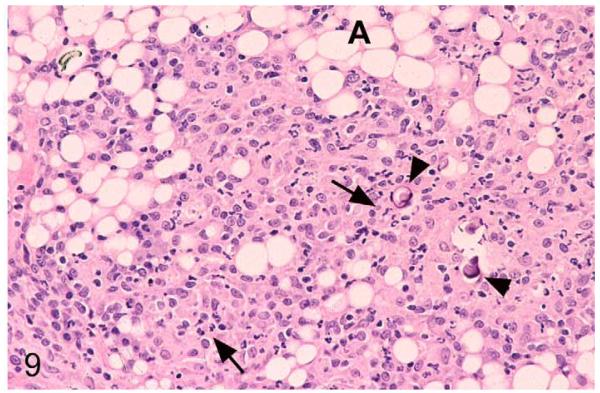
Figure 9.
M. microti tuberculous lesion in a field vole. Subcutaneous adipose tissue (A) between shoulder blades. Nondemarcated granulomatous infiltrate dominated by macrophages, with a large proportion of neutrophils (arrows). Scattered foci of mineralization are present (arrowheads). HE stain.
Figure 10.
M. microti tuberculous lesion in a field vole. Epithelioid cell in granulomatous infiltrate. (a) The cell exhibits several foci of mineralization (consistent with mitochondria) in the cytoplasm (arrows). Arrowheads: nonmineralized mitochondria. N, nucleus. (b) Cytoplasm. Beside variably swollen, nonmineralized mitochondria (arrowheads), there are 3 markedly enlarged and mineralized mitochondria, in one of which remnants of the normal mitochondrial structures are still evident (arrow). Uranyl acetate and lead citrate contrasting.
Figure 11.
M. microti tuberculous lesion in a field vole. Epithelioid macrophages (arrowheads) and multinucleated giant cells (arrows) exhibit strong lysozyme expression. Peroxidase anti-peroxidase method, Papanicolaou hematoxylin counterstain.
Figure 12.
M. microti tuberculous lesion in a field vole. Macrophages and acid-fast bacilli. Epithelioid macrophage (arrowhead) and multinucleated giant cell (arrow) with large aggregate of acid-fast bacilli within phagosome. Ziehl-Neelsen stain. Inset: Lysozyme expression in epithelioid macrophages, particularly in multinucleated giant cells. Peroxidase anti-peroxidase method, Papanicolaou hematoxylin counterstain.
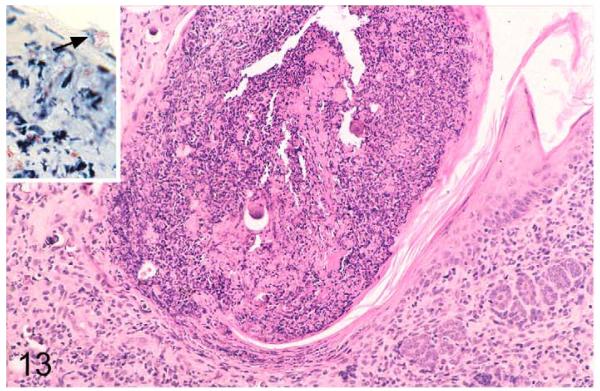
In the skin, lesions were represented by a nondemarcated, focal granulomatous dermatitis, often with involvement of the subcutaneous adipose tissue (cellulitis) and superficial ulceration and crust formation (Figs. 8, 13). Occasionally, AFB were found within these crusts (Fig. 13, inset), confirming bacterial shedding into the environment. In 1 vole with skin lesions below the left ear, the granulomatous infiltration extended into the salivary gland.
Figure 13.
Field vole with Mycobacterium microti tuberculosis. Skin lesion with granulomatous dermatitis and superficial crust formation. Hematoxylin and eosin (HE) stain. Inset: Epidermal serocellular crust with aggregate of acid-fast bacilli on the surface (arrowhead). Ziehl-Neelsen (ZN) stain.
Subcutaneous lesions between the shoulder blades and at the neck often extended into the underlying skeletal muscle layers and adipose tissue (Fig. 9) but were frequently seen without involvement of the overlying skin. In some animals, the subcutaneous adipose tissue between the shoulder blades exhibited mild focal, perivascular, granulomatous infiltration, without any evidence of AFB. Occasionally, a “fistule”-like formation was seen between the shoulder blades, with a focal infiltrate extending from the upper dermis to the underlying skeletal muscle/adipose tissue, where a more extensive infiltration was present. Some granulomatous infiltrates, however, were entirely restricted to the skeletal muscle in this area and contained large areas of necrosis and mineralization and usually myriads of AFB.
In affected lymph nodes, a focally extensive or multifocal coalescing granulomatous inflammation with extensive necrosis and mineralization was generally seen. Mineralization pearls were also present. The cellular composition was as described for the skin lesions. Inflammatory infiltrates often extended into the adjacent (adipose) tissue and, when the superficial cervical lymph node was affected, occasionally into the salivary gland (Fig. 14), likely leading to bacterial shedding with the saliva.
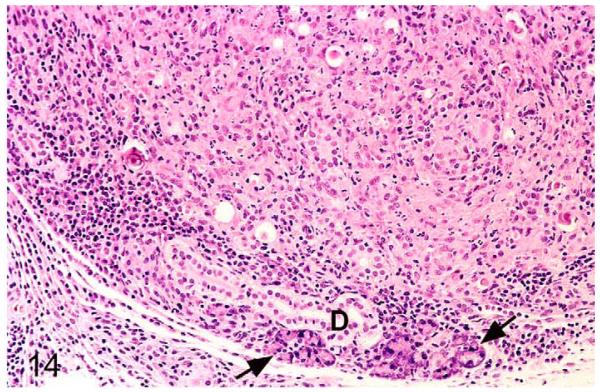
Figure 14.
Field vole with M. microti tuberculosis. Salivary gland. Most of the glandular tissue is effaced by the extensive granulomatous infiltrate. Glandular acini are seen only in the periphery (arrows). D, secretory duct. HE stain.
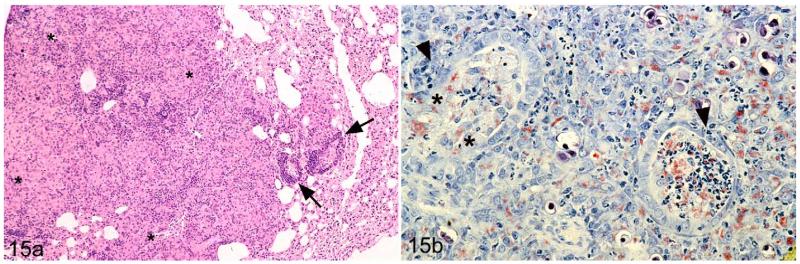
The typical focal granulomatous lesions as they were generally seen in affected lungs were occasionally associated with a (multi)focal necrotizing bronchitis and bronchiolitis with clumps of AFB in the inflammatory exudate (Fig. 15a,b), indicating discharge of bacteria with the sputum. In addition, variably intense perivascular and peribronchial B-cell–dominated lymphocyte accumulations, consistent with bronchus-associated lymphatic tissue (BALT), were observed (Fig. 15a).
Figure 15.
Field vole with M. microti tuberculous lesions in the lung. (a) Nondemarcated granulomatous infiltrate (highlighted with asterisks) with a few perivascular lymphocyte infiltrates in the periphery (arrows). HE stain. (b) Within the granulomatous infiltrate, bronchi exhibit focal loss of epithelial cells (highlighted by asterisks) and mild neutrophil and macrophage infiltration (arrowhead). The lumen of the bronchi is filled with exudate containing acid-fast bacilli, both cell free and within macrophages. ZN stain.
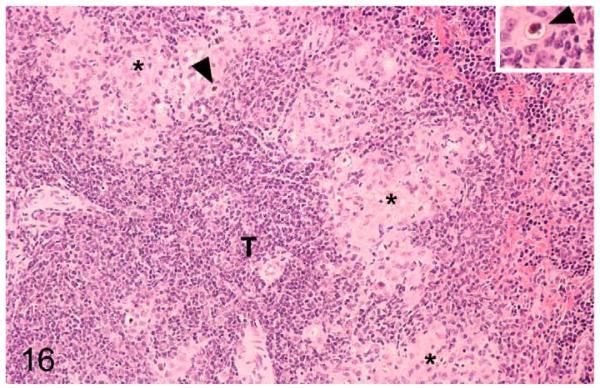
Tuberculous lesions in the spleens differed from those in other tissues. They represented small focal aggregates of macrophages and epithelioid cells with occasional neutrophils in the red pulp, immediately adjacent to T-cell zones and follicles (Fig. 16). MGCs were not observed, and AFB were very rare. However, occasional mineralization pearls were present, both cell free and within macrophages.
Figure 16.
Field vole with M. microti tuberculosis. Spleen with multifocal granulomatous infiltrates (highlighted by asterisks) in the red pulp, close to T-cell zones (T). Scattered foci of mineralization are seen (arrowheads). Inset: Higher magnification of focal mineralization. HE stain.
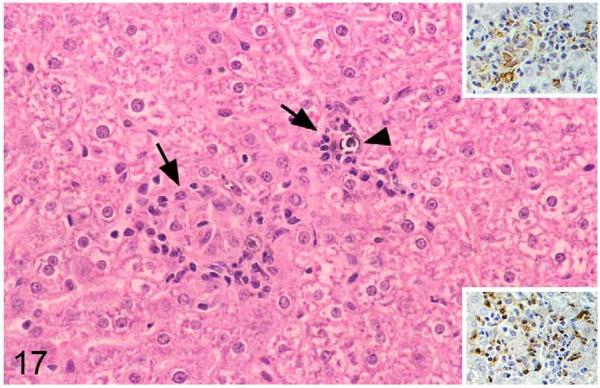
Livers exhibited disseminated lesions that were often adjacent to portal areas and were represented by small granulomatous infiltrates composed of macrophages and epithelioid cells, often together with variable numbers of MGCs and moderate numbers of lymphocytes, which were in the majority T cells (Fig. 17). AFB were very rare, but intra- and extracellular mineralization pearls were almost always observed (Fig. 17).
Figure 17.
Field vole with M. microti tuberculosis. Liver with small focal granulomatous infiltrates (arrows) composed of lysozyme-positive macrophages (upper inset) and T cells (CD3+; lower inset). Small foci of mineralization (arrowheads) are present. HE stain and peroxidase anti-peroxidase method, Papanicolaou hematoxylin counterstain.
Apart from a bilateral granulomatous nephritis and adrenalitis and a granulomatous myocarditis with the presence of abundant AFB in 1 case each, the other examined tissues did not exhibit any histological changes, and AFB were not identified.
Discussion
The present study comprises a thorough evaluation of the distribution and morphological features of M. microti–induced tuberculosis in field voles, the maintenance host of the bacterium.5,7,36 In particular in Great Britain, vole tuberculosis has been of interest as a factor affecting population dynamics of field voles in endemic areas.5,7,8,48,49 In recent years, however, its association with feline tuberculosis has been identified, and there are occasional reports of M. microti–induced tuberculosis in other species, including humans, with sporadic geographic clustering.14,36 Furthermore, a mutual geographic exclusivity of M. microti and M. bovis has been shown in the United Kingdom, indicating that M. microti may be a determinant of M. bovis epidemiology.36
In ecological studies, the prevalence of tuberculosis in field voles has usually been assessed on the basis of gross lesions.5,7,8,49 While this is certainly the most efficient approach to assess large animal cohorts and for longitudinal monitoring of the disease status, it does not reflect the true disease prevalence. We observed the typical external tuberculous skin lesions in less than 50% of field voles with the disease, of which more than 15% did not exhibit any gross lesions. It has previously been shown in studies on tuberculosis in badgers that detection rates vary depending on the examination protocol.11 Our study confirms these results and shows that for an accurate assessment of the disease prevalence in voles, a detailed histological examination of a range of tissues, including particularly liver and spleen, is essential.
However, despite the detailed histological assessment, we did not identify tuberculosis lesions in more than a quarter of the culture-positive voles. Macrophages are the primary site of mycobacterial replication and a means of dissemination. Accordingly, positive culture results might be due to low-level infection of tissue macrophages after bacteremia.12,34,37 Tissue macrophages might also be responsible for the outcome of infection in individual animals—that is, bacterial containment in macrophages or systemic inflammatory response, based on their activation and response to the mycobacteria, suggesting that a substantial proportion of voles is able to contain M. microti infection.33,47 However, mycobacterial infection without apparent gross lesions (“no visible lesions” [NVL]) is not uncommon in humans and a range of other species, particularly with M. bovis, and only with careful histological examination in particular of a wide range of lymph nodes could very small tuberculosis lesions and thereby the disease be identified.10,15,17 It therefore is possible that minute tuberculosis lesions in selected lymph nodes were not identified in the present study, since the histological examination was restricted to the superficial cervical, axillary, and brachial lymph nodes, unless gross lesions involving the lymph nodes were identified. Accordingly, it cannot be entirely ruled out that infected voles had invariably developed tuberculosis lesions (ie, the disease), and it cannot be confirmed whether they can be and remain latently infected or can clear an infection. On the other hand, we also identified a proportion of culture-negative animals with histologically confirmed tuberculosis lesions. This indicates that culture also has its limitations for the confirmation of M. microti tuberculosis and further confirms that a combined diagnostic approach is currently still required to identify the true prevalence of mycobacterial disease and/or infection in voles.
In the present study, disease/infection prevalence differed from those observed in previous studies carried out in the same endemic area, where, for example, prevalences varied between just over 20% and 50%, depending on site and time point.5,7 This reflects a known, often substantial, spatial and temporal variation and confirms that further work into interannual variation in prevalence across years and sites is needed. However, it needs to be emphasized that the present study is far more comprehensive than the previous ones, since it includes much higher sample sizes across multiple years.5,7
The distribution pattern of tuberculosis lesions in affected animals in our study confirms that vole tuberculosis is generally a systemic disease. Only in less than 10% of affected animals did we not find evidence of systemic disease (voles with lesions only in the brachial/axillary lymph node with or without involvement of a tributary area, ie, the subcutis between the shoulder blades), which indicates that diseased animals generally cannot confine the bacteria locally.
Vole tuberculosis frequently involves the skin, and we found lesions in the subcutis and/or exophytic processes with ulceration and crusts/scab formation in more than 70% of affected animals.7,8,49 These lesions did not represent a local process but were in all but 1 case associated with systemic disease. In ulcerated lesions, we occasionally found mycobacteria in the superficial crusts, indicating that the skin lesions can be a source of infection for other animals, through release of bacteria into the environment but also via direct contact.
Subcutaneous (and skin) lesions were almost exclusively observed in the cranial part of the body and were frequently seen between the shoulder blades and in the neck midline. Thorough examination of the dorsal neck between atlas and thoracic spinal cord of disease-free voles did not identify lymphatic tissue or specific (scent) glands in this location.38 Instead, the known lymph nodes draining the affected skin areas (ie, brachial/axillary and superficial cervical lymph nodes) were the most frequently involved. It therefore appears likely that the disease develops from a cutaneous infection, through skin lesions, such as bite wounds from fights or scratches that become infected from the contaminated environment. Since overt skin lesions are generally seen only in older voles, it seems also possible that they evolve at some stage from small inflammatory foci in the adipose tissue, which we also observed in some animals.36 Alternatively, some skin lesions might be the consequence of bacterial spreading from the adjacent lymph nodes or bacteremia.25,49 A more recent study provided evidence of the latter, since North American prairie voles (Microtus ochrogaster) developed “crusted caseous skin lesions” in particular between the shoulder blades, after experimental intraperitoneal infection with the vole strain ATCC 19422 at a high dose (Y. C. Manabe, personal communication, 2007).28 It remains to be elucidated whether the blood or lymphatic vessels supplying the mixed adipose tissue depots that voles exhibit at the dorsal neck and shoulder have some specific features that might “catch” infected monocytes.
Oral and/or aerogenic uptake of bacilli would be an alternative way of infection, and indeed, the presence of lesions in the superficial cervical lymph nodes without any associated skin lesions in 2 cases would be consistent with this. Similarly, Wells49 observed the development of tuberculous lesions in the “glands in the neck” in a proportion of voles housed together with an animal that had developed generalized lesions after subcutaneous infection with M. microti. He considered an oral infection from the shared feed as the likely source and showed in the same study that drinking of M. microti–infected water induces lesions not only in this location but also in lungs and mesenteric lymph nodes, thereby suggesting a combined oral and aerogenic infection. We observed extension of the inflammatory process from the superficial cervical lymph nodes into the adjacent tissue, including the salivary glands, in 2 animals, which provides evidence of bacterial shedding with the saliva.
We found tuberculous lesions in liver and spleen in more than 90% and 80% of the affected animals, respectively. This indicates that the bacteria spread readily through the lymphatic fluid and/or blood.13 They would then be taken up by specific tissue macrophages, Kupffer cells in the liver, and red pulp macrophages in the spleen, leading to the development of the observed disseminated small granulomas. Similar lesions have been reported in spleen and liver of laboratory mice after intravenous infection with M. tuberculosis and M. bovis, as well as in the spleen of M. ochrogaster voles that had been infected intraperitoneally with M. microti.1,9,28
We never found tuberculous lesions in the lungs without systemic disease (ie, involvement of spleen and/or liver as well as skin and/or nontributary lymph nodes). This suggests that lung lesions are more often a consequence of bacteremia than of aerogenic infection in naturally infected voles.13 Pulmonary lesions in the voles represented granulomas but lacked all relevant features of the classic tuberculoma that dominates in non-progressive pulmonary tuberculosis of human patients, such as the organized peripheral arrangement of follicle-like B-cell aggregates and the high vascularization of the tissue, as well as a fibrotic capsule. Instead, they were similar to the disorganized granulomas of active cavitary pulmonary tuberculosis in humans, from which bacilli are released into the sputum.37,41–43 In the voles, we also found bacilli within cell debris in the lumen of airways and, occasionally, a necrotizing bronchitis, a feature also observed in laboratory mice after intravenous M. bovis infection.9 These findings suggest that diseased voles generally cannot contain bacteria within lung lesions and can shed bacteria with the sputum.
Interestingly, none of the recent studies, including ours, on both naturally and experimentally (intraperitoneally) infected voles provided any evidence of involvement of the gastrointestinal tract. In contrast to Wells’s early studies, which describe frequent involvement of the mesenteric lymph nodes, none reported lesions in the intestine and associated lymph nodes or evidence of bacterial shedding with the feces.7,28,49 There was also no evidence of bacterial shedding with the urine, neither by culture nor by histology in our study, since we observed only lesions in the kidneys in 1 of more than 4000 examined animals.7
In the voles, the larger tuberculous lesions are granulomas with a core of caseous necrosis containing cell-free bacilli, surrounded by a variable mixture of partially bacilli-laden macrophages, epithelioid cells, and MGCs.32 Caseous necrosis in tuberculous granulomas has been interpreted in different ways. Some authors believe that it is correlated with the strength of the hypersensitivity reaction mounted by an infected animal.13 Different from badgers with tuberculosis and laboratory mice infected with M. bovis and M. tuberculosis, in which granulomas exhibit far less extensive necrosis and are believed to reflect a good cell-mediated immunity (CMI) through activated macrophages, the reaction in field voles might result from a weak CMI and be similar to that seen in postprimary and reactivation tuberculosis in humans and mouse models, respectively.13,18,20 However, other studies indicated that the necrotic areas are simply the result of hypoxia and macrophage and neutrophil death with release of neutrophil enzymes.36 We observed marked dystrophic mineralization within necrotic areas. A study on experimental tuberculosis in guinea pigs suggests that this is related to the presence of activated macrophages, since mineralization was seen in association with the accumulation of extracellular ferric iron as a consequence of transferrin receptor, H-ferritin, and lactoferrin expression on activated macrophages and neutrophils.2 Mycobacteria, like all living organisms, require iron as a cofactor for proteins and have been shown to scavenge it from the host within phagosomes but might also be able to make use of free iron. However, extracellular iron can also initiate extracellular calcification and is toxic for both cells and mycobacteria by catalyzing the generation of reactive oxygen intermediates and can thereby contribute to necrosis and the reduced bacterial viability observed in granulomas.2,37 Interestingly, M. bovis appears not to induce significant necrosis and mineralization within lesions in field voles, which suggests that the reaction mounted in response to the bacteria is influenced by the bacterium rather than the host.2,32 However, we also observed marked intracellular mineralization of macrophages within granulomas, mainly at the border to necrotic areas. Some of these cells also contained mycobacteria, but the ultrastructural examination did not identify bacilli as the mineralization core. Instead, it revealed that the mineralization started in the mitochondria, a phenomenon that is initiated by the influx of calcium into mitochondria of dying cells. In tuberculous granulomas, this is likely a consequence of hypoxia, which could also be mediated by vascular leakage.2,39,40
The dominance of macrophages and neutrophils and the limited lymphocyte contribution (ie, the lack of lymphocyte accumulations in the periphery of tuberculous infiltrates) in our study indicate a dominance and/or persistence of an innate immune response. Neutrophils in the lesions could contribute to a reduction in bacterial load. They have been shown to rapidly phagocytose M. microti and form phagolysosomes, different from macrophages, in which the bacilli inhibit phagosome-lysosome fusion by inhibition of lysosomal movements.12,34 However, they are likely to contribute also to the tissue damage due to their degranulation with death.40 T cells, which would be the hallmark of the DTH reaction, are present but do not lead to the formation of an organized granuloma, which walls off the necrotic core in humans and other animal model species of tuberculosis.34,40,41–43 It is possible that in vole tuberculosis, the proinflammatory cytokine response is only limited, which would inhibit tuberculoma formation and persistent infection but allow constant progression of the disease, a scenario that has also been proposed for some strains of M. tuberculosis.37 In field voles, granulomas contain ample MCGs, cells that generally mediate physical containment of the bacilli, inhibiting cell-to-cell spread by forming a peripheral ring of nuclei around the bacilli that aggregate centrally in the cytoplasm.6 The latter has been induced in vitro by interferon-γ in combination with interleukin-3 or granulocyte-macrophage colony-stimulating factor, cytokines that could be released by macrophages and infiltrating T cells.29
While a range of species, including humans, have been shown to be susceptible to the disease, tuberculosis due to M. microti is particularly relevant in cats in Great Britain, where it is considered a spillover from tuberculosis in small mammals, such as the field vole, in the wild.36 In cats, the disease mainly affects the skin and associated lymph nodes of the head or, though less frequently, the limbs, tail, neck, and torso, but generalized disease involving lung, liver, spleen, and mesenteric lymph nodes is also seen. Affected cats mainly live in extra-urban areas, and the case distribution confirms that the agent is endemic in certain areas, which at the same time show a low M. bovis tuberculosis incidence in cattle, badgers, and cats, suggesting that both bacteria induce protective immunity against the other.16,36 While most lesions in cats appear to be a consequence of hunting (ie, wounds inflicted by the prey animal), cases without skin lesions would indicate oral or aerogenic infection. Considering the results of the present study, infection of cats from the environment contaminated with bacteria from sputum, saliva, or skin crusts of diseased voles would also be a possibility. Wild mammalian carnivores such as foxes (Vulpes vulpes) and weasels (Mustela nivalis) that rely on field voles as their main prey are also likely to have substantial exposure to M. microti both from ingestion and their environment.3,30 Further studies would be needed to investigate the potential implications for their health status.
In humans, the disease mainly manifests as pulmonary tuberculosis. Since the first case in 1998, a total of 27 cases have been reported; both immunosuppressed and immunocompetent patients were affected.31 One report from Scotland identified some regional clustering of human (4 patients) and animal (2 cats and 1 each of badger, ferret, and llama) cases, although the genotyping showed a mixture between vole and llama type.14 Epidemiologic investigation of the reported human cases has failed to link them with infected cats or wild rodents. However, we have shown that diseased voles can shed the bacilli into the environment. At least in endemic areas, this could offer an explanation for sporadic human infections. At present, studies on the geographic distribution of M. microti in wild rodents in Europe are limited to Great Britain.5,7,8 They were initiated due to the interest of our group in the population dynamics of wild rodents with endemic pathogens generally.48,49 Field voles (M. agrestis) are widely distributed throughout Europe, including Russia to southeast Siberia, with the exception of Ireland, Iceland, and southernmost Europe, and with fluctuation in the population density in 3- to 4-year cycles.25 However, the presence of the disease might not be recognized easily in endemic areas with low disease prevalence despite the frequent external lesions; also, Wells48 assumed that it was very unusual to find dead voles in the field because they were rapidly devoured by other voles. Nonetheless, since geographic clustering has been shown, a systematic approach could clarify whether the field vole is the maintenance host for M. microti also in other regions in Europe or whether other common small rodents have to be considered.36
Acknowledgements
We thank all those who were involved in the field work and the laboratory work for this project, particularly Mr Richard Fox, now Abbey Veterinary Services, Newton Abbot, UK. Thanks are also due to the technicians in the Histology Laboratory and the Electron Microscopy Unit, Veterinary Laboratory Services, School of Veterinary Science, University of Liverpool for excellent technical support.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Wellcome Trust (075202/Z/04/Z) and was licensed under Home Office project license PPL40/1813. S. Burthe was supported by the Natural Environmental Research Council (Studentship number NER/S/A/2000/03445) and M. Abo Rokia was supported by the Ministry of Higher Education, Libya.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Actor JK, Olsen M, Jagannath C, et al. Relationship and survival, organism containment, and granuloma formation in acute murine tuberculosis. J Interferon Cytokine Res. 1999;19(10):1183–1193. doi: 10.1089/107999099313136. [DOI] [PubMed] [Google Scholar]
- 2.Basaraba RJ, Bielefeldt-Ohmann H, Eschelback EK, et al. Increased expression of host iron-binding proteins precedes iron accumulation and calcification or primary lung lesions in experimental lung lesions in experimental tuberculosis in the guinea pig. Tuberculosis. 2008;88(1):69–79. doi: 10.1016/j.tube.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt MJ, Lambin X. Movement patterns of a specialist predator, the weasel Mustela nivalis exploiting asynchronous cyclic field vole Microtus agrestis populations. Acta Theriol. 2007;52(1):13–25. [Google Scholar]
- 4.Brodin P, Eiglmeier K, Marmiesse M, et al. Bacterial artificial chromosome-based comparative genomic analysis identifies Mycobacterium microti as a natural ESAT-6 deletion mutant. Infect Immun. 2002;70(10):5568–5578. doi: 10.1128/IAI.70.10.5568-5578.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burthe S, Bennett M, Kipar A, et al. Tuberculosis (Mycobacterium microti) in wild field vole populations. Parasitology. 2008;135(3):309–317. doi: 10.1017/S0031182007003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrd TF. Multinucleated giant cell formation induced by IFN-γ/IL-3 is associated with restriction of virulent Mycobacterium tuberculosis cell to cell invasion in human monocyte monolayers. Cell Immunol. 1998;188(2):89–96. doi: 10.1006/cimm.1998.1352. [DOI] [PubMed] [Google Scholar]
- 7.Cavanagh R, Begon M, Bennett M, et al. Mycobacterium microti infection (vole tuberculosis) in wild rodent populations. J Clin Microbiol. 2002;40(9):3281–3285. doi: 10.1128/JCM.40.9.3281-3285.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavanagh R, Lambin X, Ergon T, et al. Disease dynamics in cyclic populations of field voles (Microtus agrestis): cowpox virus and vole tuberculosis (Mycobacterium microti) Proc Biol Sci. 2004;271(1541):859–867. doi: 10.1098/rspb.2003.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers MA, Gavier-Widen D, Hewison RG. Histopathogenesis of experimental Mycobacterium bovis infection in mice. Res Vet Sci. 2006;80(1):62–70. doi: 10.1016/j.rvsc.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Corner LA, O’Meara D, Costello E, et al. The distribution of Mycobacterium bovis infection in naturally infected badgers. Vet J. 2012;194(2):166–172. doi: 10.1016/j.tvjl.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Crawshaw TR, Griffiths IB, Clifton-Hadley RS. Comparison of a standard and a detailed post-mortem protocol for detecting Mycobacterium bovis in badgers. Vet Rec. 2008;163(160):473–477. doi: 10.1136/vr.163.16.473. [DOI] [PubMed] [Google Scholar]
- 12.D’Arcy Hart P, Young MR, Gordon AH, et al. Inhibition of phagosome-lysosome fusion in macrophages by certain mycobacteria can be explained by inhibition of lysosomal movements observed after phagocytosis. J Exp Med. 1987;166(4):933–946. doi: 10.1084/jem.166.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dannenberg AM, Jr, Collins FM. Progressive pulmonary tuberculosis is not due to increasing numbers of viable bacilli in rabbits, mice and guinea pigs, but is due to a continuous host response to mycobacterial products. Tuberculosis. 2001;81(3):229–242. doi: 10.1054/tube.2001.0287. [DOI] [PubMed] [Google Scholar]
- 14.Emmanuel FX, Seager AL, Doig C, et al. Human and animal infections with Mycobacterium microti, Scotland. Emerg Infect Dis. 2007;13(12):1924–1927. doi: 10.3201/eid1312.061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavier-Widén D, Cooke MM, Gallagher J, et al. A review of infection if wildlife hosts with Mycobacterium bovis and the diagnostic difficulties of the ‘no visible lesion’ presentation. N Z Vet J. 2009;57(3):122–131. doi: 10.1080/00480169.2009.36891. [DOI] [PubMed] [Google Scholar]
- 16.Gunn-Moore DA, McFarland SE, Brewer JI, et al. Mycobacterial disease in cats in Great Britain, I: culture results, geographical distribution and clinical presentation of 339 cases. J Fel Med Surg. 2011;13(12):934–944. doi: 10.1016/j.jfms.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez-Pando R, Jeyanathan M, Mengistu G, et al. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet. 2000;356(9248):2133–2138. doi: 10.1016/s0140-6736(00)03493-0. [DOI] [PubMed] [Google Scholar]
- 18.Higgins DA. The skin inflammatory response of the badger (Meles meles) Br J Exp Pathol. 1985;66(6):643–653. [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes D, Kipar A, Leeming G, et al. Experimental infection of laboratory-bred bank voles (Myodes glareolus) with murid herpesvirus 4. Arch Virol. 2012;157(11):2207–2212. doi: 10.1007/s00705-012-1397-5. [DOI] [PubMed] [Google Scholar]
- 20.Hunter RL, Jagannath C, Actor JK. Pathology of postprimary tuberculosis in humans and mice: contradiction of long-held beliefs. Tuberculosis. 2007;87(4):267–278. doi: 10.1016/j.tube.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Kamerbeek J, Schouls L, Kolk A, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35(4):907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keshav S, Chung O, Milon G, et al. Lysozyme is an inducible marker of macrophage activation in murine tissues as demonstrated by in situ hybridization. J Exp Med. 1991;174(5):1049–1058. doi: 10.1084/jem.174.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kipar A, Baumgärtner W, Vogl C, et al. Immunohistochemical characterization of inflammatory cells in brains of dogs with granulomatous meningoencephalitis. Vet Pathol. 1998;35(1):43–52. doi: 10.1177/030098589803500104. [DOI] [PubMed] [Google Scholar]
- 24.Kremer K, van Soolingen D, van Embden J, et al. Mycobacterium microti: more widespread than previously thought. J Clin Microbiol. 1998;36(9):2793–2794. doi: 10.1128/jcm.36.9.2793-2794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kryštufek B, Vohralík V, Zima J, et al. Microtus agrestis. Version 2013.1. IUCN; [Accessed August 17, 2013]. 2013. IUCN Red List of Threatened Species. www.iucnredlist.org. [Google Scholar]
- 26.Lambin X, Petty SJ, MacKinnon JL. Cycle dynamics in field vole populations and generalist predation. J Anim Ecol. 2000;69:106–118. [Google Scholar]
- 27.Magdalena J, Supply P, Locht C. Specific differentiation between Mycobacterium bovis BSG and virulent strains of the Mycobacterium tuberculosis complex. J Clin Microbiol. 1998;36(9):2471–2476. doi: 10.1128/jcm.36.9.2471-2476.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manabe YC, Scott CP, Bishai WR. Naturally attenuated, orally administered Mycobacterium microti as a tuberculosis vaccine is better than subcutaneous Mycobacterium bovis BCG. Infect Immun. 2002;70(3):1566–1570. doi: 10.1128/IAI.70.3.1566-1570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNally AK, Anderson JM. Interleukin-4 induces foreign body giant cells from human monocytes/macrophages: differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am J Pathol. 1995;147(5):1487–1499. [PMC free article] [PubMed] [Google Scholar]
- 30.O’Mahony D, Lambin X, MacKinnon JL, et al. Generalist fox predation on cyclic field vole populations in Britain. Ecography. 1999;22:575–581. [Google Scholar]
- 31.Panteix G, Gutierrez MC, Boschiroli ML, et al. Pulmonary tuberculosis due to Mycobacterium microti: a study of six recent cases in France. J Med Microbiol. 2010;59(pt 8):984–989. doi: 10.1099/jmm.0.019372-0. [DOI] [PubMed] [Google Scholar]
- 32.Robb-Smith AHT. Notes on the morphology of infection by the vole acid-fast bacillus. Med Res Council Spec Rep Ser. 1946;259:43–48. [Google Scholar]
- 33.Szeliga J, Daniel D, Yang CH, et al. Granulocyte-macrophage colony stimulating factor–mediated innate immune responses in tuberculosis. Tuberculosis. 2008;88(1):7–20. doi: 10.1016/j.tube.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith CC, Barr RM, Alexander J. Studies on the interaction of Mycobacterium microti and Mycobacterium lepraemurium with mouse polymorphonucelar leukocytes. J Gen Microbiol. 1979;112(1):185–189. doi: 10.1099/00221287-112-1-185. [DOI] [PubMed] [Google Scholar]
- 35.Smith NH, Kremer K, Inwald J, et al. Ecotypes of the Mycobacterium tuberculosis complex. J Theor Biol. 2006;239(2):220–225. doi: 10.1016/j.jtbi.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 36.Smith NH, Cranshaw T, Parry J, et al. Mycobacterium microti: more diverse than previously thought. J Clin Microbiol. 2009;47(8):2551–2559. doi: 10.1128/JCM.00638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart GR, Robertson BD, Young DB. Tuberculosis: a problem with persistence. Nat Rev Microbiol. 2003;1(2):97–105. doi: 10.1038/nrmicro749. [DOI] [PubMed] [Google Scholar]
- 38.Terszowski G, Müller SM, Bleul CC, et al. Evidence for a functional second thymus in mice. Science. 2006;312(5771):284–287. doi: 10.1126/science.1123497. [DOI] [PubMed] [Google Scholar]
- 39.Trump BF, Berezesky IK, Sato T, et al. Cell calcium, cell injury and cell death. Environ Health Perspect. 1984;57:281–287. doi: 10.1289/ehp.8457281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner OC, Basaraba RJ, Orme IM. Immunopathogenesis of pulmonary granulomas in the guinea pig after infection with Mycobacterium tuberculosis. Infect Immun. 2003;71(2):864–871. doi: 10.1128/IAI.71.2.864-871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulrichs T, Kosmiadi GA, Trusov V, et al. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J Pathol. 2004;204(2):217–228. doi: 10.1002/path.1628. [DOI] [PubMed] [Google Scholar]
- 42.Ulrichs T, Kosmiadi GA, Jörg S, et al. Differential organization of the local immune response in patients with active cavitary tuberculosis or with nonprogressive tuberculoma. J Infect Dis. 2005;192(1):89–97. doi: 10.1086/430621. [DOI] [PubMed] [Google Scholar]
- 43.Ulrichs T, Kaufmann SHE. New insights into the function of granulomas in human tuberculosis. J Pathol. 2006;208(2):261–269. doi: 10.1002/path.1906. [DOI] [PubMed] [Google Scholar]
- 44.van Embden JDA, Cave MD, Crawford JT, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31(2):406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Soolingen D, Hoofenboezem T, de Haas PE, et al. A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa. Int J Syst Bacteriol. 1997;47(4):1236–1245. doi: 10.1099/00207713-47-4-1236. [DOI] [PubMed] [Google Scholar]
- 46.van Soolingen D, van der Zanden AG, de Haas PE, et al. Diagnosis of Mycobacterium microti infections among humans by using novel genetic markers. J Clin Microbiol. 1998;36(7):1840–1845. doi: 10.1128/jcm.36.7.1840-1845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viegas MS, do Carmo A, Silva T, et al. CD38 plays a role in effective containment of mycobacteria within granulomata and polarization of Th1 immune responses against Mycobacterium avium. Microbes Infect. 2007;9(7):847–854. doi: 10.1016/j.micinf.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Wells AQ, Oxon DM. Tuberculosis in wild voles. Lancet. 1937;1:1221. [Google Scholar]
- 49.Wells AQ. The murine type of tubercle bacillus (the vole acid-fast bacillus) Spec Rep Ser Med Council London. 1946;259:1–48. [Google Scholar]