Abstract
Aim
Long-chain polyunsaturated fatty acids (LCPUFAs) are immunomodulatory, but their role in allergy development is controversial. We investigated whether proportions of LCPUFAs in serum phospholipids were related to allergic diagnosis, seafood intake and LCPUFA proportions in cord blood.
Methods
Serum was obtained from 148 birth cohort children at 13 years of age. Forty had atopic eczema, 53 had respiratory allergy, and 55 were nonallergic. Proportions of LCPUFAs were determined in serum phospholipids; cord blood from 128 of the individuals was previously analysed. Seafood intake was estimated using questionnaires.
Results
Allergic and nonallergic individuals did not differ significantly regarding individual LCPUFAs. However, arachidonic acid over docosahexaenoic acid (DHA) ratio was higher in allergic, compared with nonallergic, adolescents. In nonallergic individuals, LCPUFA proportions in cord serum and adolescent serum correlated weakly. In individuals with atopic eczema and respiratory allergy, these correlations were weak or absent. A moderate correlation between seafood intake and serum DHA was seen in nonallergic individuals and those with respiratory allergy, but not in those with atopic eczema.
Conclusion
Serum LCPUFA pattern was similar in allergic and nonallergic adolescents. Fatty acid metabolism may be altered in atopic eczema subjects, suggested by poor correlations between fatty acid intake and serum levels.
Keywords: Allergy, Asthma, Atopic eczema, Fatty acids, Polyunsaturated fatty acids
Introduction
Polyunsaturated fatty acids (PUFAs) are found in phospholipid membranes, including those surrounding serum lipoproteins, thrombocytes and cells. PUFAs are grouped into the n-3 and n-6 series, depending on the position of the double bonds. Some long-chain polyunsaturated fatty acids (LCPUFAs) are intracellular messengers and substrates for the production of mediators, such as prostaglandins and thromboxanes 1. Both n-3 and n-6 LCPUFAs strongly oppose activation of T cells, in particular those of the Th1 type 2–4. We recently found that high proportions of LCPUFAs in the cord serum of newborn infants were positively associated with the development of sensitisation and respiratory allergy during childhood 5. Our interpretation was that LCPUFAs hampered immune activation in response to neonatal microbial colonisation, thereby preventing immune maturation and natural tolerance development.
The serum fatty acid pattern in a foetus reflects what has been transferred through the placenta from the maternal circulation during pregnancy 6. In children and adults, serum LCPUFAs derive mainly from the diet, but also from endogenous production from essential precursors of the omega-6 and omega-3 series, that is, 18:2 n-6 (linoleic acid, LA) and 18:3 n-3 (alpha-linolenic acid, ALA), respectively. These essential PUFAs are mainly found in vegetable oils. LCPUFAs of the n-3 series are mainly found in fish and other seafood, while n-6 LCPUFAs are found in both fish and meat. LCPUFAs are incorporated into membranes, used as an energy source and act as substrates for the production of a range of bioactive compounds 7. For example, arachidonic acid is the precursor of prostaglandins, leukotrienes and thromboxanes 1. Consequently, bodily consumption of LCPUFAs may be elevated in inflammatory processes, including allergic inflammation during which prostaglandins and leukotrienes are produced from mast cells and macrophages in response to allergen exposure. This phenomenon was demonstrated in an experimental respiratory allergy model in mice, in which a rapid decrease in serum levels of both n-3 and n-6 LCPUFAs accompanied the inflammatory reaction triggered by the allergen challenge 8. We previously reported that women with respiratory allergy and atopic eczema had lower proportions of n-3 LCPUFAs in breastmilk than nonallergic women, despite having at least the same high fish intake, which could suggest greater consumption of LCPUFAs in allergic than healthy individuals 9.
Key notes
Long-chain polyunsaturated fatty acids (LCPUFAs) have immunomodulatory effects, but their role in allergy development is controversial.
The LCPUFA pattern in serum at 13 years of age was related to LCPUFA proportions at birth and present seafood intake in nonallergic individuals, but the correlations were weaker or nonexistent in subjects with respiratory allergy or atopic eczema.
Our results suggest that fatty acid metabolism is modified in individuals with atopic eczema.
The present study examined serum phospholipid LCPUFA patterns in 13-year-old adolescents with either respiratory allergy, atopic eczema or without any allergic symptoms. The individuals had previously been characterised with respect to LCPUFA patterns in cord blood obtained at parturition 5. We could thereby relate the LCPUFA pattern at 13 years of age to the pattern in the cord blood and examine whether allergic and nonallergic individuals differed when it came to changes in LCPUFA patterns over time. By assessing seafood intake using a food frequency questionnaire, we could examine the relation between seafood intake and serum n-3 LCPUFA levels in allergic and nonallergic individuals.
Methods
A total of 148 subjects aged 13 years were selected from a birth cohort consisting of all 1228 children born during a one-year period from February 1996 to January 1997 at the Östersund Hospital in Jämtland, Sweden. Pregnant women were recruited in gestational week 18, and the children were followed from birth until 13 years of age, with regular assessments of allergic sensitisation by skin prick tests at the ages of one and four and allergic symptoms by questionnaires at the ages of one four and seven, as previously described 5,10. At 13 years of age, 794 adolescents filled out a new questionnaire regarding allergic symptoms and were skin prick tested for milk, eggs, fish, wheat, soy, cat, dog, horse, timothy grass and birch using standardised extracts (ALK, Hörsholm, Denmark; 10 histamine equivalent prick units potency). A single nurse conducted all the skin prick tests, and reproducibility was repeatedly checked according to international recommendations 11.
Of the 794 adolescents who filled out the questionnaire and received a skin prick test, 130 had respiratory allergy, but no atopic manifestations from other organs, and at least one positive reaction in the skin prick test. Respiratory allergy was defined as fulfilling one or more of the following criteria: wheeze in the past year, doctor's diagnosis of asthma, treatment with an asthma inhaler or a positive answer to the question ‘Have you had any signs of pollen allergy or allergy to furred pets during the last 12 months?’ Another 79 adolescents had atopic eczema without any other allergic disease manifestation. Atopic eczema was defined as a pruritic, chronic or chronically relapsing noninfectious dermatitis with typical features and distribution, fulfilling three of the main criteria suggested by Hanifin and Rajka 12. We also identified 332 adolescents who were free of any allergic symptoms and had not displayed any positive reaction in the skin prick test during any of the follow-ups during childhood.
From the three clinical groups, we randomly selected 53 cases with respiratory allergy, but no other allergic disease, 40 cases with atopic eczema, but no other allergic disease, and 55 nonallergic and nonsensitised controls. Of these 148 adolescents, 128 were previously investigated regarding fatty acid proportions in cord serum obtained at birth 5. The atopic eczema group was mainly skin prick negative (36/40), while all the adolescents in the respiratory allergy group had to have at least one positive skin prick test.
Analysis of fatty acids in serum
Venous blood (10 mL) was drawn from the adolescents, allowed to clot and centrifuged. Serum was separated, aliquoted and frozen within 3 h. It was stored at −80 °C until analysed.
Lipids were extracted from serum with chloroform and methanol (1:2) 13. The phospholipid fraction was separated on aminopropyl solid-phase extraction columns (Isolute NH2, 6 mL, 50 mg, IST, Mid Glamorgon, UK) 14, and the fatty acids were then converted to methyl esters using acetyl chloride (10%) dissolved in methanol 15. After methylation, the fatty acids were separated by gas chromatography (Hewlett Packard 5890, Waldbronn, Germany) on a HP Ultra 1 column (50 m × 0.32 mm × 0.52 μm DF, J&W Scientific, California, USA). Detection was carried out by flame ionisation, and the Borwin software (Le Fontanil, France) was used for evaluation. Polyunsaturated fatty acids that were found in sufficient amount and could clearly be separated were selected for statistical analyses (20:2 n-6, 20:3 n-6, 20:4 n-3, 20:4 n-6, 20:5 n-3, 22:4 n-6, 22:5 n-6, 22:5 n-3 and 22:6 n-3). We also analysed the total proportions of n-3 and n-6 LCPUFAs as well as the total proportion of all LCPUFAs, by summing the proportions of the nine individual LCPUFA species.
Assessment of dietary intake of seafood
In relation to blood sampling, the adolescents were asked to fill out a food frequency questionnaire (FFQ) with the aid of an accompanying parent. The questionnaire was based on the validated Northern Sweden FFQ 16 and contained 52 questions covering intake of selected food items that could be expected to affect fatty acid pattern in serum, for example fatty fish, lean fish, shellfish and n-3/n-6 supplements. Options for intake frequencies included never, once a month, once a week and daily, as well as a comment field where respondents could report frequencies in detail. Frequencies were converted to intake in grams using the 2001 Swedish National Food Agency's weight table.
Ethic statement
The study was conducted according to the second Declaration of Helsinki (http://www.wma.net/en/30publications/10policies/b3/index.html) and was approved by the local ethical committee in Umeå, Sweden (Dnr 09-110M). At 13 years of age, the adolescents were able to provide their own verbal approval for the skin prick tests and for the fatty acid analyses in the new serum samples. In addition, their parents provided a written consent form on behalf of the children. Participation was voluntary, and the subjects were free to withdraw, at any time and without any stated reason, without being disadvantaged in any way.
Statistical analysis
Multivariate analysis
We used partial least squares discriminant analysis (PLS-DA) 17 to find a model able to separate the three clinical classes: nonallergic, atopic eczema and respiratory allergy, based on the proportion of each of nine specified n-3 and n-6 LCPUFAs, as well the sum of n-3 LCPUFAs, n-6 LCPUFAs, total LCPUFAs, and the ratios of arachidonic acid (AA) over docosahexaenoic acid (DHA) and total n-6 LCPUFAs over total n-3 LCPUFAs (Fig. 1). If a PLS-DA model is achieved, the variables that contribute to the separation of the classes can be identified. This method permits an evaluation of differences in variable values without the risk of mass significance and need for normal distribution of data or strict independence among variables as in conventional statistics. PLS-DA was performed using Simca 13.0 (Umetrics, Umeå, Sweden).
Figure 1.
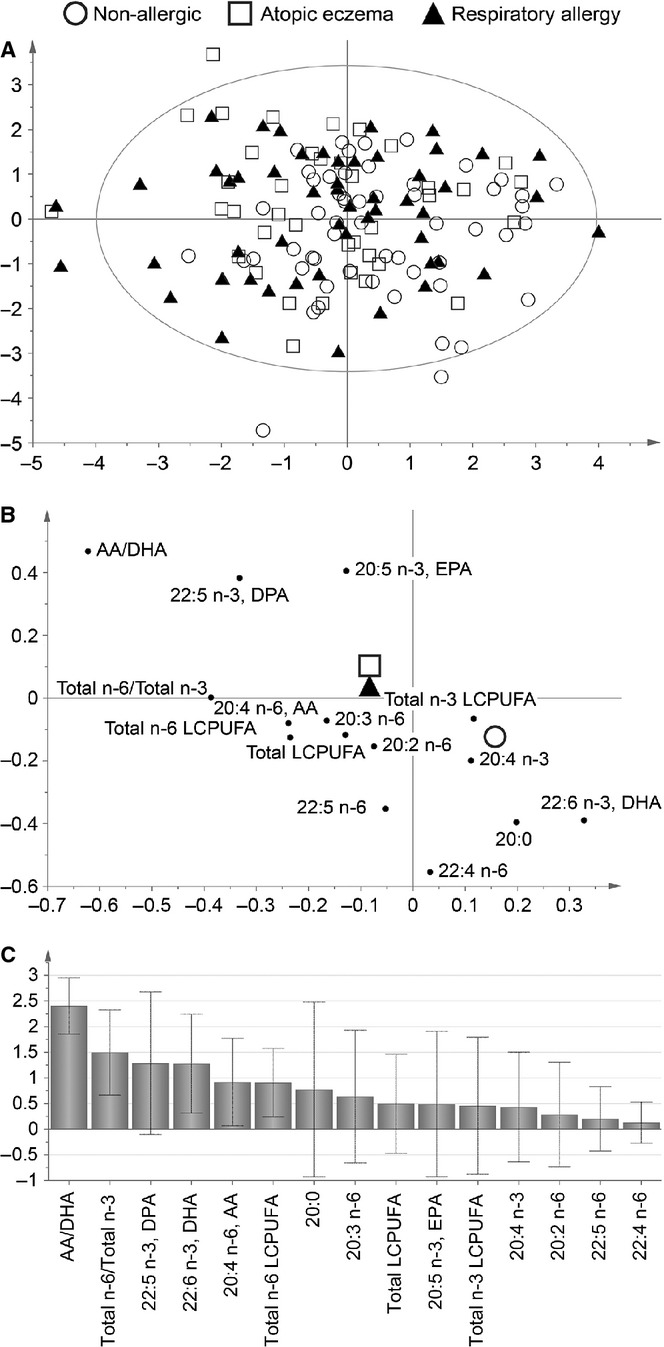
Partial least squares discriminant analysis (PLS-DA), showing the separation of the three groups, nonallergic, atopic eczema and respiratory allergy, according to fatty acid profile in serum at 13 years of age. The PLS-DA score plot (A) shows an overview of the separation between the three clinical groups. Each symbol represents one individual, and the position of the symbol is achieved through a combination of this individual's value of all variables. The PLS-DA loading plot (B) display to what extent the different variables contribute to the separation of the three groups; higher the bar, higher the discriminatory power. The variable of importance (VIP) plot (C) shows to what degree the different variables contribute to the group separation.
Univariate analysis
Chi-square test was used to analyse differences in background variables between the selected individuals and the groups from which they were selected. Intake of seafood (not normally distributed) was compared pairwise between the diagnostic groups using Mann–Whitney U-test. Paired t-test was used to compare proportions of fatty acids in the same individuals between cord blood and blood collected at 13 years of age, and data from each diagnostic group were calculated separately. Pearson's correlation test was used for correlations between normally distributed variables, such as fatty acid proportions in serum, while Spearman's rank test was used to correlate intake of seafood (not normally distributed) with serum fatty acid pattern. All univariate statistical analyses were performed using IBM SPSS Statistics version 19 (IBM Corporation, New York, USA), and p ≤ 0.05 was considered significant.
Results
In this study, allergic 13-year-old subjects were compared to nonallergic subjects of the same age regarding fatty acid proportions in serum phospholipids. In all, 148 subjects were chosen based on their clinical diagnosis at 13 year of age, that is, respiratory allergy (n = 53), atopic eczema (n = 40) or no allergy (n = 55). Selected adolescents did not differ statistically from those in the groups from which they were selected (Table 1). Atopic eczema was disproportionately more common among female individuals (p = 0.024) and respiratory allergy among male individuals (p < 0.001) (Table 1).
Table 1.
Characteristics of the study population
| Nonallergic | Atopic eczema* | Respiratory allergy† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All, % (n = 332) | Randomly selected (n = 55) | p-value‡ | All, % (n = 79) | Randomly selected (n = 40) | p-value‡ | All, % (n = 130) | Randomly selected (n = 53) | p-value‡ | |
| Gender (female) | 56 (177/330) | 65 (36/55) | 0.16 | 70 (55/79) | 70 (28/40) | 1.0 | 33 (43/130) | 32 (18/53) | 0.91 |
| Have older siblings§ | 60 (181/302) | 69 (38/55) | 0.35 | 58 (46/79) | 60 (24/40) | 0.59 | 65 (85/130) | 62 (33/53) | 0.51 |
| Exclusive breastfeeding at 4 months§ | 71 (211/299) | 70 (35/50) | 0.82 | 78 (59/76) | 76 (29/38) | 0.52 | 73 (92/126) | 69 (35/51) | 0.30 |
| Maternal allergic heredity¶ | 33 (106/329) | 36 (20/55) | 0.55 | 52 (41/79) | 45 (18/40) | 0.48 | 55 (72/130) | 57 (30/53) | 0.88 |
| Asthma | 6 (20/329) | 5 (3/55) | 0.86 | 23 (18/79) | 20 (8/40) | 0.73 | 17 (22/130) | 25 (13/53) | 0.24 |
| Rhinitis | 11 (37/329) | 16 (9/55) | 0.28 | 24 (19/79) | 20 (8/40) | 0.62 | 33 (43/130) | 40 (21/53) | 0.40 |
| Eczema | 23 (75/329) | 22 (12/55) | 0.87 | 38 (30/79) | 33 (13/40) | 0.56 | 32 (41/130) | 28 (15/53) | 0.67 |
| Paternal allergic heredity¶ | 32 (105/324) | 31 (17/55) | 0.83 | 37 (29/79) | 25 (10/40) | 0.65 | 48 (62/129) | 50 (26/52) | 0.81 |
| Asthma | 11 (35/324) | 11 (6/55) | 0.98 | 8 (6/79) | 8 (3/40) | 0.99 | 16 (20/129) | 15 (8/52) | 0.98 |
| Rhinitis | 15 (47/324) | 15 (8/55) | 0.99 | 19 (15/79) | 15 (6/40) | 0.59 | 32 (41/129) | 36 (19/52) | 0.54 |
| Eczema | 16 (52/324) | 7 (4/55) | 0.09 | 25 (20/79) | 23 (9/40) | 0.74 | 19 (25/129) | 23 (12/52) | 0.58 |
| Cat during first year in life§ | 19 (64/331) | 24 (13/55) | 0.46 | 23 (19/79) | 25 (10/40) | 0.91 | 12 (16/130) | 13 (7/53) | 0.87 |
| Dog during first year in life¶ | 30 (99/331) | 20 (11/55) | 0.13 | 29 (23/79) | 30 (12/40) | 0.92 | 16 (21/130) | 15 (8/53) | 0.86 |
| Living in urban area** | 7 (18/242) | 3 (1/37) | 0.32 | 7 (4/56) | 4 (1/28) | 0.52 | 12 (12/103) | 9 (4/43) | 0.68 |
Defined as a pruritic, chronic or chronically relapsing noninfectious dermatitis with typical features and distribution three of the main criteria suggested by Hanifin and Rajka 18, diagnosed at 13 years of age.
Fulfilling ≥1 of the following criteria: wheeze in the past year, asthma inhalation treatment or a positive answer to the question: ‘Have you had any signs of pollen allergy or allergy to furred pets during the last 12 months?’, diagnosed at 13 years of age.
Chi-square test between the whole group and the randomly selected group.
Answered at 1 year of age.
Defined as an affirmative answer to the question ‘Has the child's mother/father ever had asthma, hay fever or eczema?’, answered at 13 years of age.
Answered at recruitment in mid-pregnancy.
LCPUFA pattern in serum of allergic and nonallergic adolescents
Serum from 13-year-old individuals with respiratory allergy, atopic eczema or no allergy was analysed regarding LCPUFA proportions. We used the multivariate pattern recognition method PLS-DA to investigate whether the three diagnostic groups could be separated based on serum LCPUFA pattern. As shown in Figure 1A, the three groups were poorly separated from one another, suggesting that the proportions of different LCPUFAs in serum did not differ much between the different groups (Fig. 1B). The contribution of each variable to the group separation is shown in Figure 1C (variable of importance plot). The plot shows that the AA/DHA ratio and the total n-6/total n-3 ratio differed most between allergic and nonallergic individuals, being higher in the allergic groups than in the nonallergic group (Fig. 1B). Univariate analysis confirmed that the AA/DHA ratio was significantly elevated in subjects with respiratory allergy (2.6 ± 0.64) and atopic eczema (2.6 ± 0.58) compared with nonallergic subjects (2.3 ± 0.47) (p = 0.018 and 0.023, respectively). The difference in ratio of total n-6 LCPUFA/total n-3 LCPUFA was not confirmed by univariate analysis (respiratory allergy: 2.3 ± 0.48, atopic eczema: 2.3 ± 0.39, p = 0.068 and 0.185, respectively, compared with nonallergic subjects, 2.2 ± 0.40).
Changes in serum fatty acids between birth and 13 years of age in allergic and nonallergic adolescents
Cord blood serum samples had been collected from 128 of the 148 adolescents at birth and analysed for LCPUFA pattern 5. Figure 2 shows the changes in proportion of the major n-3 and n-6 LCPUFAs between birth and 13 years of age in these individuals. The analyses were carried out separately for the three diagnostic groups, and the three single fatty acids found in the highest proportions, that is, DHA, dihomo-γ-linolenic acid (DGLA) and AA, as well as total n-3 LCPUFA, total n-6 LCPUFA and total LCPUFA, are shown. The proportion of total n-3 LCPUFA increased significantly in all three groups (nonallergic, atopic eczema and respiratory allergy), but more so in the nonallergic group. Conversely, total n-6 LCPUFA proportions decreased significantly in all three groups, but most strongly in the allergic groups. For DHA, the major n-3 LCPUFA found in serum phospholipids, the proportions increased over time from cord serum to adolescent serum in nonallergic subjects, whereas it decreased in the two allergic groups. The same was true for the total proportion of LCPUFAs among serum phospholipids, which increased between birth and 13 years of age in nonallergic individuals, while it decreased significantly both in the atopic eczema and respiratory allergy groups (Fig. 2). We had previously reported higher levels of both n-3 and n-6 LCPUFAs in cord blood of individuals who later developed allergy 5. As nonallergic individuals tended to increase their n-3 LCPUFA levels more than the allergic groups and decrease their n-6 LCPUFA levels less, the differences between the groups were evened out, which resulted in the lack of significant differences at the group level at 13 years of age (Fig. 1).
Figure 2.
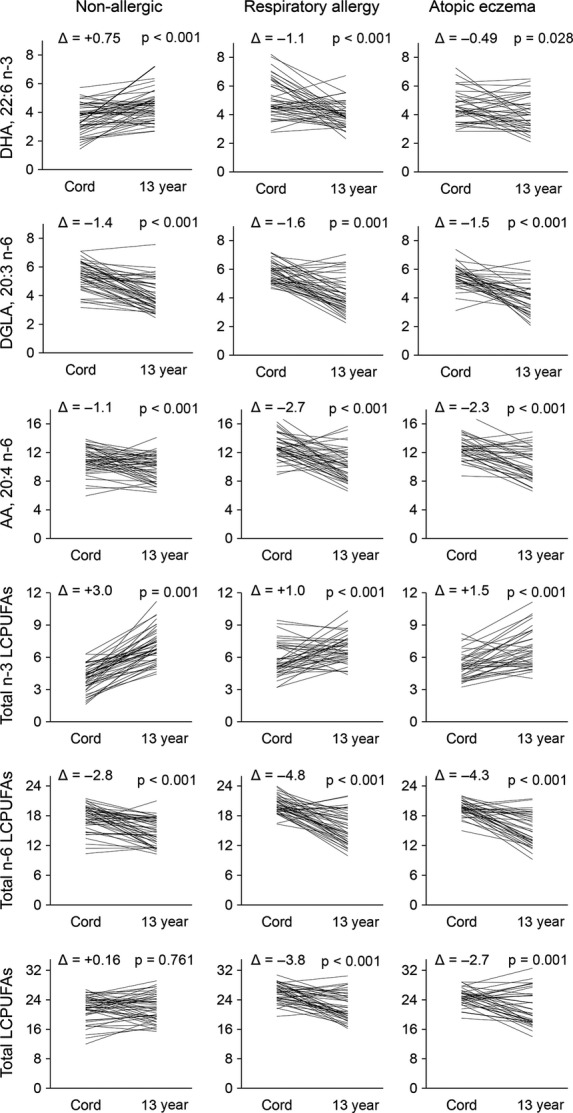
Line chart showing the change of fatty acid proportions in serum phospholipids [% of serum phospholipids] in each individual between cord serum drawn at birth and adolescent serum drawn at 13 years of age for each clinical group. The p-values are from paired t-test. Δ shows the mean difference between proportion in cord serum and proportion in adolescent serum. Abbreviations and explanations: LCPUFA, long-chain polyunsaturated fatty acids; total n-3 LCPUFA, sum of 20:4, 20:5, 22:5 and 22:6; total n-6 LCPUFA, sum of 20:2, 20:3, 20:4, 22:4 and 22:5; total LCPUFA, sum of total n-3 and total n-6 LCPUFA.
Correlation between serum fatty acids at birth and at 13 years of age in allergic and nonallergic adolescents
We also investigated whether the individuals who had the highest proportions of a particular LCPUFA at birth also had the highest proportions 13 years later and vice versa. Correlations were analysed separately for the three diagnostic groups for the three single fatty acids that are dominating in proportion in serum (DHA, DGLA and AA) as well as total n-3 LCPUFA, total n-6 LCPUFA and total LCPUFA (Table 2). In the nonallergic group, two variables (DGLA and total LCPUFA) showed a modest (>0.4) correlation and three variables (DHA, total n-3 LCPUFA and total n-6 LCPUFA) weak correlation (>0.3) between the proportions in cord serum and in serum drawn at 13 years of age. By contrast, in the respiratory allergy group, there were no correlations between the individuals' measures of any LCPUFA types between birth and at 13 years of age. In the atopic eczema group, one variable (total n-3 LCPUFA) showed a weak correlation (>0.3) between the proportion in cord serum and in serum drawn at 13 years of age (Table 2).
Table 2.
Correlations between proportions of LCPUFAs in serum phospholipids obtained at birth and at 13 years of age in the same individuals‡
| Nonallergic (n = 48†) | Respiratory allergy (n = 43†) | Atopic eczema (n = 37†) | |
|---|---|---|---|
| 22:6 n-3 (DHA) | 0.347* | 0.050 | 0.310 |
| 20:3 n-6 (DGLA) | 0.441** | 0.116 | −0.105 |
| 20:4 n-6 (AA) | 0.268 | −0.040 | 0.313 |
| Total n-3 LCPUFA | 0.352* | 0.183 | 0.391* |
| Total n-6 LCPUFA | 0.345* | −0.020 | 0.170 |
| Total LCPUFA | 0.437*** | 0.110 | 0.241 |
LCPUFA = long-chain polyunsaturated fatty acids. Total n-3 LCPUFA is the sum of 20:4, 20:5, 22:5 and 22:6. Total n-6 LCPUFA is the sum of 20:2, 20:3, 20:4, 22:4 and 22:5. Total LCPUFA is the sum of total n-3 and total n-6 LCPUFA.
p ≤ 0.05.
p ≤ 0.01.
p ≤ 0.001.
Number of individuals with fatty acid proportions analysed both at birth and 13 years later.
Pearson's correlation coefficient.
Correlation between intake of seafood and proportions of LCPUFAs in serum phospholipids in allergic and nonallergic adolescents
The proportion of DHA in serum phospholipids was slightly higher in nonallergic 13-year-old adolescents than in those with allergies (Fig. 1). LCPUFAs of the n-3 series are foremost found in seafood, that is, both fatty and lean fish, as well as other seafood. Intake of seafood at 13 years of age was assessed by a food frequency questionnaire, and the intake of seafood (in grams per week) was calculated for the three groups. As shown in Table 3, there were no significant differences in intake of lean fish, fatty fish or total seafood between allergic and nonallergic subjects.
Table 3.
Intake of seafood and fish oil capsules at 13 years of age*
| Nonallergic (n = 53)† | Respiratory allergy (n = 49)† | Atopic eczema (n = 39)† | p (respiratory vs control)‡ | p (eczema vs control)‡ | |
|---|---|---|---|---|---|
| Lean fish | 77 ± 57 | 72 ± 61 | 85 ± 58 | 0.63 | 0.41 |
| Fatty fish | 73 ± 58 | 61 ± 57 | 86 ± 63 | 0.32 | 0.34 |
| Total seafood§ | 158 ± 93 | 142 ± 106 | 180 ± 102 | 0.32 | 0.45 |
| Fish oil capsules | 0.49 ± 1.7 | 0.51 ± 2.2 | 0.56 ± 2.5 | 0.83 | 0.79 |
Shown as mean ± SD [g/week].
Number of subjects that have filled in a complete food frequency questionnaire in each group.
p-value obtained by nonparametric Mann–Whitney U-test.
Including fatty and lean fish, as well as shellfish.
We correlated the proportions of DHA and total n-3 LCPUFAs in serum phospholipids with the reported intake frequencies of total seafood, in the three diagnostic groups (Fig. 3). In nonallergic subjects, higher intake of seafood resulted in increased serum proportions of DHA (r = 0.46, p < 0.001), as well as total n-3 LCPUFA (r = 0.46, p < 0.001) among serum phospholipids (Fig. 3, left panel). In subjects with respiratory allergy, there was a weak, but still significant, correlation between intake of seafood and DHA (r = 0.29, p = 0.036) and a weak nonsignificant positive correlation between intake of seafood and total n-3 LCPUFA proportions (r = 0.24, p = 0.086) in serum phospholipids (Fig. 3, middle panel). In subjects with atopic eczema, however, intake of seafood did not result in any increase in either serum proportions of DHA (r = 0.16, p = 0.319) or total n-3 LCPUFAs (r = 0.03, p = 0.845) (Fig. 3, right panel). We also correlated the serum proportions of DHA and total n-3 LCPUFA to the intake of fatty fish, rather than the total seafood intake. Among nonallergic individuals, fatty fish intake correlated moderately with DHA and total n-3 in serum (r = 0.30, p = 0.026 for DHA and r = 0.30, p = 0.028 for total n-3 LCPUFA). This pattern was also seen in individuals with respiratory allergy (r = 0.32, p = 0.022 for DHA and r = 0.33, p = 0.017 for total n-3 LCPUFA) but not among individuals with atopic eczema (r = 0.18, p = 0.26 for DHA and r = 0.041, p = 0.80 for total n-3 LCPUFA).
Figure 3.
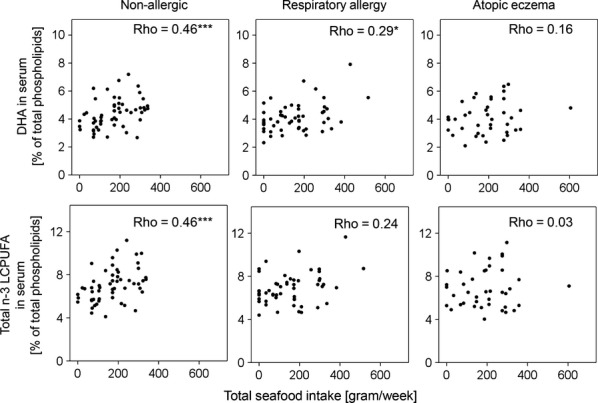
Correlations between intake of total seafood [gram/week] and proportions of DHA and total n-3 LCPUFAs in serum [% of total serum phospholipids] for each studied group. *p ≤ 0.05, ***p ≤ 0.001.
EPA in serum phospholipids correlated with fatty fish intake and total seafood intake in a similar manner as did DHA and total n-3 LCPUFAs. For nonallergic individuals, EPA showed a nonsignificant correlation with fatty fish intake (r = 0.241, p = 0.076) and a significant correlation with total seafood intake (r = 0.391, p = 0.003). This was also seen for subjects with respiratory allergy in whom EPA was nonsignificantly correlated with fatty fish intake (r = 0.254, p = 0.070) and significantly correlated with total seafood intake (r = 0.220, p = 0.118). In subjects with atopic eczema, EPA correlated neither with fatty fish intake (r = 0.000, p = 0.998) nor with total seafood intake (r = 0.056, p = 0.719).
Discussion
We have recently shown that adolescents with atopic eczema or respiratory allergy at 13 years of age were characterised by higher proportions of many LCPUFAs in phospholipids of serum obtained at birth, compared with individuals who remained nonsensitised and nonallergic during their first 13 years of life 5. Here, we investigate whether such differences in LCPUFA pattern could be detected in serum at 13 years of age. We used a multivariate approach to achieve this first aim. Partial least square discriminant analysis (PLS-DA) can be used to separate predetermined groups of observations (in this case individuals with different diagnoses at 13 years of age: nonallergic, respiratory allergy or atopic eczema) based on the values of all measured parameters (here the proportions of LCPUFA species). It was clear that the three diagnostic groups (nonallergic, respiratory allergy and atopic eczema) could not be separated based on their fatty acid pattern in serum at 13 years of age. A similar result was recently found by Standl et al. 18, who analysed fatty acids in cord blood and in blood collected at two, six and 10 years in the LISAplus birth cohort. At 10 years of age, which is close to the age of the subjects in this study, no differences were found between subjects with asthma, hay fever or eczema and nonallergic controls regarding total n-3 LCPUFA, total n-6 LCPUFA or n-6/n-3 ratio.
Fatty acids in cord blood are transported from the maternal circulation across the placenta during pregnancy. Some fatty acids are transported actively, including several n-3 PUFAs and n-6 PUFAs, while others diffuse passively, including saturated fatty acids and monounsaturated fatty acids 6. Fatty acid composition in cord serum is therefore a function of the efficiency of the placental fatty acid transport proteins, the elongation of essential fatty acids to LCPUFAs by the foetus or by the concentration of fatty acids in the maternal circulation. In children and adults, the fatty acid composition of serum phospholipids is mainly determined by dietary intake of fatty acid rich food. Hence, the proportions of cord and adolescent blood LCPUFAs are determined by different factors. The longer n-3 and n-6 LCPUFAs can also be synthesised from the shorter essential linoleic and α-linolenic acid, but only at low efficiency 19. The capacity to elongate medium-sized fatty acids to LCPUFA is determined by genetic factors 20.
It is not very well known how the proportions of LCPUFAs in serum change from birth to adolescence. Here, we show that the proportion of n-3 LCPUFAs increase over time, while the proportion of n-6 LCPUFAs decrease over time. The increase in n-3 LCPUFAs was more pronounced among nonallergic individuals than among allergic individuals. Furthermore, the two allergic groups showed a significant decrease in total LCPUFA proportions between birth and 13 years of age, while this was not seen in the nonallergic group. Infants who later developed allergy were born with higher proportions of a range of LCPUFAs, and a higher total proportion of LCPUFA, than individuals who remained nonallergic 5. The decrease in total LCPUFA proportions (from birth to adolescence) in the allergic groups eradicated the differences between allergic and nonallergic subjects at birth. Hence, at 13 years of age, allergic and nonallergic individuals did not differ in the proportion of total LCPUFAs in serum phospholipids.
We analysed the correlation between fatty acid proportions at birth and at 13 years of age in the same individuals. In the nonallergic group, we found significant correlations between birth and adolescence, for DHA, DGLA as well as for total n-3 LCPUFA, total n-6 LCPUFA and the total LCPUFA proportions, that is, individuals who had high proportions at birth also tended to have high proportions at 13 years of age. It should be recognised, however, that a regression coefficient of 0.4 means that only 16% of the difference in proportions of a certain LCPUFA at 13 years of age may be predicted from the proportions at birth. The correlations between birth and 13-year proportions of different LCPUFAs were even weaker in the atopic eczema group and nonexisting in the respiratory allergy group.
Several studies have explored the association between diet and allergy. Fish intake is associated with reduced risk of asthma in adults 21–23 and children 24, and early introduction of fish into the diet is associated with reduced risk of development of eczema 25–27. However, one study reported higher prevalence of asthma among children who consumed fish one to two times a week than among children who consumed fish less often 28. In the present study, we did not observe any difference in fish intake between allergic and nonallergic individuals.
Several studies have shown that dietary intake of n-3 LCPUFAs is reflected in serum 29. However, to our knowledge, no studies have analysed if the intake of seafood (rich in n-3 LCPUFA) correlate with the proportions of n-3 LCPUFA serum phospholipids in allergic subjects and if it differs depending on allergic manifestation. Therefore, we analysed the correlation between intake of seafood and the proportion of n-3 LCPUFAs in serum in both nonallergic adolescents and in those with eczema and respiratory allergy. We found that there was a correlation between intake of seafood and the proportion of n-3 LCPUFA in serum phospholipids in nonallergic individuals and in subjects with respiratory allergy, but not in subjects with atopic eczema. Thus, nonallergic individuals who consumed high levels of seafood had higher proportions of EPA, DHA and total n-3 LCPUFA among their serum phospholipids than those who consumed little seafood. This was not seen in individuals with atopic eczema, whose LCPUFA serum proportions did not correlate with fish intake. We have previously found that women with both respiratory allergy and atopic eczema have lower proportions of n-3 LCPUFAs in serum and breastmilk, as compared to nonallergic women or women with isolated respiratory allergy, despite a greater intake of fish in the first group 9. Similar result was found by Dunder et al. 30 who reported that children with atopic dermatitis had lower levels of EPA and DHA than nonallergic controls, despite a similar fish intake. Accordingly, in the present study, we found that allergic individuals had lower DHA levels than the nonallergic despite similar seafood intake. Moreover, LCPUFAs was observed to be consumed during allergic inflammation in an experimental mouse model of respiratory allergy 8. These findings support the notion that the fatty acid metabolism differs in allergic and nonallergic persons. We propose that bodily consumption of LCPUFAs during allergic inflammation might explain these findings. Another possible explanation is that allergic individuals would be more prone to report their intake, as compared to nonallergic individuals. People with a chronic disease might be more conscious than healthy people of diet and other factors that are seen as affecting health.
Acknowledgments
We thank Nils-Gunnar Carlsson for technical assistance and Anna Bernholm for blood sampling and performing the skin prick tests.
Funding
The work was supported by grants from the Swedish Research Council (project number 521-213-3154); the Swedish Research Council for Environmental, Agricultural Sciences and Spatial Planning (FORMAS), Stockholm, Sweden (project number 216-2009-752); the Västra Götaland Region, Sweden; the Centre for Environment and Sustainability, GMV, Gothenburg, Sweden; the Research and Development Departments of the Jämtland and Norrbotten County Councils, Sweden; and the ThC Bergh Foundation.
Conflict of Interests
The authors declare no financial or commercial conflict of interests.
References
- 1.Murakami M. Lipid mediators in life science. Exp Anim. 2011;60:7–20. doi: 10.1538/expanim.60.7. [DOI] [PubMed] [Google Scholar]
- 2.Soyland E, Nenseter MS, Braathen L, Drevon CA. Very long chain n-3 and n-6 polyunsaturated fatty acids inhibit proliferation of human T-lymphocytes in vitro. Eur J Clin Invest. 1993;23:112–21. doi: 10.1111/j.1365-2362.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- 3.Zurier RB, Rossetti RG, Seiler CM, Laposata M. Human peripheral blood T lymphocyte proliferation after activation of the T cell receptor: effects of unsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 1999;60:371–5. doi: 10.1016/s0952-3278(99)80015-5. [DOI] [PubMed] [Google Scholar]
- 4.Wallace FA, Miles EA, Evans C, Stock TE, Yaqoob P, Calder PC. Dietary fatty acids influence the production of Th1- but not Th2-type cytokines. J Leukocyte Biol. 2001;69:449–57. [PubMed] [Google Scholar]
- 5.Barman M, Johansson S, Hesselmar B, Wold AE, Sandberg A-S, Sandin A. High Levels of Both n-3 and n-6 Long-chain polyunsaturated fatty acids in cord serum phospholipids predict allergy development. PLoS ONE. 2013;8:e67920. doi: 10.1371/journal.pone.0067920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham P, McDermott L. Long chain PUFA transport in human term placenta. J Nutr. 2009;139:636–9. doi: 10.3945/jn.108.098608. [DOI] [PubMed] [Google Scholar]
- 7.Ratnayake WM, Galli C. Fat and fatty acid terminology, methods of analysis and fat digestion and metabolism: a background review paper. Ann Nutr Metab. 2009;55:8–43. doi: 10.1159/000228994. [DOI] [PubMed] [Google Scholar]
- 8.Johansson S, Lonnqvist A, Ostman S, Sandberg AS, Wold AE. Long-chain polyunsaturated fatty acids are consumed during allergic inflammation and affect T helper type 1 (Th1)- and Th2-mediated hypersensitivity differently. Clin Exp Immunol. 2010;160:411–9. doi: 10.1111/j.1365-2249.2010.04107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson S, Wold AE, Sandberg AS. Low breast milk levels of long-chain n-3 fatty acids in allergic women, despite frequent fish intake. Clin Exp Allergy. 2011;41:505–15. doi: 10.1111/j.1365-2222.2010.03678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandin A, Björkstén B, Bråbäck L. Development of atopy and wheezing symptoms in relation to heredity and early pet keeping in a Swedish birth cohort. Pediatr Allergy Immunol. 2004;15:316–22. doi: 10.1111/j.1399-3038.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- 11.International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Phase II Modules of the International Study of Asthma and Allergies in Childhood (ISAAC) Munster, Germany: University of Munster; 1998. [DOI] [PubMed] [Google Scholar]
- 12.Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;92(Suppl):44–7. [Google Scholar]
- 13.Lee CM, Trevino B, Chaiyawat M. A simple and rapid solvent extraction method for determining total lipids in fish tissue. J AOAC Int. 1996;79:487–92. [PubMed] [Google Scholar]
- 14.Kaluzny MA, Duncan LA, Merritt MV, Epps DE. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J Lipid Res. 1985;26:135–40. [PubMed] [Google Scholar]
- 15.Lepage G, Roy CC. Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J Lipid Res. 1984;25:1391–6. [PubMed] [Google Scholar]
- 16.Johansson I, Hallmans G, Wikman A, Biessy C, Riboli E, Kaaks R. Validation and calibration of food-frequency questionnaire measurements in the Northern Sweden Health and Disease cohort. Public Health Nutr. 2002;5:487–96. doi: 10.1079/phn2001315. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson L, Johansson E, Kettaneh-Wold N, Trygg J, Wikström C, Wold S. Multi- and megavariate data analysis, Part 1 Basic principles and applications. Umeå, Sweden: Umetrics AB; 2006. 2nd revised and enlarged ed. [Google Scholar]
- 18.Standl M, Demmelmair H, Koletzko B, Heinrich J. Cord blood LC-PUFA composition and allergic diseases during the first 10 yr. Results from the LISAplus study. Pediatr Allergy Immunol. 2014 doi: 10.1111/pai.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenna JT, Salem N, Jr, Sinclair AJ, Cunnane SC. alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Xie L, Innis SM. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J Nutr. 2008;138:2222–8. doi: 10.3945/jn.108.096156. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Xun P, Zamora D, Sood A, Liu K, Daviglus M, et al. Intakes of long-chain omega-3 (n−3) PUFAs and fish in relation to incidence of asthma among American young adults: the CARDIA study. Am J Clin Nutr. 2013;97:173–8. doi: 10.3945/ajcn.112.041145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laerum BN, Wentzel-Larsen T, Gulsvik A, Omenaas E, Gislason T, Janson C, et al. Relationship of fish and cod oil intake with adult asthma. Clin Exp Allergy. 2007;37:1616–23. doi: 10.1111/j.1365-2222.2007.02821.x. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto S, Miyake Y, Sasaki S, Tanaka K, Ohya Y, Matsunaga I, et al. Fat and fish intake and asthma in Japanese women: baseline data from the Osaka Maternal and Child Health Study. Int J Tuberc Lung Dis. 2007;11:103–9. [PubMed] [Google Scholar]
- 24.Hodge L, Salome CM, Peat JK, Haby MM, Xuan W, Woolcock AJ. Consumption of oily fish and childhood asthma risk. Med J Aust. 1996;164:137–40. doi: 10.5694/j.1326-5377.1996.tb122010.x. [DOI] [PubMed] [Google Scholar]
- 25.Alm B, Aberg N, Erdes L, Mollborg P, Pettersson R, Norvenius SG, et al. Early introduction of fish decreases the risk of eczema in infants. Arch Dis Child. 2009;94:11–5. doi: 10.1136/adc.2008.140418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hesselmar B, Saalman R, Rudin A, Adlerberth I, Wold A. Early fish introduction is associated with less eczema, but not sensitization, in infants. Acta Paediatr. 2010;99:1861–7. doi: 10.1111/j.1651-2227.2010.01939.x. [DOI] [PubMed] [Google Scholar]
- 27.Kull I, Bergström A, Lilja G, Pershagen G, Wickman M. Fish consumption during the first year of life and development of allergic diseases during childhood. Allergy. 2006;61:1009–15. doi: 10.1111/j.1398-9995.2006.01115.x. [DOI] [PubMed] [Google Scholar]
- 28.Takemura Y, Sakurai Y, Honjo S, Tokimatsu A, Gibo M, Hara T, et al. The relationship between fish intake and the prevalence of asthma: the Tokorozawa childhood asthma and pollinosis study. Prev Med. 2002;34:221–5. doi: 10.1006/pmed.2001.0978. [DOI] [PubMed] [Google Scholar]
- 29.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–80. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Dunder T, Kuikka L, Turtinen J, Rasanen L, Uhari M. Diet, serum fatty acids, and atopic diseases in childhood. Allergy. 2001;56:425–8. doi: 10.1034/j.1398-9995.2001.056005425.x. [DOI] [PubMed] [Google Scholar]


