Abstract
Blocking the CD40–CD154 interaction is reported to be effective for transplantation management and autoimmune disease models in rodents and nonhuman primates. However, clinical trials with anti-CD154 mAbs were halted because of high incidence of thromboembolic complications. Thus, we generated and characterized a fully human anti-CD40 mAb ASKP1240, as an alternative to anti-CD154 mAb. In vitro ASKP1240 concentration-dependently inhibited human peripheral blood mononuclear cell proliferation induced by soluble CD154. In addition, ASKP1240 did not destabilize platelet thrombi under physiological high shear conditions while mouse anti-human CD154 mAb (mu5C8) did. And ASKP1240 itself did not activate platelet and endothelial cells. In vivo administration of ASKP1240 (1 or 10 mg/kg, intravenously) to cynomolgus monkeys, weekly for 3 weeks, significantly attenuated both delayed-type hypersensitivity and specific antibody formation evoked by tetanus toxoid. The immunosuppressive effect was well correlated with the CD40 receptor saturation. Thus, these results suggest that ASKP1240 is immunosuppressive but not prothromboembolic, and as such appears to be a promising therapeutic candidate for the management of solid organ transplant rejection and autoimmune diseases therapy.
Keywords: CD40, costimulation, immunosuppressive therapy, mAbs
Introduction
The CD40 molecule is mainly expressed on antigen-presenting cells such as macrophages, and dendritic cells (DCs) as well as on B lymphocytes and appears to play an important role in immunological responses 1. Blocking the CD40–CD154 interaction has shown therapeutic effects in several experimental disease models, including organ rejection after transplantation 2, atherosclerosis 3 and autoimmune diseases 4–7. Although several humanized anti-CD154 mAbs (hu5C8, IDEC-131 and ABI793) have been developed and shown to be markedly efficacious in nonhuman primate renal allograft models 8–12, there are significant obstacles to further clinical development. In particular, in early clinical trials with hu5C8 or IDEC-131 there were thromboembolic events 13–15. The mechanism is not fully understood 16–19, but recent studies have suggested that CD154 functions to stabilize arterial thrombi in a CD40-independent manner through its integrin binding KGD (Lys-Gly-Asp) sequence 20,21. It is assumed that targeting the CD40–CD154 pathway via CD40 rather than CD154 might allow an immunosuppressive effect, while leaving the CD154–integrin interactions necessary to regulate thrombus stability unaltered. Several chimeric mAbs against CD40 (chi220 and ch5D12) have been developed as alternatives to anti-CD154 mAbs and were also found to be effective in renal allograft models 22–24 as well as autoimmune disease models in nonhuman primates 25. However, these mAbs were immunogenic reducing their suitability for drug development.
Therefore, we generated a fully human anti-CD40 antagonistic mAb (ASKP1240) from trans-chromosome mice 26. This is an IgG4 masking antibody that shows neither antibody-dependent cell-mediated cytotoxicity (ADCC) nor complement-dependent cytotoxicity (CDC) 27. This ASKP1240 antibody was recently reported to significantly prolong kidney, liver and islet graft survival in nonhuman primates 28–31. The current study characterized this antibody with respect to its effects on soluble human CD154 (shCD154) induced cellular proliferation. In addition, the potential for prothromboembolic effects was assessed in vitro using human platelets and endothelial cells. Finally, the immunosuppressive activity and safety of ASKP1240 were examined in cynomolgus monkeys.
Materials and Methods
ASKP1240 antibody generation
Fully human anti-CD40 antibodies were generated using the KM mouse™ technology 26. These mice were immunized with soluble human extracellular domain CD40 protein and the splenocytes were fused with SP20 cells (ATCC, Rockville, MD). A SMART RACE cDNA Amplification Kit (Clontech Laboratories, Palo Alto, CA) was used for the cloning of the human antibody variable region. Human heavy and light chain variable sequences were subsequently cloned into IgG4 antibody expression vector. The expression vector was transfected into Chinese hamster ovary cells and the antibody ASKP1240 was expressed and purified.
ADCC assay
Blood samples were collected from human healthy volunteers and peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation. 51Cr-labeled Raji cells (ATCC) were incubated in triplicate with 100 µg/mL indicated antibodies and PBMCs at effector-target ratio of 100:1 at 37°C. After 4-h incubation, the radioactivity in the supernatants was counted. The percentage of specific lysis was calculated according to the following formula: % lysis = 100 × (ER − SR)/(MR − SR), where ER, SR and MR represent experimental, spontaneous and maximum 51Cr-release, respectively.
CDC assay
51Cr-labeled Raji cells were incubated with 100 µg/mL indicated antibodies and 10% normal human serum at 37°C. After 2-h incubation, the radioactivity in the supernatants was counted. The percentage of specific lysis was calculated as described above.
Internalization assay by flow cytometry
To measure the clearance of immunocomplexes from the cell surface, Ramos cells (ATCC) were incubated with fluorescein isothiocyanate (FITC)-labeled ASKP1240 or FITC-labeled anti-CD40 agonistic mAb (clone G28.5; ATCC) in RPMI1640 supplemented with 10% fetal bovine serum (FBS) for 15 min at 4°C. The stained cells were washed, and then incubated for varying durations in culture medium at 37°C. At the given time points, cells were removed from the culture and washed with phosphate-buffered saline (PBS) containing 0.1% NaN3 to block internalization. Next, the cells were incubated with phycoerythrin (PE)-labeled anti-human IgG (BioLegend, San Diego, CA) or PE-labeled anti-mouse IgG (BioLegend) for 30 min at 4°C. The geometric mean fluorescence intensity (geoMFI) was measured by flow cytometry and analyzed using CellQuest software (ver. 5.1.1; BD Biosciences, Franklin Lakes, NJ).
Cross-reactivity in the peripheral blood
Peripheral blood samples from mice (B6C3F1), rats (Sprague–Dawley), rabbits (Japanese White), cynomolgus monkeys and humans were incubated with FITC-labeled ASKP1240. The erythrocytes/platelets, lymphocyte, monocytes and granulocytes were identified by their light scatter characteristics and lineage-specific mAbs (anti-mouse CD3e, 145-2C11, BD; anti-CD45R/B220, RA3-6B2, BD; anti-mouse CD41, MWReg30, BD; anti-rat CD3, G4.18, BD; anti-rat CD45RA, OX-33, BD; anti-rat CD42d, RPM.4, BD; anti-rabbit T lymphocyte, KEN-5, AbD Serotec, Kidlington, UK; anti-rabbit CD41, P2, Beckman Coulter, Brea, CA; anti-monkey CD3, SP34 BD; anti-monkey CD20, H29, Beckman Coulter; anti-monkey CD4a1, HIP8, BD; anti-human CD3, UCHT1, Beckman Coulter; anti-human CD19, J4.119, Beckman Coulter; anti-human CD41, P2, BD). The fluorescent activity of each cell population was determined by flow cytometry.
Affinity analysis
CD40 complementary DNA clones were isolated from human, cynomolgus monkey and rabbit tissues. Glutathione-S-transferase (GST)-fused CD40 recombinant proteins were prepared using HEK293F cells. Affinity evaluation was carried out using a BIAcore 3000 (GE Healthcare, Piscataway, NJ). The flow cell of Sensor Chip CM5 (GE Healthcare) was coupled with anti-GST antibody by amine coupling. The recombinant CD40 GST fusion proteins in HBS-EP buffer were injected at a flow rate of 10 µL/min, followed by ASKP1240 at concentrations of 0.16–10 µg/mL (flow rate of 10 µL/min). The association and dissociation reactions were recorded for 4 and 15 min, respectively. For regeneration, 10 mM glycine–HCl (pH 2.2) was injected at a 20 µL/min flow rate for 2 min. The kinetic parameters were calculated using BIAEVALUATION software version 4.0 (GE Healthcare).
PBMC proliferation
Three individual PBMC samples from each species (human, monkey and rabbit) were collected as described above and the cells were re-suspended in RPMI1640 supplemented with 10% FBS, and were cultured with 1 µg/mL Flag-tagged shCD154 (Alexis Biochemicals, Lausanne, Switzerland) and 10 µg/mL anti-Flag M2 mAb (Sigma–Aldrich, St. Louis, MO) to cross-link shCD154 in the presence or absence of various concentrations of ASKP1240 for 48 h at 37°C. After incubation, the cells were pulse labeled with [methyl 3H]-thymidine (1 µCi/well; GE Healthcare). Sixteen hours later the cells were harvested on microglass filter strips and the incorporated radioactivity was determined by liquid scintillation. Their IC50 were estimated using GraphPad Prism version 4.0 (GraphPad Software, La Jolla, CA).
Delayed-type hypersensitivity (DTH) reaction and antibody production in vivo
ASKP1240, at doses of 0 (placebo), 0.1, 1 and 10 mg/kg, was administered intravenously to 5 males/group on days 0, 7 and 14. For sensitization, the animals were anesthetized with ketamine then injected with 10 Lf/mL tetanus toxoid (TTx; Denka Seiken, Tokyo, Japan) vaccine into the skin on the back intradermally (50 µL/site × 12 sites) and thigh intramuscularly (0.6 mL/body) once on day 0. Three weeks later, 10 µL TTx solution, at a concentration of 0, 1, 3 or 10 Lf/mL, was injected into the thoracic skin. Skin reactions at the injection sites were photographed and evaluated for erythematous and edematous reactions 48 h after challenge. Reaction scores based on erythema and edema were determined according to the Draize skin test. The total score was the sum of the erythema and edema scores at an individual injection site. Anti-TTx IgG and IgM levels were determined by enzyme-linked immunosorbent assay (ELISA). Plates were coated with TTx; then serially diluted monkey serum from the test animals was added. The plates were incubated at room temperature, and peroxidase-conjugated goat anti-monkey IgG or IgM antibodies (Abcam, Cambridge, MA) were added for detection. The titer was defined as the dilution magnitude of sera at which the ELISA optical density dropped to the control level.
Receptor competition assay
ASKP1240 was administered intravenously once at dose levels of 0 (placebo), 0.1, 1, 3 and 10 mg/kg to four male cynomolgus monkeys per group. Sampling points were set between days −11 and 49 according to dose level. The collected blood samples were lysed and stained with biotinylated ASKP1240 plus PE-labeled streptavidin (BD) and allophycocyanin-labeled anti-CD20 mAb (clone 2H7; BD). The geoMFI of PE in CD20+ lymphocytes was monitored by flow cytometory. ASKP1240 was also determined by an ELISA, as described before 28.
Platelet preparation and activation
Platelet-rich plasma (PRP) was generated by centrifugation citrated human blood from healthy donors at 150g for 10 min. Platelets were stimulated with 0.2 U/mL human thrombin (Sigma–Aldrich) at 37°C without stirring. After activation, platelets were fixed with 1% paraformaldehyde, washed and reacted with a platelet-specific PE-labeled anti-CD41 mAb (clone 5B12; DAKO, Carpinteria, CA) and FITC-labeled ASKP1240 or FITC-labeled mu5C8. The fluorescence was measured by flow cytometry.
In vitro experiment on platelet thrombus formation
Blood samples were collected from three healthy human volunteers and used within 1 h of blood collection. A silicone gasket with a flowpath height of 127 µm was placed between a parallel plate perfusion chamber (GlycoTech, Rockville, MD) and a human type III fibrillar collagen coated Petri dish (Sigma–Aldrich). Blood sample was divided into four tubes and added with 100 µg/mL indicated antibodies: ASKP1240, human isotype antibody, mu5C8 (ATCC) or mouse isotype antibody. The antibody-treated blood samples were aspirated through the chamber by a syringe pump at a constant flow stress to produce wall shear stresses of 500/s and 1500/s. Seven minutes after the initial platelet–surface interaction, platelet thrombi generated on a collagen-coated surface were fixed with paraformaldehyde. After fixation, the Petri dish was stained with PE-labeled anti-CD41 mAb (DAKO) and viewed with a fluorescent microscope.
Change of human platelet and endothelial cell activation marker
PRP was prepared from blood sample by centrifugation. The PRP was incubated with either media alone, Flag-tagged shCD154 (1 µg/mL) plus anti-Flag M2 mAb (10 µg/mL), ASKP1240 (1 mg/mL) or thrombin receptor activating peptide (SFLLRN, 100 µM; Sigma–Aldrich) as a positive control for platelet activation, for 45 min at 37°C. Following incubation, samples were fixed in 1% p-formaldehyde for 30 min on ice, washed, stained with FITC-labeled anti-CD41 (DAKO) and PE-labeled anti-CD62P (clone CLBThromb/6; Beckman Coulter) and then analyzed by flow cytometory.
The measurement of endothelial cell activation markers was carried out by the following methods. Confluent human umbilical vein endothelial cells were incubated with either media alone, shCD154 (1 µg/mL), ASKP1240 (1 mg/mL) or tumor necrosis factor (TNF)α (100 U/mL; Sigma–Aldrich) as a positive control for endothelial activation for 4 h at 37°C. Following incubation, the cells were collected by trypsinization, stained with PE-labeled anti-CD62E (clone 68-5H11; BD) or PE-labeled anti-CD142 (clone HTF-1; BD) and fluorescence activated cell sorting analyses were performed.
In vivo safety assessment
For the safety study, cynomolgus monkeys were allocated to four groups, each containing six males and six females. ASKP1240, at doses of 0 (placebo), 1, 10 and 100 mg/kg, was administered weekly for 4 weeks from day 0. Complete gross necropsies were performed on three males and three females from each group on day 28 (end of the dosing period). The remaining three males and three females in each group were monitored for an additional 4 weeks (end of the recovery period). The number of circulating CD20+ B cells was determined flow cytometrically. The anti-ASKP1240 titer was also measured by BIAcore as described previously 28.
Animal welfare
The experiments using cynomolgus monkeys were performed in facilities at Shin Nippon Biomedical Laboratories (SNBL; Kagoshima, Japan). This study was approved by the Institutional Animal Care and Use Committee of SNBL and performed in accordance with the ethics criteria contained in the bylaws of the committee.
Results
Cytotoxicitity of ASKP1240
The constant region of ASKP1240 is converted to IgG4 to reduce ADCC and CDC activities that might injure CD40 positive cells (i.e. B cells, monocytes, platelet, endothelial cells, etc.). One hundred micrograms per milliliter of ASKP1240 IgG4 did not induce CD40 expressing cell lysis via ADCC and CDC activity in contrast with rituximab and IgG1 version of ASKP1240 (Figure 1).
Figure 1.
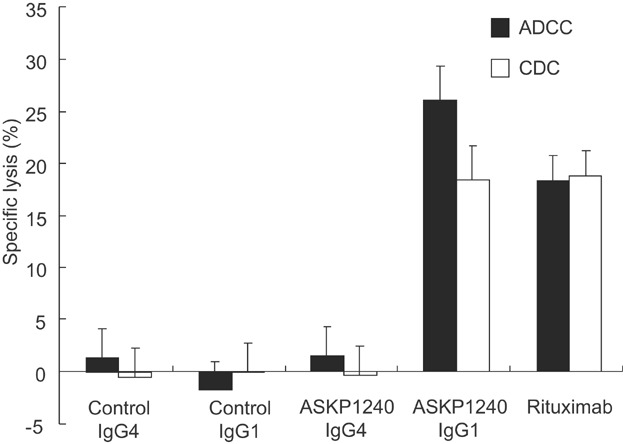
Cytotoxic assay of ASKP1240. Antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) activities of 100 µg/mL of antibodies were evaluated by chromium release assay. The percentage of specific lysis of B-lymphoma cells (Raji) is represented as the mean ± SD of three wells.
Internalization assay of ASKP1240
Since CD40 ligation can lead to internalization of cell surface CD40 32, a determination was made as to whether ASKP1240 can elicit internalization of CD40. In this experiment, internalized antigen showed FITC fluorescence only, but residual cell surface antigen showed both PE and FITC fluorescence. Although binding of the anti-CD40 agonistic mAb, G28.5, leads to an immediate decrease in PE fluorescence, ASKP1240 affected neither FITC nor PE fluorescence levels (Figure 2).
Figure 2.
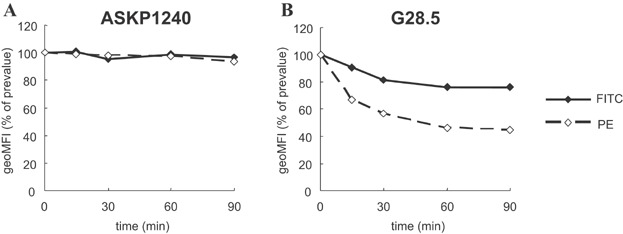
Modulation of CD40 antigens on B cell lines analyzed by flow cytometry. Ramos cells were incubated with FITC-labeled ASKP1240 (A) or FITC-labeled G28.5 (B) in culture medium at 37°C. At the given time points, cells were washed with PBS containing NaN3 and then labeled with PE-labeled secondary antibodies. The fluorescence of FITC represents simultaneous measurements of cell surface and internalized immunocomplexes, while that of PE represents only cell surface immunocomplexes. The percentage of geoMFI at indicated time point relative to prevalue is plotted over time. FITC, fluorescein isothiocyanate; geoMFI, geometric mean fluorescence intensity; PBS, phosphate-buffered saline; PE, phycoerythrin.
Cross-reactivity and binding affinity of ASKP1240
The cross-reactivity of ASKP1240 to mouse, rat, rabbit, cynomolgus monkey or human CD40 on blood cells was assessed flow cytometrically. Specific binding of FITC-labeled ASKP1240 was detected in human, cynomolgus monkey and rabbit CD40, indicating that the cynomolgus monkey and rabbit have the potential to be pharmacologically reactive to ASKP1240. The ASKP1240 reactive blood cell populations were also identified (i.e. B cells, monocytes and platelets) (Table1). Equilibrium and kinetic-binding analyses were performed on ASKP1240 using surface plasmon resonance. The ASKP1240 molecule has approximately sixfold higher avidity (KD) for human and cynomolgus monkey CD40 than for rabbit CD40. Kinetic binding analysis revealed the binding rates (ka) of ASKP1240 for human, cynomolgus monkey and rabbit CD40 were similar, though dissociation rates (kd) were not. The dissociation rates of ASKP1240 for human and cynomolgus monkey CD40 were approximately sixfold slower than that for rabbit CD40 (Table2).
Table 1.
Cross-reactivity assay
| Human | Monkey | Rabbit | Rat | Mouse | |
|---|---|---|---|---|---|
| Lymphocyte | ND | ND | + | ND | ND |
| T cell | − | − | − | − | − |
| B cell | + | + | ND | − | − |
| Monocyte | + | + | + | − | − |
| Granulocyte | − | − | − | − | − |
| Erythrocyte | − | − | − | − | − |
| Platelet | + | + | − | − | − |
The cross-reactivity of ASKP1240 to mouse, rat, rabbit, cynomolgus monkey or human CD40 on blood cells was assessed. Human peripheral blood was stained with biotinylated ASKP1240 plus streptavidin-PE or FITC-ASKP1240, and the fluorescent activity of the erythrocytes, platelets, T lymphocytes, B lymphocytes, monocytes and granulocytes, from each species, was determined by flow cytometry. ND, not done.
Table 2.
Summary of equilibrium and apparent kinetic constants
| Immobilized protein | Analyte | ka (×105), M−1 S−1 | kd (×10−4), S−1 | KD, nM |
|---|---|---|---|---|
| Human CD40 | ASKP1240 | 1.22 ± 0.12 | 0.29 ± 0.01 | 0.24 ± 0.02 |
| Monkey CD40 | ASKP1240 | 1.26 ± 0.03 | 0.36 ± 0.03 | 0.28 ± 0.03 |
| Rabbit CD40 | ASKP1240 | 1.32 ± 0.03 | 1.90 ± 0.33 | 1.44 ± 0.26 |
ka, association constant; kd; dissociation constant; KD, equilibrium dissociation constant.
The mean ± SD of three independent experiments is shown.
Effect of ASKP1240 on PBMC proliferation induced by shCD154
The effect of ASKP1240 on PBMC proliferation induced by shCD154 was compared using human, cynomolgus monkey and rabbit blood samples. The ASKP1240 mAb inhibited PBMC proliferation induced by shCD154 in a concentration-dependent manner in all three species with IC50 estimated to be 5.8, 25.1 and 91.8 ng/mL, for human, monkey and rabbit samples, respectively (Figure 3).
Figure 3.
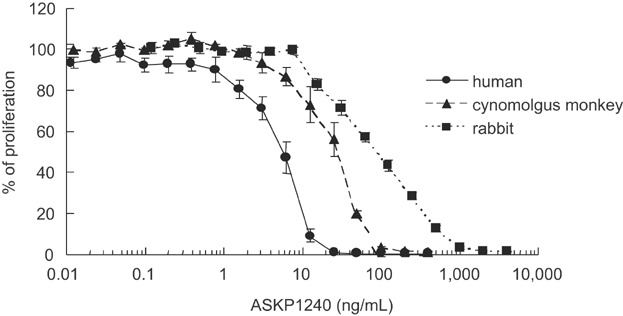
Inhibition of immune cell activation induced by shCD154 in vitro. Inhibition of PBMC proliferation stimulated with shCD154 in human, cynomolgus monkey and rabbit samples. The effects of increasing the concentration of ASKP1240 were evaluated. Cell proliferation was measured by incorporation of 3H-thymidine. The percentage of proliferation relative to the ASKP1240 (−) control is plotted as the mean ± SE of three individuals. PBMC, peripheral blood mononuclear cell; shCD154, soluble human CD154.
Effects of ASKP1240 on DTH reactions and anti-TTx antibody productions in cynomolgus monkeys in vivo
The ASKP1240 inhibited DTH reaction in a dose-dependent manner (Figure 4A). Histopathologically, vasculitis and inflammatory cell infiltration including mononuclear cells and acidophils were observed in the dermis and subcutis at the challenge sites injected with TTx in the control group, but not at the sites injected with physiological saline. The incidences and degrees of histological changes decreased dose dependently (data not shown). Anti-TTx antibodies were also assessed over time, through day 23. ASKP1240 dose-dependently inhibited anti-TTx IgG and anti-TTx IgM productions (Figure 4B and C) with complete inhibition in antibody formation seen at doses of 10 mg/kg.
Figure 4.
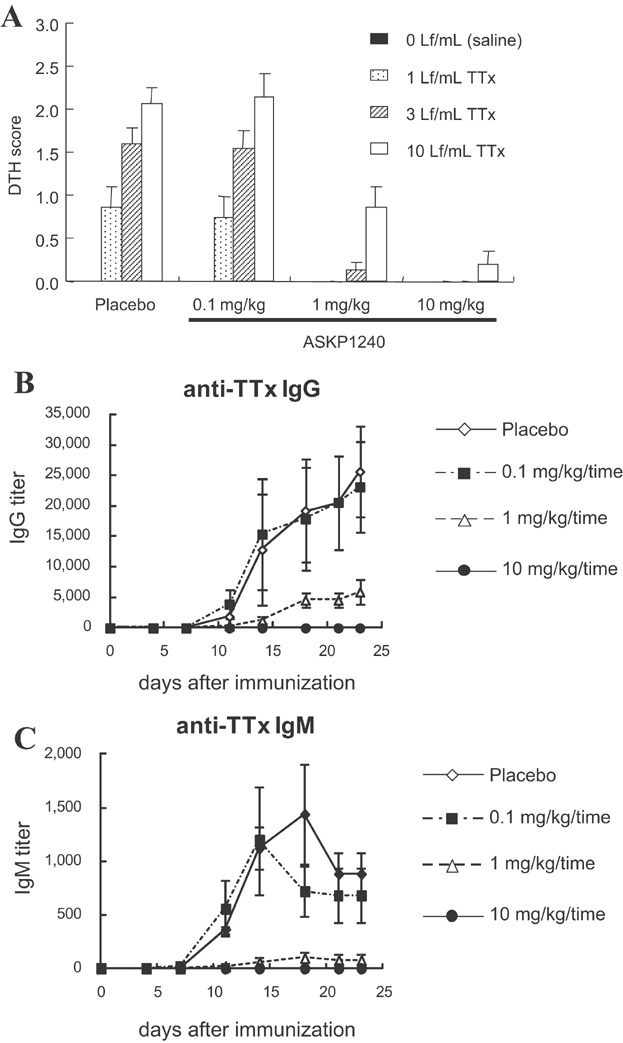
Inhibition of DTH reactions and antibody productions in vivo. (A) Animals were immunized once with TTx on day 0 and challenged with injections into the thoracic skin of serially diluted TTx on day 21. ASKP1240 or placebo was administered intravenously on days 0, 7 and 14. At 48 h after challenge, skin reactions at the injection site were scored as described in the Materials and Methods section. The DTH scores are expressed as the mean ± SE of five individuals. (B, C) Animals were immunized once with TTx on day 0 and blood samples were obtained twice per week for serum titration. Anti-TTx IgG (B) and anti-TTx IgM (C) titers were measured by ELISA. The IgG and IgM titers are expressed as the means ± SE of five individuals. DTH, delayed-type hypersensitivity; ELISA, enzyme-linked immunosorbent assay; TTx, tetanus toxoid.
Pharmacokinetics and receptor occupancy
After single administration, the serum ASKP1240 concentrations increased dose dependently, and decreased rapidly in the 0.1 mg/kg group, and slowly in the 1, 3 and 10 mg/kg groups (Figure 5A). The receptor occupancy was monitored as a pharmacodynamic (PD) marker. The binding of biotinylated ASKP1240 completely diminished just after the administration of ASKP1240. Although the free CD40 receptor recovered immediately in 0.1 mg/kg group, 1, 3 and 10 mg/kg sustained full receptor occupancy for 5, 10 and 14 days, respectively. The recovery of free CD40 receptor also delayed in a dose-dependent manner (Figure 5B). The CD40 antigen was almost completely occupied when the serum ASKP1240 concentrations were ≥100 ng/mL (Figure 5C).
Figure 5.
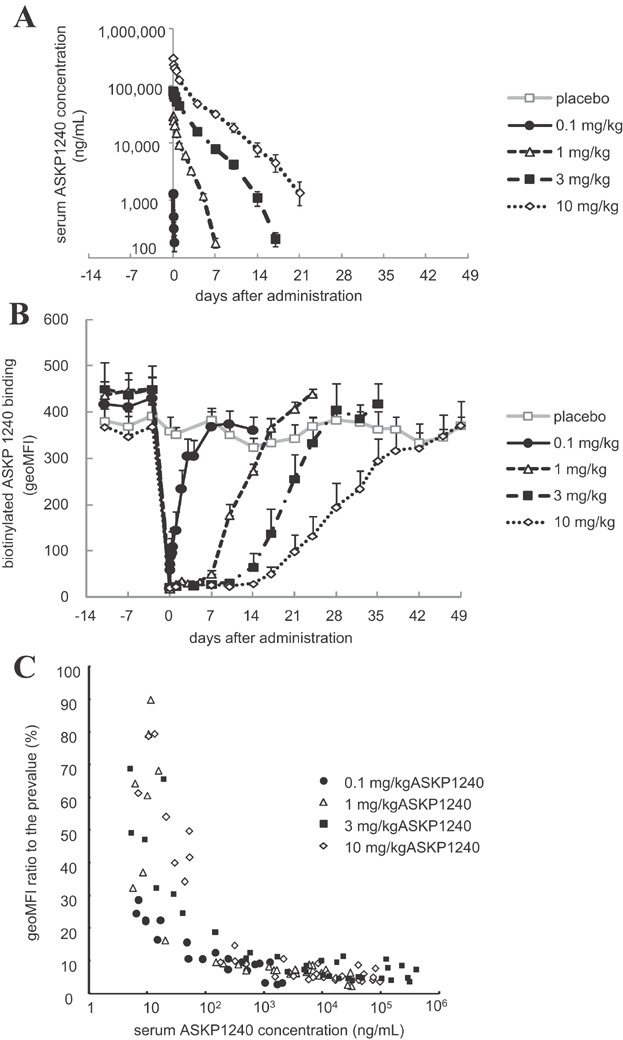
Pharmacokinetics and receptor occupancy ASKP1240 were administered intravenously once on day 0. The serum ASKP1240 level was measured by an ELISA. To evaluate the receptor occupancy, the peripheral CD20+ cells in the collected blood samples were stained with biotinylated ASKP1240 plus PE-labeled streptavidin. The serum ASKP1240 concentration and the geoMFI of PE in the CD20+ lymphocytes are expressed as the means ± SE of three individuals (A, B). The correlation between the serum ASKP1240 concentration and receptor occupancy was plotted (C). ELISA, enzyme-linked immunosorbent assay; geoMFI, geometric mean fluorescence intensity; PE, phycoerythrin.
Influence of anti-CD40 and anti-CD154 antibodies on human platelet thrombus formation in vitro
Flow cytometric analysis showed that ASKP1240 bound to both unstimulated and stimulated platelets. Conversely, the mouse anti-human CD154 mAb, mu5C8, bound only thrombin-stimulated platelets (Figure 6A). A recent study indicated that CD154 contributes to the stabilization of the formed platelet thrombi via an αIIbβ3-dependent (CD40 independent) mechanism 20. Indeed, although blood from CD154-deficient mice shows normal hemostasis processes under low shear stress conditions, the formed thrombi frequently ruptured and embolized under high shear stress conditions. Therefore, the influence of anti-CD154 and ASKP1240 on the platelet thrombus stability was evaluated by a perfusion assay. Under low shear stress condition (500/s), ASKP1240 and mu5C8-treated blood samples formed normal platelet thrombi on the collagen-coated surface. However, under high shear stress condition (1500/s) only smaller thrombi remained in mu5C8-treated blood sample, though normal platelet thrombi were observed in ASKP1240-treated blood sample (Figure 6B).
Figure 6.
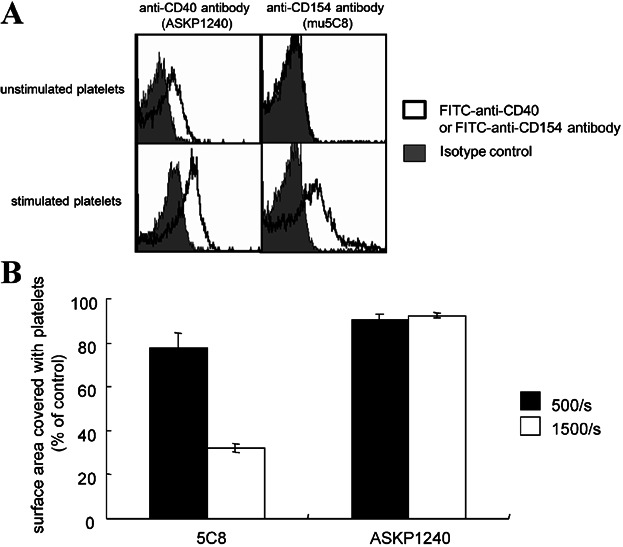
Influence of anti-CD40 antibody and anti-CD154 antibody on human platelet thrombus formation in vitro. (A) The expression levels of CD40 and CD154 in unstimulated and thrombin-stimulated human platelets were analyzed by flow cytometry (solid line histogram). The background was defined using their corresponding isotype control antibodies (gray-filled histogram). (B) Influence of ASKP1240 and anti-CD154 antibody (mu5C8) on thrombus formation. The blood samples incubated with ASKP1240, human isotype antibody, mu5C8 or mouse isotype antibody were perfused over bovine type III collagen-coated Petri dishes in a flat perfusion chamber at wall shear rates of 500/s and 1500/s. Platelet thrombi generated on a collagen-coated surface were fixed using paraformaldehyde and stained with PE-labeled anti-CD41 antibody. The stained surfaces were photographed and the area stained with PE-labeled anti-CD41 antibody was calculated. Percentages compared with their corresponding isotype control antibody-treated samples are shown as the mean ± SD. The graph is the representative of three experiments with similar results is shown. PE, phycoerythrin.
Influence of ASKP1240 on human platelet and endothelial cell activation markers
Since some anti-CD40 mAbs and CD154 reportedly activate platelets and vascular endothelial cells 33–35, we evaluated the influence of ASKP1240 on these cells. Positive control reagent (SFLLRN or TNFα) and shCD154 up-regulated cell activation markers, such as CD62P (P-selectin), CD62E (E-selectin) and CD142 (tissue factor). On the other hand, ASKP1240 by itself did not up-regulate their activation markers, and rather inhibited cell activation by shCD154 (Figure 7).
Figure 7.
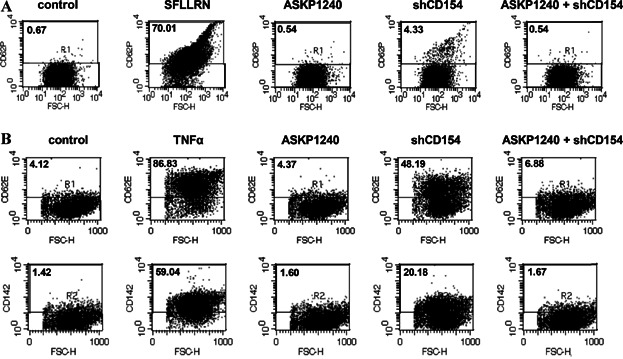
Influence of ASKP1240 on human platelet and endothelial cell activation markers. (A) Effect of ASKP1240 on platelet P-selectin (CD62P) expression. Diluted PRP was incubated with indicated reagents. Shown are dot plots obtained from flow cytometory analyses demonstrating the cells incubated only media, 100 µM of thrombin receptor activating peptide (SFLLRN), 1 mg/mL of ASKP1240, 1 µg/mL of shCD154 or 1 mg/mL of ASKP1240 plus 1 µg/mL of shCD40. (B) Effect of ASKP1240 on endothelial cell E-selectin (CD62E) and tissue factor (CD142) expression. Shown are dot plots obtained from flow cytometory analyses demonstrating the cells incubated with only media, 100 U/mL of TNFα, 1 mg/mL of ASKP1240, 1 µg/mL of shCD154 or 1 mg/mL of ASKP1240 plus 1 µg/mL of shCD40. PRP, platelet-rich plasma; shCD154, soluble human CD154; TNF, tumor necrosis factor.
Repeated dose safety assessment
No clinical signs or other abnormalities including thrombosis-related events were observed during dosing, at 1 week following the end of the dosing, or at the end of the 4-week recovery period. No ASKP1240-related changes were noted in body weight, food consumption, urinalysis, hematology or blood chemistry measurements in any group (data not shown). There were no significant decreases in the circulating B lymphocytes counts (Figure 8). During the study period, no evidence suggesting the production of anti-ASKP1240 antibodies was noted in any animal in any group at any measurement point (data not shown).
Figure 8.
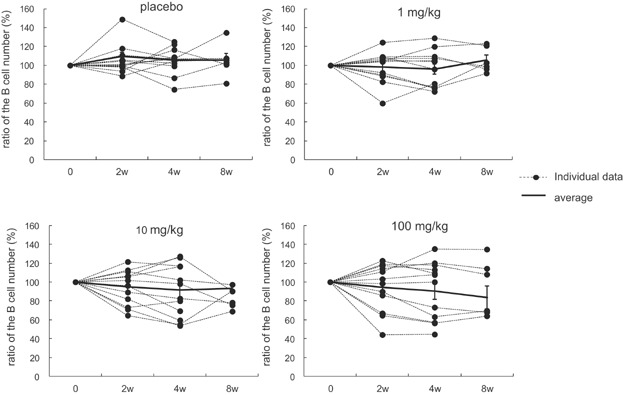
Influence of ASKP1240 on the circulating B cell number. B cells were measured as CD20 positive cells by flow cytometry at 2, 4 (at the end of dosing period) and 8 (at the end of the recovery period) weeks after immunization. The percentage of the B cell number remaining relative to the predosing value is plotted over time. Each dot represents individual data, and the solid line represents the means ± SE of 12 individuals (6 individuals at 8 weeks).
Discussion
T cell activation is a multistep process involving T cell receptor engagement by antigen/major histocompatibility complex as well as involvement of costimulatory molecules. Two of the best characterized costimulatory pathways are the interactions between B7-CD28 and CD40–CD154. Blockade of these pathways has exhibited extraordinary efficacy in several transplantation and autoimmune disease models 2–12. B7-CD28 blockers, such as abatacept and belatacept, are in fact now on the market. On the other hand, the development of CD40–CD154 blockers has not made substantial progress. The main reason is that several anti-CD154 mAbs (5C8, IDEC-131 and ABI793) showed unexpected high incidence of agent-associated thromboembolic complications 13–15. As alternatives, mAbs against CD40 (chi220 and ch5D12) have been developed. These mAbs were effective and no thromboembolic events were reported in nonhuman primate models of either organ transplantation or autoimmune disease. However, chi220, IgG1 with high cytotoxicity, reduced the number of circulating B cells and was associated with cytomegalovirus infection. Furthermore, both mAbs are chimeric and have proven to be highly immunogenic 22,23. Thus, in order to circumvent this problem, we generated an antagonistic fully human IgG4 anti-CD40 mAb, ASKP1240, in trans-chromosome mice 26. Our rationale was as follows: (1) a fully human antibody is expected to have low immunogenicity, (2) IgG4 has a masking property, without depleting CD40 positive cells, such as B cells, DCs, macrophages, platelets, etc. and (3) IgG4 has low binding affinity for FC gamma receptors (FcγRs), which can cross-link the antibodies and induce agonistic activities 36. Therefore, ASKP1240 is expected to exert its immunomodulatory action without the unwanted effects of anti-drug antibody emergence or the depletion of CD40 positive cells.
It is reported that costimulatory blocker with higher avidity resulted in more potent efficacy 37. The ASKP1240 mAb was shown to bind to human CD40 with high affinity (KD = 0.24 nM) and to effectively block the interaction of CD40 with CD154. Its binding activity and blocking potency for human and cynomolgus monkey CD40 were similar.
In the in vivo DTH and antibody production models, weekly administration of ASKP1240 at dose of 10 mg/kg, completely suppressed both the cell-mediated and antibody immune responses, while weekly administration of 1 mg/kg exerted a suboptimal effect. This is reasonable considering the result of receptor competition assay and indicates that receptor occupancy can be used as PD marker of ASKP1240. Although free CD40 antigen started to increase from around 5 days after the administration in 1 mg/kg group, it is expected that weekly administration of 10 mg/kg sustained complete receptor occupancy during this study. Certainly the amount of free CD40 antigen in 1 mg/kg would be very few, but we might have to aware of the accessibility of ASKP1240 for precise pharmacological prediction. Peripheral blood cell would be the easier target for antibody drug to access compared to lymph nodes and tissues. From the pharmacokinetic/PD study, the peripheral CD40 antigens were found to be almost completely occupied when the serum ASKP1240 concentrations were ≥100 ng/mL, and were maintained at a nadir when the serum ASKP1240 concentrations were ≥1000 ng/mL.
In the safety study, the weekly administration of ASKP1240 at doses up to 100 mg/kg did not lead to significant reduction in the number of circulating B cells or to any adverse events, including thrombosis. Anti-ASKP1240 antibodies were not detected in any animal in any group at any measurement point. Previous studies using mAbs directed against CD154 were associated with an increased risk of thromboembolic complications. Although the mechanism remains controversial, it was reported that shCD154 may stabilize arterial thrombi in vivo by an αIIbβ3-dependent (CD40-independent) mechanism via outside-in signaling through its integrin binding KGD sequence 20. Furthermore, CD154-deficient mice have been shown to form unstable thrombi, whereas this defect is not observed in CD40-deficient mice 20,37. Instability of thrombi can be a risk factor for thromboembolic complications because it occurs when formed thrombi detach from blood vessels and circulate as emboli. The present study showed that ASKP1240 did not affect the platelet thrombus formation nor did it destabilize platelet thrombi under high-flow condition. Conversely, while mu5C8 did not inhibit platelet thrombus formation on collagen under low-flow condition, it did destabilize it under high-flow condition. Some investigators have speculated that thromboembolic complications by anti-CD154 mAbs are attributable to platelet activation via platelet FcγR or complement deposition, rather than the αIIbβ3-dependent mechanism 16. Even if this is the case, ASKP1240 is expected to be safe because prethrombotic anti-CD154 mAbs, such as hu5C8, IDEC-131 and ABI793, are comprised of recombinant IgG1, while ASKP1240 is IgG4, which has an extremely lower affinity for platelet FcγR and extremely lower complement activation capacity than IgG1 27. In addition, although some anti-CD40 agonistic mAbs and CD154 reportedly act on platelets and vascular endothelial cells to up-regulate their activation markers, such as P-selectin, E-selectin and tissue factors 33–35, ASKP1240 by itself did not up-regulate these markers. In addition to these in vitro and in vivo studies presented here, ASKP1240-related thrombosis was not observed among 72 renal transplantation recipient monkeys treated with ASKP1240. These experimental data, taken together, suggest that ASKP1240 itself is not inherently prothromboembolic.
In conclusion, the novel fully human antagonistic anti-CD40 mAb, ASKP1240, inhibited shCD154-stimulated cellular proliferation in vitro demonstrating its ability to block CD40–CD154 interactions on a cellular level. In vivo, ASKP1240 administration suppressed indicators of both cell-mediated and antibody immune responses (DTH and anti-TTx antibody responses). In vitro platelet studies suggest that ASKP1240 is not prothromboembolic in that it did not inhibit collagen-induced stable thrombus formation nor did it destabilize the thrombus once formed under flow conditions. Finally, the safety studies showed that the weekly administration of ASKP1240 at doses up to 100 mg/kg was not immunogenic, and resulted in no significant toxicity. Based on these findings, it is concluded that the fully human antagonistic CD40 mAb, ASKP1240, is a good candidate for further clinical development in transplant and autoimmune diseases.
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Y. Miyao, S. Sakuma and F. Kinugasa are employees of Astellas Pharma, Inc.
Glossary
- ADCC
antibody-dependent cell-mediated cytotoxicity
- CDC
complement-dependent cytotoxicity
- DCs
dendritic cells
- DTH
delayed-type hypersensitivity
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- FcγR
Fc gamma receptor
- FITC
fluorescein isothiocyanate
- geoMFI
geometric mean fluorescence intensity
- GST
glutathione-S-transferase
- PBMC
peripheral blood mononuclear cell
- PBS
phosphate-buffered saline
- PD
pharmacodynamic
- PE
phycoerythrin
- PRP
platelet-rich plasma
- shCD154
soluble human CD154
- TNF
tumor necrosis factor
- TTx
tetanus toxoid
References
- van Kooten C, Banchereau J. CD40–CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- Nathan MJ, Yin D, Eichwald EJ, Bishop DK. The immunobiology of inductive anti-CD40L therapy in transplantation: Allograft acceptance is not dependent upon the deletion of graft-reactive T cells. Am J Transplant. 2002;2:323–332. doi: 10.1034/j.1600-6143.2002.20406.x. [DOI] [PubMed] [Google Scholar]
- Remskar M, Li H, Chyu KY, Shah PK, Cercek B. Absence of CD40 signaling is associated with an increase in intimal thickening after arterial injury. Circ Res. 2001;88:390–394. doi: 10.1161/01.res.88.4.390. [DOI] [PubMed] [Google Scholar]
- Daikh DI, Finck BK, Linsley PS, Hollenbaugh D, Wofsy D. Long-term inhibition of murine lupus by brief simultaneous blockade of the B7/CD28 and CD40/gp39 costimulation pathways. J Immunol. 1997;159:3104–3108. [PubMed] [Google Scholar]
- Gerritse K, Laman JD, Noelle RJ, et al. CD40–CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA. 1996;93:2499–2504. doi: 10.1073/pnas.93.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durie FH, Fava RA, Foy TM, Aruffo A, Ledbetter JA, Noelle RJ. Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science. 1993;261:1328–1330. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- Liu Z, Geboes K, Colpaert S, et al. Prevention of experimental colitis in SCID mice reconstituted with CD45RBhigh CD4+ T cells by blocking the CD40–CD154 interactions. J Immunol. 2000;164:6005–6014. doi: 10.4049/jimmunol.164.11.6005. [DOI] [PubMed] [Google Scholar]
- Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston EH, Xu H, Dhanireddy KK, et al. IDEC-131 (anti-CD154), sirolimus and donor-specific transfusion facilitate operational tolerance in non-human primates. Am J Transplant. 2005;5:1032–1041. doi: 10.1111/j.1600-6143.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- Schuler W, Bigaud M, Brinkmann V, et al. Efficacy and safety of ABI793, a novel human anti-human CD154 monoclonal antibody, in cynomolgus monkey renal allotransplantation. Transplantation. 2004;77:717–726. doi: 10.1097/01.tp.0000116563.72763.83. [DOI] [PubMed] [Google Scholar]
- Kanmaz T, Fechner JJ, Jr, Torrealba J, et al. Monotherapy with the novel human anti-CD154 monoclonal antibody ABI793 in rhesus monkey renal transplantation model. Transplantation. 2004;77:914–920. doi: 10.1097/01.tp.0000116392.72152.75. [DOI] [PubMed] [Google Scholar]
- Knechtle SJ, Hamawy MM, Hu H, Fechner JH, Jr, Cho CS. Tolerance and near-tolerance strategies in monkeys and their application to human renal transplantation. Immunol Rev. 2001;183:205–213. doi: 10.1034/j.1600-065x.2001.1830116.x. [DOI] [PubMed] [Google Scholar]
- Vincenti F. What's in the pipeline? New immunosuppressive drugs in transplantation. Am J Transplant. 2002;2:898–903. doi: 10.1034/j.1600-6143.2002.21005.x. [DOI] [PubMed] [Google Scholar]
- Larsen CP, Knechtle SJ, Adams A, Pearson T, Kirk AD. A new look at blockade of T-cell costimulation: A therapeutic strategy for long-term maintenance immunosuppression. Am J Transplant. 2006;6:876–883. doi: 10.1111/j.1600-6143.2006.01259.x. [DOI] [PubMed] [Google Scholar]
- Prasad KS, Andre P, Yan Y, Phillips DR. The platelet CD40L/GP IIb–IIIa axis in atherothrombotic disease. Curr Opin Hematol. 2003;10:356–361. doi: 10.1097/00062752-200309000-00006. [DOI] [PubMed] [Google Scholar]
- Langer F, Ingersoll SB, Amirkhosravi A, et al. The role of CD40 in CD40L- and antibody-mediated platelet activation. Thromb Haemost. 2005;93:1137–1146. doi: 10.1160/TH04-12-0774. [DOI] [PubMed] [Google Scholar]
- De Reys S, Blom C, Lepoudre B, et al. Human platelet aggregation by murine monoclonal antiplatelet antibodies is subtype-dependent. Blood. 1993;81:1792–1800. [PubMed] [Google Scholar]
- Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- Andre P, Prasad KS, Denis CV, et al. CD40L stabilizes arterial thrombi by a beta3 integrin-dependent mechanism. Nat Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- Prasad KS, Andre P, He M, Bao M, Manganello J, Phillips DR. Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc Natl Acad Sci USA. 2003;100:12367–12371. doi: 10.1073/pnas.2032886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haanstra KG, Ringers J, Sick EA, et al. Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation. 2003;75:637–643. doi: 10.1097/01.TP.0000054835.58014.C2. [DOI] [PubMed] [Google Scholar]
- Pearson TC, Trambley J, Odom K, et al. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation. 2002;74:933–940. doi: 10.1097/00007890-200210150-00006. [DOI] [PubMed] [Google Scholar]
- Adams AB, Shirasugi N, Jones TR, et al. Development of a chimeric anti-CD40 monoclonal antibody that synergizes with LEA29Y to prolong islet allograft survival. J Immunol. 2005;174:542–550. doi: 10.4049/jimmunol.174.1.542. [DOI] [PubMed] [Google Scholar]
- Laman JD, 't Hart BA, Brok H, et al. Protection of marmoset monkeys against EAE by treatment with a murine antibody blocking CD40 (mu5D12) Eur J Immunol. 2002;32:2218–2228. doi: 10.1002/1521-4141(200208)32:8<2218::AID-IMMU2218>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ishida I, Tomizuka K, Yoshida H. Production of human monoclonal and polyclonal antibodies in TransChromo animals. Cloning Stem Cells. 2002;4:91–102. doi: 10.1089/153623002753632084. [DOI] [PubMed] [Google Scholar]
- van der Pol W, van de Winkel JG. IgG receptor polymorphisms: Risk factors for disease. Immunogenetics. 1998;48:222–232. doi: 10.1007/s002510050426. [DOI] [PubMed] [Google Scholar]
- Imai A, Suzuki T, Sugitani A, et al. A novel fully human anti-CD40 monoclonal antibody, ASKP1240, for kidney transplantation in cynomolgus monkeys. Transplantation. 2007;84:1020–1028. doi: 10.1097/01.tp.0000286058.79448.c7. [DOI] [PubMed] [Google Scholar]
- Aoyagi T, Yamashita K, Suzuki T, et al. A human anti-CD40 monoclonal antibody, ASKP1240, for kidney transplantation in cynomolgus monkeys: Induction and maintenance therapy. Am J Transplant. 2009;9:1732–1741. doi: 10.1111/j.1600-6143.2009.02693.x. [DOI] [PubMed] [Google Scholar]
- Oura T, Yamashita K, Suzuki T, et al. Long-term hepatic allograft acceptance based on CD40 blockade by ASKP1240 in nonhuman primates. Am J Transplant. 2012;12:1740–1754. doi: 10.1111/j.1600-6143.2012.04014.x. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Yamashita K, Suzuki T, et al. ASKP1240, a fully human anti-CD40 monoclonal antibody, prolongs pancreatic islet allograft survival in nonhuman primates. Am J Transplant. 2013;13:1976–1988. doi: 10.1111/ajt.12330. [DOI] [PubMed] [Google Scholar]
- Press OW, Farr AG, Borroz KI, Anderson SK, Martin PJ. Endocytosis and degradation of monoclonal antibodies targeting human B-cell malignancies. Cancer Res. 1989;49:4906–4912. [PubMed] [Google Scholar]
- Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res. 2003;92:1041–1048. doi: 10.1161/01.RES.0000070111.98158.6C. [DOI] [PubMed] [Google Scholar]
- Slupsky JR, Kalbas M, Willuweit A, Henn V, Kroczek RA, Müller-Berghaus G. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb Haemost. 1998;80:1008–1014. [PubMed] [Google Scholar]
- Miller DL, Yaron R, Yellin MJ. CD40L–CD40 interactions regulate endothelial cell surface tissue factor and thrombomodulin expression. J Leukoc Biol. 1998;63:373–379. doi: 10.1002/jlb.63.3.373. [DOI] [PubMed] [Google Scholar]
- Xu Y, Szalai AJ, Zhou T, et al. Fc gamma Rs modulate cytotoxicity of anti-Fas antibodies: Implications for agonistic antibody-based therapeutics. J Immunol. 2003;171:562–568. doi: 10.4049/jimmunol.171.2.562. [DOI] [PubMed] [Google Scholar]
- Crow AR, Leytin V, Starkey AF, Rand ML, Lazarus AH. CD154 (CD40 ligand)-deficient mice exhibit prolonged bleeding time and decreased shear-induced platelet aggregates. J Thromb Haemost. 2003;1:850–852. doi: 10.1046/j.1538-7836.2003.t01-1-00115.x. [DOI] [PubMed] [Google Scholar]


