Abstract
Iron deficiency anemia (IDA) is the most common form of anemia worldwide. Although oral iron is used as first-line treatment, many patients are unresponsive to or cannot take oral iron. This Phase III, open-label, non-inferiority study compared the efficacy and safety of ferumoxytol, a rapid, injectable intravenous (IV) iron product with low immunological reactivity and minimal detectable free iron, with IV iron sucrose in adults with IDA of any cause. Patients (N = 605) were randomized 2:1 to receive ferumoxytol (n = 406, two doses of 510 mg 5 ± 3 days apart) or iron sucrose (n = 199, five doses of 200 mg on five nonconsecutive days over 14 days) and followed for 5 weeks. Ferumoxytol demonstrated noninferiority to iron sucrose at the primary endpoint, the proportion of patients achieving a hemoglobin increase of ≥2 g dL−1 at any time from Baseline to Week 5 (ferumoxytol, 84.0% [n = 406] vs. iron sucrose, 81.4% [n = 199]), with a noninferiority margin of 15%. Ferumoxytol was superior to iron sucrose (2.7 g dL−1 vs. 2.4 g dL−1) in the mean change in hemoglobin from Baseline to Week 5 (the alternative preplanned primary endpoint) with P = 0.0124. Transferrin saturation, quality-of-life measures, and safety outcomes were similar between the two treatment groups. Overall, ferumoxytol demonstrated comparable safety and efficacy to iron sucrose, suggesting that ferumoxytol may be a useful treatment option for patients with IDA in whom oral iron was unsatisfactory or could not be used. Am. J. Hematol. 89:646–650, 2014. © 2014 Wiley Periodicals, Inc.
Introduction
Worldwide, anemia has a prevalence of ∼25% 1. In the United States (US), iron deficiency anemia (IDA) continues to be one of the most common types of anemia, affecting ∼1–2% of men and 2–5% of women 2. IDA is associated with a range of adverse health and quality-of-life (QOL)-related issues including impaired cognitive development and performance, reduced work capacity, lower resistance to infection, increased morbidity and mortality related to childbearing, restricted infant and child growth, and impaired endocrine function 3. There are many causes of IDA including digestive diseases and blood loss from the gastrointestinal (GI) tract, which are common causes in adult men and postmenopausal women, and excessive menstrual loss, which is the most common cause in women of childbearing age 4–7. Because of the clinical impact of anemia, patients with IDA require prompt and effective iron replacement treatment to increase iron stores and raise hemoglobin (Hgb) levels to improve or maintain their QOL 6,8,9.
Although oral iron is the first-line treatment for patients with IDA, many patients are unable to tolerate, may not respond to, or cannot absorb oral iron 10,11. In these patients, an alternative to oral iron is required to effectively manage and treat IDA 12. Prior to the recent approval of ferric carboxymaltose in the US, the only approved intravenous (IV) iron formulations for the treatment of IDA in patients without chronic kidney disease (CKD), that is, IDA of any underlying cause, were the iron dextrans 13. The iron dextran products require the administration of a test dose and, per their US Food and Drug Administration (FDA)-approved prescribing information, are limited to low individual doses (100 mg), therefore requiring multiple doses to administer a typical full 1-g treatment course 14. However, larger doses may often be administered as infusions over a number of hours; for example, the UK labeling allows for infusion of up to 20 mg iron/kg body weight infused over 4–6 hr 15. The test dose is required by the FDA only for the first dose, whereas the UK Medicines and Healthcare Products Regulatory Agency (MHRA) requires a slower rate of administration for the first 25 mg of iron dextran for every dose 16, stating that “the first 25 mg of iron should be infused over a period of 15 minutes, the patient must be kept under close medical observation during this period” 15.
For these reasons, some health care providers may be reluctant to use the IV iron dextrans to treat patients with IDA who cannot tolerate oral iron 17. Thus, there remains a need for additional safe and effective therapies for these patients in the US.
Ferumoxytol (Feraheme®, AMAG Pharmaceuticals Inc., Waltham, MA) was approved in June 2009 by the FDA for the treatment of IDA in adult patients with CKD 18. Ferumoxytol is a colloidal superparamagnetic iron oxide coated with a semi-synthetic carbohydrate specifically designed to minimize immunological reactivity 19,20. Ferumoxytol can be injected rapidly IV at doses of 510 mg with no test dose, and therefore a full treatment course (1.02 g) can be administered with only two clinic visits.
There are few randomized, controlled clinical trials that have directly compared IV iron products in patients with IDA of any underlying cause 21–23. Recently, two Phase III trials have been completed that evaluated ferumoxytol for the treatment of IDA of any cause in patients with a history of unsatisfactory oral iron therapy or in whom oral iron could not be used, and a supplemental new drug application has been submitted to the FDA. The first study compared ferumoxytol with placebo 24. Here, we present the results of the second trial (http://ClinicalTrials.gov identifier: NCT01114204), which compared the efficacy and safety of ferumoxytol with that of iron sucrose for the treatment of IDA in patients with a history of unsatisfactory oral iron therapy or in whom oral iron could not be used. These data demonstrate that ferumoxytol has safety and efficacy that was comparable to iron sucrose, suggesting that ferumoxytol may be a useful treatment option for these patients with IDA.
Methods
Study design and patient eligibility
This open-label, active-controlled, multicenter, global, Phase III study was designed to demonstrate noninferiority and was conducted in accordance with the ethical principles of Good Clinical Practice and in compliance with the Declaration of Helsinki. The study protocol was reviewed and approved by the institutional review boards or ethics committees at each study site. All patients provided written informed consent prior to study entry.
The study enrolled male and female patients ≥18 years of age, with Baseline Hgb >7 to <10 g dL−1, transferrin saturation (TSAT) <20%, and history of unsatisfactory oral iron therapy or intolerance to oral iron. Serum ferritin was not utilized as an entry criterion because, although indicative of iron deficiency if very low (e.g., <30 ng mL−1), it is an acute-phase reactant and may be artifactually elevated in the face of iron deficiency in patients with concurrent inflammation (such as those with cancer, heart failure, or autoimmune diseases). Patients were excluded if they had an estimated glomerular filtration rate <30 mL min−1; serum ferritin >600 ng mL−1; history of allergy to IV iron or two or more classes of drugs; females who were pregnant, planning to become pregnant, or who were breastfeeding; recent parenteral or oral iron therapy; a cause of anemia other than iron deficiency; recent or planned blood transfusions; recent or anticipated erythropoiesis-stimulating agent therapy initiation, disruption, or dose change >20%; recent major surgery or invasive intervention within 4 weeks prior to screening; or recent initiation or change in therapy to control bleeding.
Treatment plan
The study consisted of a 14-day screening period, a treatment period of 5 days for ferumoxytol or 14 days for iron sucrose, and a 5-week follow-up period. Patients were randomized 2:1 to receive either ferumoxytol, administered as two injections of 510 mg given over 30–60 seconds 5 days apart, or iron sucrose, administered as five infusions or injections of 200 mg on five nonconsecutive days over a 14-day period. A test dose of a 20-mg injection or 25-mg infusion of iron sucrose was given to iron sucrose-naïve patients prior to their first dose, in compliance with the labeling requirements for some of the countries where the study was conducted. No pretreatment was given prior to the study drug, and the study drug was administered at least 1 hr before chemotherapy.
Iron sucrose (Venofer®, American Regent, Shirley, NY) was chosen as the comparator in this study because, although it is approved in the US for IDA in patients with CKD only 25, it is approved outside of the US for IDA of any cause. In addition, unlike the IV iron dextrans that are approved in the US for this indication, iron sucrose does not have a black box safety warning. At the time of the study, ferric carboxymaltose was not approved for this indication in the US.
Study endpoints
Efficacy endpoints. The primary efficacy endpoint of this study was the proportion of patients achieving a ≥2-g dL−1 increase in Hgb at any time from Baseline to Week 5. Based on requests from different health authorities, a prespecified alternate primary endpoint analysis (the mean change in Hgb from Baseline to Week 5) was also performed. Secondary efficacy endpoints included the proportion of patients achieving an Hgb level ≥12 g dL−1 at any time from Baseline to Week 5; time to an Hgb increase of ≥2 g dL−1 or to Hgb ≥12 g dL−1 from Baseline to Week 5; and the mean change in TSAT from Baseline to Week 5. In addition, the mean change in the patient-reported outcome (PRO) for the Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-Fatigue) 26 scale score from Baseline to Week 5 was also assessed. Furthermore, two additional prespecified exploratory PRO endpoints were included: the mean change from Baseline to Week 5 in the energy domain of the QOL Linear Analogue Scale Assessment (LASA-Energy) and the Vitality domain of the 36-Item Short-Form General Health Survey 27 (SF-36-Vitality) scores.
Subgroup analyses
The proportion of patients achieving a ≥2-g dL−1 increase in Hgb at any time from baseline to Week 5 and the mean change in Hgb from Baseline to Week 5 were also analyzed in five predefined subgroups based on the patients' primary underlying cause of their IDA, as attributed by the investigators (i.e., patients with abnormal uterine bleeding (AUB), cancer, GI disorders, postpartum anemia, or other conditions [e.g., patients with nutritional deficiency, heart failure, and rheumatoid arthritis]).
Safety endpoints
Safety endpoints, assessed throughout treatment and at the 5-week follow-up period, included: the incidence of treatment-emergent adverse events (TEAEs), serious adverse events (SAEs), severe adverse events (AEs), any AEs leading to withdrawal of study treatment, AEs leading to study withdrawal, and AEs leading to death. In addition, two composite endpoints were assessed, which were agreed upon a priori with the regulatory agency: AEs of special interest (predefined as moderate-to-severe hypotension occurring on the day of dosing and moderate-to-severe hypersensitivity reactions occurring within 48-hr post dose), and a composite cardiovascular endpoint (predefined as nonfatal myocardial infarction, heart failure, moderate-to-severe hypertension, and hospitalization due to any cardiovascular event). Safety assessments also included vital signs, physical examination, and laboratory parameters.
Statistical analyses
Efficacy analysis. For the primary efficacy endpoint, a sample size of 600 subjects (400 exposed to ferumoxytol and 200 exposed to iron sucrose) was calculated to provide 94% power for the assessment of the noninferiority of ferumoxytol and iron sucrose, assuming a two-sided alpha of 0.05, an efficacy rate of 60%, and a noninferiority margin of 15% for testing the difference between treatment groups.
The efficacy analyses were performed on the intent-to-treat (ITT) population (all randomized patients who had any exposure to the study drug [ferumoxytol or iron sucrose]). The point estimate and 95% confidence interval (CI) for the treatment difference of categorical endpoints are presented using the large sample size assumption. Noninferiority was first tested and then concluded if the lower bound of the 95% CI was greater than or equal to the predefined noninferiority margin (−0.15), and superiority if the lower bound was >0. Baseline Hgb for each subject was the Day 1 Hgb value (prior to injection of study drug); the screening Hgb value or most recent Hgb value prior to Day 1 was used for any patients missing a Day 1 Hgb value. Patients with no Hgb values reported post-Baseline were conservatively classified as not having achieved a ≥2-g dL−1 increase in Hgb.
For secondary efficacy endpoints for treatment differences with continuous endpoints, the P value and 95% CIs were obtained using an analysis of covariance model, adjusting for Baseline Hgb and primary underlying condition. For treatment differences with continuous endpoints, the treatment difference, P value, and 95% CIs were obtained using an analysis of covariance model, adjusted for Baseline Hgb and primary underlying condition.
Mean change from Baseline to Week 5 in Hgb, TSAT, and FACIT-Fatigue score was presented by treatment group, and the point estimates, 95% CIs, and P value for the treatment difference were calculated. Because no patients with postpartum anemia were enrolled, no analysis was performed for this subgroup.
Safety analysis
The safety population, which included all randomized subjects who received any amount of study drug, was based on actual treatment received. All AEs were reported descriptively as patient numbers and incidence percentages; no statistical comparisons were performed on the safety data.
Results
Patient characteristics
A total of 605 patients from 96 sites were enrolled in this study between August 2010 and November 2011. Patients were randomized to one of the two treatment arms (ferumoxytol, n = 406; iron sucrose, n = 199) (Supporting Information, Fig. 1).
Baseline demographics were similar between the two treatment groups (Supporting Information, Table 1). The mean ± standard deviation age of the overall study population (N = 605) was 48.2 ± 14.81 years, and the majority of patients were female (n = 502; 83.0%) and predominantly white (n = 510; 84.3%). Minor differences in age, sex, and race between the ferumoxytol and iron sucrose treatment groups were not clinically meaningful.
Approximately two-thirds of patients (66.8%) entered the study in the high Baseline Hgb subgroup (>8.5 to <10 g dL−1), and the remainder entered the study in the low Baseline Hgb subgroup (>7 to ≤8.5 g dL−1). Baseline values for TSAT and serum ferritin were 5.9% ± 10.1% and 24.5 ± 78.1 ng mL−1, respectively.
Efficacy
Primary efficacy endpoints
Ferumoxytol was shown to be non-inferior to iron sucrose. Eighty-four percent of ferumoxytol-treated patients had an Hgb increase of ≥2 g dL−1 at any time from Baseline to Week 5 compared with 81.4% of patients treated with IV iron sucrose in the ITT population (noninferiority margin: −15%; Fig. 1).
Figure 1.
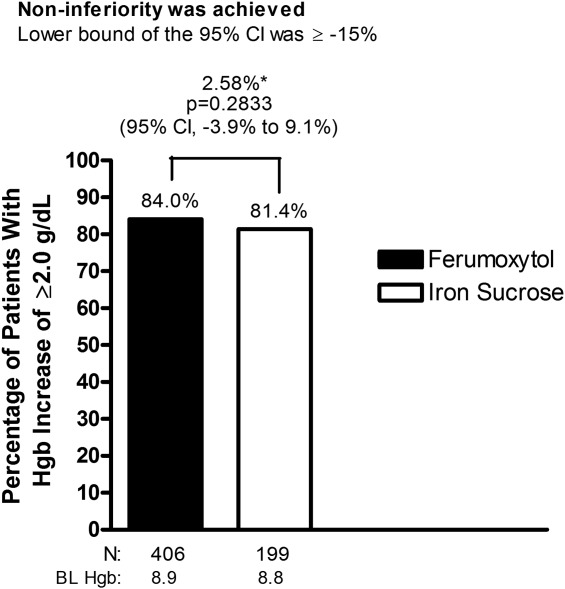
Proportion of patients with ≥2-g dL−1 increase in Hgb at any time from Baseline to Week 5 (intent-to-treat population). *Treatment difference. BL, Baseline; CI, confidence interval; Hgb, hemoglobin.
Secondary efficacy endpoints
At each time point examined (Weeks 3, 4, and 5), ferumoxytol-treated patients had a greater increase in Hgb values following treatment compared with those treated with iron sucrose, leading to a consistently higher mean Hgb level at each time point ( Fig. 2).
Figure 2.
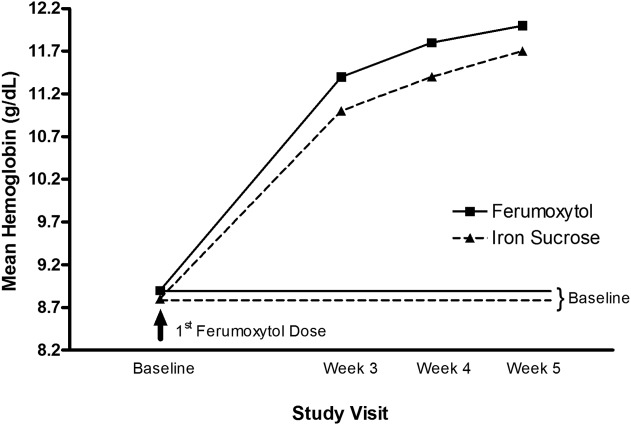
Hemoglobin values over time in patients receiving ferumoxytol or iron sucrose for up to 5 weeks (intent-to-treat population).
Ferumoxytol was shown to be superior to iron sucrose in the mean increase in Hgb from Baseline to Week 5 (the alternate, preplanned primary efficacy endpoint), with a treatment difference of 0.3 g dL−1 (ferumoxytol, 2.7 vs. iron sucrose, 2.4 g dL−1; P = 0.0124) ( Fig. 3).
Figure 3.
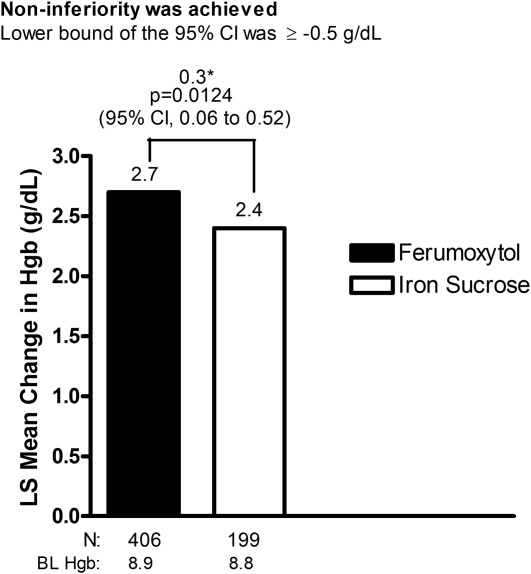
LS mean change in Hgb from Baseline to Week 5 (intent-to-treat population). *Treatment difference. BL, baseline; CI, confidence interval; Hgb, hemoglobin; LS, least-squares.
Ferumoxytol demonstrated noninferiority to iron sucrose in the proportion of patients achieving an Hgb level ≥12 g dL−1 at any time from Baseline up to Week 5. A total of 66.7% of ferumoxytol-treated patients achieved an Hgb level ≥12 g dL−1 at any time from Baseline to Week 5 compared with only 48.2% of iron sucrose-treated patients (P < 0.0001).
Ferumoxytol demonstrated a significantly shorter median time to an Hgb increase of ≥2 g dL−1 or to an Hgb level of ≥12 g dL−1 from Baseline than iron sucrose (16 days vs. 22 days, respectively; P < 0.0001) ( Fig. 2).
Ferumoxytol-treated patients showed a statistically significant (P = 0.0048) greater increase in TSAT from Baseline to Week 5 (14.5%) compared with the increase in iron sucrose-treated patients (10.6%).
Patient-reported endpoints
FACIT-Fatigue scores at Baseline were comparable for both IV iron treatment groups (ferumoxytol [27.8 ± 11.19] and iron sucrose [28.7 ± 10.78]. At Week 5, significant improvements in FACIT-Fatigue scores from Baseline were demonstrated for both ferumoxytol (41.7 ± 10.05) and iron sucrose (41.5 ± 9.51). In addition, both the ferumoxytol and iron sucrose groups showed significant increases in SF-36-Vitality scores (13.0 vs. 12.5, respectively; P > 0.05) and LASA-Energy scores (21.9 vs. 21.3; P > 0.05) from Baseline to Week 5.
Effect of underlying condition on Hgb increase
In the AUB, GI, and Other subgroups, ferumoxytol achieved noninferiority for the proportion of patients with a >2.0-g dL−1 increase in Hgb and for the mean change in Hgb at any time from Baseline to Week 5.
The proportion of ferumoxytol-treated patients in the cancer subgroup with a >2.0-g dL−1 Hgb increase at Week 5 was also higher (54.8%) compared with iron sucrose-treated patients (38.5%), although ferumoxytol did not meet the criteria for noninferiority, probably due to the small number of patients in this subgroup (n = 31; 7.6%). For the same reason, the mean improvement in Hgb in the cancer subgroup with ferumoxytol was comparable to that of iron sucrose (+1.9 g dL−1 for both), but did not meet the noninferiority criteria. There were no patients with postpartum anemia enrolled in the study.
Safety
Overall, ferumoxytol was well tolerated and had a safety profile comparable to that of iron sucrose. TEAEs (ferumoxytol, 41.4%; iron sucrose, 44.2%) and drug-related TEAEs (ferumoxytol, 14.3%; iron sucrose, 16.1%) were reported at similar rates in the two treatment groups (Table 1).
TABLE 1.
Summary of AEs During the Study
| Ferumoxytol (n = 406) | Iron sucrose (n = 199) | Total (N = 605) | ||||
|---|---|---|---|---|---|---|
| AE category | Events, n | Patients, n (%) | Events, n | Patients, n (%) | Events, n | Patients, n (%) |
| All TEAEs | 360 | 168 (41.4) | 224 | 88 (44.2) | 584 | 256 (42.3) |
| Treatment-related AEsa | 115 | 58 (14.3) | 73 | 32 (16.1) | 188 | 90 (14.9) |
| SAEs | 24 | 17 (4.2) | 6 | 5 (2.5) | 30 | 22 (3.6) |
| Related SAEs | 5 | 2 (0.5) | 0 | 0 (0.0) | 5 | 2 (0.3) |
| Protocol-defined AEs of special interestb | 15 | 11 (2.7) | 12 | 10 (5.0) | 27 | 21 (3.5) |
| Composite cardiovascular AE endpointc | 5 | 4 (1.0) | 4 | 2 (1.0) | 9 | 6 (1.0) |
| TEAEs resulting in temporary discontinuation of study medication | 1 | 1 (0.2) | 1 | 1 (0.5) | 2 | 2 (0.3) |
| TEAEs resulting in permanent discontinuation of study medication | 11 | 6 (1.5) | 8 | 5 (2.5) | 19 | 11 (1.8) |
| TEAEs resulting in study discontinuation | 3 | 3 (0.7) | 2 | 2 (1.0) | 5 | 5 (0.8) |
| Death | 1 | 1 (0.2) | 0 | 0 (0.0) | 1 | 1 (0.2) |
AEs, adverse events; SAEs, serious adverse events; TEAEs, treatment-emergent adverse events.
Treatment-related AEs were those classified by the investigator as related to the study drug.
AEs of special interest include hypotension and hypersensitivity.
Composite cardiovascular AE endpoint included nonfatal myocardial infarction, heart failure, moderate-to-severe hypertension, and hospitalization due to any cardiovascular cause.
Note: Patients were counted once within the same system organ class or preferred term; percentages are based on the number of patients in each treatment group.
SAEs were observed at a slightly higher rate in the ferumoxytol treatment group compared with the iron sucrose treatment group (4.2% vs. 2.5%, respectively). Except for uterine hemorrhage, which occurred in two ferumoxytol-treated patients (0.5%) and was not considered to be related to study treatment, all other SAEs in both treatment groups were individual events that occurred in single patients. There was no clustering of SAEs noted to suggest a specific safety signal. Two ferumoxytol-treated patients (0.5%) had SAEs deemed by the investigators to be related to study drug, including anaphylactic reaction in one patient and one event each of hypertension, angioedema, urticaria, and tachycardia in one patient.
There was one patient death reported in the ferumoxytol group that was considered by the investigator to be unrelated to treatment in a subject with a pancreatic tumor causing duodenal obstruction who died postoperatively.
AEs of special interest
Patients in both treatment groups experienced protocol-defined AEs of special interest. Iron sucrose-treated patients had a higher incidence (5.0%) of protocol-defined AEs of special interest compared with ferumoxytol-treated patients (2.7%). The incidence of Composite Cardiovascular Adverse Event Endpoint AEs (nonfatal myocardial infarction, heart failure, moderate-to-severe hypertension, and hospitalization due to any cardiovascular cause) was comparable between the two treatment groups. Overall, no trends or unexpected safety events were identified in this study between the two IV iron treatment groups.
Discussion
Results of this Phase III open-label study show that in the population of patients with IDA and a history of unsatisfactory oral iron therapy or in whom oral iron could not be used, ferumoxytol, delivered as two IV doses of 510 mg, provided a clinically meaningful and statistically significant increase in Hgb from baseline, a statistically significantly greater increase in Hgb levels compared with iron sucrose, and met the predefined criteria for noninferiority to iron sucrose. In the AUB, GI, and Other subgroups, ferumoxytol similarly demonstrated noninferiority to iron sucrose for the proportion of patients with a ≥2.0-g dL−1 increase in Hgb at any time from Baseline to Week 5 and in the mean change in Hgb from Baseline to Week 5. The proportion of ferumoxytol-treated patients in the Cancer subgroup with a >2.0-g dL−1 Hgb increase at Week 5 was also higher than iron sucrose-treated patients, but did not meet the noninferiority margin criteria; this was likely due to the relatively low number of patients with cancer enrolled in this study reflecting their higher priority to participate in therapeutic trials of anticancer agents.
The clinical benefit of ferumoxytol treatment in terms of increasing Hgb was further supported by the consistent, positive results from multiple PRO QOL instruments. Ferumoxytol was shown to improve scores from Baseline on the FACIT-Fatigue, LASA-Energy, and SF-36-Vitality with improvements in fatigue, energy, and vitality that were similar to those seen with iron sucrose. The PRO improvements from Baseline to Week 5 were clinically meaningful and exceeded the minimal important difference (MID) previously reported for these measures 28,29. For the LASA-Energy, the MID was previously estimated as 9.61 29. For the SF-36-Vitality, a difference of 5.0 points has been identified as the MID, and a decrease of 5–10 points has been correlated with increased risk of negative outcomes 30,31.
Ferumoxytol was also shown to be well tolerated when administered to patients with IDA. No new or unexpected safety signals were observed in patients treated with ferumoxytol. The majority of AEs were similar between the ferumoxytol and iron sucrose treatment groups. The frequency and types of AEs with ferumoxytol were consistent with those observed in the postmarketing experience with ferumoxytol in patients with IDA and CKD and in previous ferumoxytol IDA studies 11,32–34.
In addition to its potential efficacy and tolerability benefits, ferumoxytol can be administered as a rapid IV injection, does not require a test dose prior to administration, and requires fewer administrations to deliver a full 1-g treatment course than most of the other IV irons available in the US 35. Unlike other IV irons, which generally require multiple office visits (five or more), ferumoxytol can be administered as two doses of 510 mg via IV injection in under 1 min, and therefore has the potential to improve treatment compliance 11,36.
Limitations of this study include the open-label, nonblinded design, which is a potential source of bias; however, because the study had no placebo arm and two active treatment arms, bias is likely to be minimal. The study was not powered to detect significant differences between treatments in the predefined subgroups. The dose regimens administered in the two treatment groups were different, which could lead to differences in efficacy and tolerability, but the regimens reflect how these agents are used in clinical practice based on their labels.
In conclusion, the results of this randomized, active-controlled, multicenter study suggest that the therapeutic usefulness of ferumoxytol may extend beyond its currently approved indication (i.e., the treatment of IDA in adult patients with CKD) to a broader population of IDA patients with a history of unsatisfactory oral iron therapy or in whom oral iron cannot be used 11,32,33,37. In this study, ferumoxytol administered as two IV doses of 510 mg each was shown to be well tolerated and effective in treating IDA. Ferumoxytol may, therefore, provide an important new treatment option for patients with IDA and a history of unsatisfactory oral iron therapy or in whom oral iron could not be used.
Acknowledgments
Maria McGill, RPh, CMPP, and Mary Hines of inScience Communications, Springer Healthcare provided medical writing support funded by AMAG Pharmaceuticals, Inc.
Author Contributions
D.H. contributed patients; performed the clinical trial; discussed the outline; wrote, edited, and proofread the manuscript; and agreed upon the data presented.
W.S. designed and oversaw the execution of the trial; analyzed the data; wrote, edited, and proofread the manuscript; and agreed upon the data presented.
K.B. designed and oversaw the execution of the trial; analyzed the data; wrote, edited and proofread the manuscript; and agreed upon the data presented.
Z.L. designed and oversaw the execution of the trial; analyzed the data; performed statistical analysis; wrote, edited, and proofread the manuscript; and agreed upon the data presented.
A.U. contributed patients; performed the clinical trial; discussed the outline; wrote, edited, and proofread the manuscript; and agreed upon the data presented.
L.F.A. designed and oversaw the execution of the trial; analyzed the data; wrote, edited, and proofread the manuscript; and agreed upon the data presented.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
References
- 1.World Health Organization. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia. Geneva: World Health Organization; 2008. [Google Scholar]
- 2.Looker AC, Dallman PR, Carroll MD, et al. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- 3.Smith RE., Jr The clinical and economic burden of anemia. Am J Manag Care. 2010;16:S59–S66. [PubMed] [Google Scholar]
- 4.Croker JR, Beynon G. Gastro-intestinal bleeding—A major cause of iron deficiency in the elderly. Age Ageing. 1981;10:40–43. doi: 10.1093/ageing/10.1.40. [DOI] [PubMed] [Google Scholar]
- 5.Niv E, Elis A, Zissin R, et al. Iron deficiency anemia in patients without gastrointestinal symptoms—A prospective study. Fam Pract. 2005;22:58–61. doi: 10.1093/fampra/cmh705. [DOI] [PubMed] [Google Scholar]
- 6.Bermejo F, Garcia-Lopez S. A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J Gastroenterol. 2009;15:4638–4643. doi: 10.3748/wjg.15.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayraktar UD, Bayraktar S. Treatment of iron deficiency anemia associated with gastrointestinal tract diseases. World J Gastroenterol. 2010;16:2720–2725. doi: 10.3748/wjg.v16.i22.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells CW, Lewis S, Barton JR, Corbett S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm Bowel Dis. 2006;12:123–130. doi: 10.1097/01.MIB.0000196646.64615.db. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Iron deficiency anaemia: Assessment, prevention, and control: A guide for programme managers. Geneva, Switzerland: World Health Organization; 2001. p. 114. [Google Scholar]
- 10.de Silva AD, Tsironi E, Feakins RM, Rampton DS. Efficacy and tolerability of oral iron therapy in inflammatory bowel disease: A prospective, comparative trial. Aliment Pharmacol Ther. 2005;22:1097–1105. doi: 10.1111/j.1365-2036.2005.02700.x. [DOI] [PubMed] [Google Scholar]
- 11.Provenzano R, Schiller B, Rao M, et al. Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:386–393. doi: 10.2215/CJN.02840608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vifor. 2013. Venofer solution for injection or concentrate for solution for infusion: Summary of product characteristics http://www.medicines.org.uk/emc/medicine/24168/SPC/Venofer+(iron+sucrose)/
- 13.Barton JC, Barton EH, Bertoli LF, et al. Intravenous iron dextran therapy in patients with iron deficiency and normal renal function who failed to respond to or did not tolerate oral iron supplementation. Am J Med. 2000;109:27–32. doi: 10.1016/s0002-9343(00)00396-x. [DOI] [PubMed] [Google Scholar]
- 14.INFed®. Morristown, NY: Watson Pharma Inc; 2009. (iron dextran injection USP): US Prescribing Information. [Google Scholar]
- 15.Electronic Medicines Compendium (eMC) 2014. Cosmofer. Summary of Product Characteristics. Available at: http://www.medicines.org.uk/emc/medicine/14139/SPC/CosmoFer. (accessed 21 Jan )
- 16.Medicines and Healthcare Products Regulatory Agency. 2013;7(1) Intravenous iron and serious hypersensitivity reactions: New strengthened recommendations to manage and minimise risk. Drug Safety Update vol, issue August : A1. Available at: http://www.mhra.gov.uk/Safetyinformation/DrugSafetyUpdate/CON300398. (accessed 21 Jan 2014) [Google Scholar]
- 17.Auerbach M, Rodgers GM. Intravenous iron. N Engl J Med. 2007;357:93–94. doi: 10.1056/NEJMc070203. [DOI] [PubMed] [Google Scholar]
- 18.Feraheme (ferumoxytol) Injection for Intravenous (IV) Use: US Prescribing Information. Lexington, MA: AMAG Pharmaceuticals Inc; 2009. [Google Scholar]
- 19.Rosner MH, Auerbach M. Ferumoxytol for the treatment of iron deficiency. Expert Rev Hematol. 2011;4:399–406. doi: 10.1586/ehm.11.31. [DOI] [PubMed] [Google Scholar]
- 20.Pai AB, Garba AO. Ferumoxytol: A silver lining in the treatment of anemia of chronic kidney disease or another dark cloud? J Blood Med. 2012;3:77–85. doi: 10.2147/JBM.S29204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evstatiev R, Marteau P, Iqbal T, et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141:846–853. doi: 10.1053/j.gastro.2011.06.005. e841–e842. [DOI] [PubMed] [Google Scholar]
- 22.Bisbe E, Garcia-Erce JA, Diez-Lobo AI, Munoz M. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesthesia. 2011;107:477–478. doi: 10.1093/bja/aer242. [DOI] [PubMed] [Google Scholar]
- 23.Onken JE, Bregman DB, Harrington RA, et al. A multicenter, randomized, active-controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion. 2014;54:306–315. doi: 10.1111/trf.12289. [DOI] [PubMed] [Google Scholar]
- 24.Vadhan-Raj S, Strauss W, Ford D, et al. Efficacy and safety of IV ferumoxytol for adults with iron deficiency anemia previously unresponsive to or unable to tolerate oral iron. Am J Hematol. 2014;89:7–12. doi: 10.1002/ajh.23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venofer (iron sucrose injection, USP) US Prescribing Information. Shirley, NY: American Regent Inc; 2011. [Google Scholar]
- 26.Smith E, Lai JS, Cella D. Building a measure of fatigue: The functional assessment of chronic illness therapy fatigue scale. Pm R. 2010;2:359–363. doi: 10.1016/j.pmrj.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 28.Cella D, Eton DT, Lai JS, et al. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 29.Patrick DL, Gagnon DD, Zagari MJ, et al. Assessing the clinical significance of health-related quality of life (HrQOL) improvements in anaemic cancer patients receiving epoetin alfa. Eur J Cancer. 2003;39:335–345. doi: 10.1016/s0959-8049(02)00628-7. [DOI] [PubMed] [Google Scholar]
- 30.Bjorner JB, Wallenstein GV, Martin MC, et al. Interpreting score differences in the SF-36 Vitality scale: Using clinical conditions and functional outcomes to define the minimally important difference. Curr Med Res Opin. 2007;23:731–739. doi: 10.1185/030079907x178757. [DOI] [PubMed] [Google Scholar]
- 31.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 32.Singh A, Patel T, Hertel J, et al. Safety of ferumoxytol in patients with anemia and CKD. Am J Kidney Dis. 2008;52:907–915. doi: 10.1053/j.ajkd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Spinowitz BS, Kausz AT, Baptista J, et al. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol. 2008;19:1599–1605. doi: 10.1681/ASN.2007101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwenk MH. Ferumoxytol: A new intravenous iron preparation for the treatment of iron deficiency anemia in patients with chronic kidney disease. Pharmacotherapy. 2010;30:70–79. doi: 10.1592/phco.30.1.70. [DOI] [PubMed] [Google Scholar]
- 35.McCormack PL. Ferumoxytol: In iron deficiency anaemia in adults with chronic kidney disease. Drugs. 2012;72:2013–2022. doi: 10.2165/11209880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.Rawal A, Katsulis P, Stutz L, et al. Presented at the National Kidney Foundation Spring Clinical Meeting. Lake Buena Vista, FL: 2013. Comparison of intravenous iron therapy for the treatment of anemia in CKD. April 2–6. [Google Scholar]
- 37.Spinowitz BS, Schwenk MH, Jacobs PM, et al. The safety and efficacy of ferumoxytol therapy in anemic chronic kidney disease patients. Kidney Int. 2005;68:1801–1807. doi: 10.1111/j.1523-1755.2005.00598.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


