Abstract
The purpose of the current study was to investigate the effect of the recently synthesized mitochondrially-targeted H2S donor, AP39 [10-oxo-10-(4-(3-thioxo-3H-1,2-dithiol-5yl)phenoxy)decyl) triphenylphosphonium bromide], on bioenergetics, viability, and mitochondrial DNA integrity in bEnd.3 murine microvascular endothelial cells in vitro, under normal conditions, and during oxidative stress. Intracellular H2S was assessed by the fluorescent dye 7-azido-4-methylcoumarin. For the measurement of bioenergetic function, the XF24 Extracellular Flux Analyzer was used. Cell viability was estimated by the combination of the MTT and LDH methods. Oxidative protein modifications were measured by the Oxyblot method. Reactive oxygen species production was monitored by the MitoSOX method. Mitochondrial and nuclear DNA integrity were assayed by the Long Amplicon PCR method. Oxidative stress was induced by addition of glucose oxidase. AP39 (30 – 300 nM) to bEnd.3 cells increased intracellular H2S levels, with a preferential response in the mitochondrial regions. AP39 exerted a concentration-dependent effect on mitochondrial activity, which consisted of a stimulation of mitochondrial electron transport and cellular bioenergetic function at lower concentrations (30–100 nM) and an inhibitory effect at the higher concentration of 300 nM. Under oxidative stress conditions induced by glucose oxidase, an increase in oxidative protein modification and an enhancement in MitoSOX oxidation was noted, coupled with an inhibition of cellular bioenergetic function and a reduction in cell viability. AP39 pretreatment attenuated these responses. Glucose oxidase induced a preferential damage to the mitochondrial DNA; AP39 (100 nM) pretreatment protected against it. In conclusion, the current paper documents antioxidant and cytoprotective effects of AP39 under oxidative stress conditions, including a protection against oxidative mitochondrial DNA damage.
Keywords: mitochondria, bioenergetics, DNA repair, oxidative stress, cytoprotection
Introduction
Emerging mitochondrial roles of hydrogen sulfide (H2S) include antioxidant effects, modulation of mitochondrial cell death pathways and the regulation of cellular bioenergetics reviewed in [1–4]. With respect to antioxidant/cell death modulating responses, multiple studies have demonstrated that H2S donors can maintain mitochondrial integrity, reduce the release of mitochondrial death signals and attenuate mitochondrially-regulated cell death responses of various types [5–13]. With respect to the regulatory role of H2S on cellular bioenergetic responses, recent data show that H2S, in lower concentrations, serves as a physiological electron donor and as an inorganic source of energy in mammalian cells; via these pathways, H2S acts as an alternative supporter of mitochondrial electron transport and ATP generation [4,14–17]. However, the mitochondrial effects of H2S typically follow bell-shaped curves, whereby elevation of H2S concentrations beyond a certain concentration range becomes cytotoxic, genotoxic, pro-oxidant, and suppresses mitochondrial electron transport (reviewed in [17]). Closely related to the concentration-dependency of biological H2S responses, the rate of H2S generation is also critical: fast-releasing and slow-releasing H2S donors can affect different biochemical pathways and can exert different, even opposing, cellular responses [18,19].
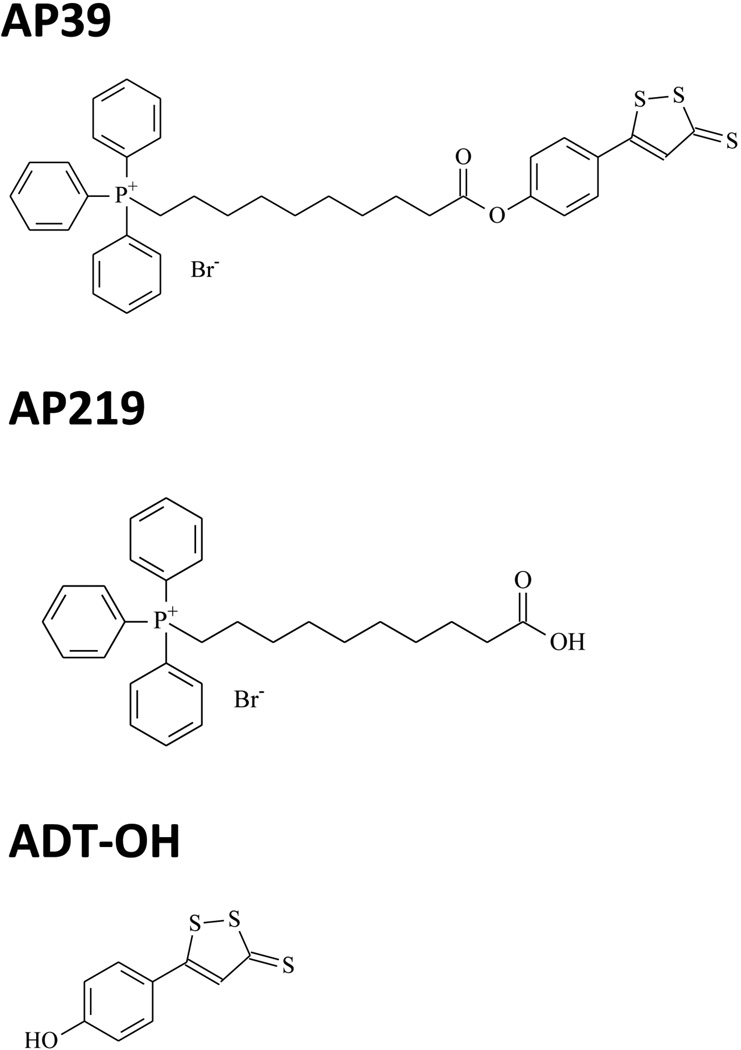
The goal of the current study was to characterize the H2S donor AP39. AP39 consists of a mitochondria-targeting motif, triphenylphosphonium (TPP+), coupled to a H2S-donating moiety (dithiolethione) by an aliphatic linker (Fig. 1). The purpose of the synthesis of this structure is to target the H2S to the mitochondria, by exploiting the well-known tendency of TPP+ to accumulate in mitochondria [20]. We have also compared the effects of AP39 with those of AP219, a structurally related molecule, which does not have the H2S donating moiety, as well as with ADT-OH (5-phydroxyphenyl-1,2-dithione-3-thione), the H2S donor moiety that is incorporated into the AP39 structure (Fig. 1). The effect of AP39 in endothelial cells was studied both under basal conditions and under conditions of oxidative stress. In addition to detecting overall cell viability, we have also assessed changes in mitochondrial electron transport/cellular bioenergetics and in mitochondrial DNA damage.
Fig. 1. Chemical structures of AP39 and the two control molecules AP219 and ADT-OH.
AP219 is an AP39-like scaffold that does not have the H2S donor group and is the predicted product of AP39, if the compound undergoes hydrolysis by intracellular esterases. ADT-OH is the H2S donor moiety used in AP39, without the mitochondrially targeted TPP+ group.
Material and Methods
Materials
AP39, ADT-OH and AP219 were synthesized in-house as described [21–23]. Antimycin A, 7-azido-4-methylcoumarin (AzMC), carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), 2-deoxyglucose, oligomycin and rotenone were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
The murine brain microvascular endothelial cell line, bEnd.3 (ATTC #CRL-2299, Manassas, VA) was maintained in DMEM containing 1g/l glucose supplemented with 10% fetal bovine serum, 1% non-essential amino acids, 100 IU/ml penicillin, and 100 µg/ml streptomycin at 37 °C in 10% CO2.
Detection and cellular localization of H2S
40,000 of bEnd.3 cells were seeded in Lab-Tek II chamber coverglass system (Nalgen Nunc International) and incubated at 37°C and 10% CO2 humidified incubator overnight. The H2S-sensitive fluorescent dye 7-azido-4-methylcoumarin (AzMC) [24] was incorporated in a cell-based assay [25] to detect H2S production. The cells were loaded with AzMC and MitoTracker Red CMXRos (Invitrogen M7512) fluorogenic dyes at 10 µM and 50 nM final concentrations, respectively, for 30 min. Various concentrations of AP39, or the control compounds AP219 or ADT-OH, were added with fresh media and cells were further incubated for 1h. In another set of the experiments, cells were pretreated with the mitochondrial uncoupling agent FCCP (0.5 µM) for 1h; then AP39 was added and H2S imaging was performed. Cells were washed three times with PBS and the specific fluorescence of the various dyes was visualized using a Nikon eclipse 80i inverted microscope with a Photometric CoolSNAP HQ2 camera and the NIS-Elements BR 3.10 software.
Bioenergetic analysis in cultured cells
The XF24 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA) was used to measure bioenergetic function, as originally described [26,27], and as employed by us in prior studies [13,16]. The measurement of oxygen consumption rate (OCR) after oligomycin (1.5 µg/ml) was used to assess ATP production rate and the measurement of OCR after FCCP (0.5 µM) was used to assess maximal mitochondrial respiratory capacity. 2-deoxyglucose (100 mM) was used to estimate cellular glycolytic dependency and antimycin A (2 µg/ml) and rotenone (2 µM) were used to inhibit the flux of electrons through complex III and I, to detect residual non-mitochondrial oxygen consumption rate, which is considered to be due to cytosolic oxidase enzymes. Simultaneously with the OCR measurements, extracellular acidification rate (ECAR), an index of glycolytic function, was also measured.
MTT assay
The MTT method was performed as described [28]. Briefly, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) was added to the cells at a final concentration of 0.5 mg/ml and cells were cultured at 37 °C for 1 hour. The cells were washed with PBS and the formazan dye was dissolved in DMSO. The amount of converted formazan dye was measured at 570 nm with a background measurement at 690 nm on a Powerwave reader (Biotek). The method detects Complex II-dependent mitochondrial activity, and is often used to estimate mitochondrial function and/or cell viability.
LDH assay
Lactate dehydrogenase (LDH) release was determined as a cytotoxicity assay, a secondary measurement for determination of cell death, as described [28]. Briefly, 30 µl of supernatant was saved before addition of MTT and mixed with 100 µl freshly prepared LDH assay reagent containing 85 mM lactic acid, 1 mM nicotinamide adenine dinucleotide (NAD+), 0.27 mM N-methylphenazonium methyl sulfate (PMS), 0.528 mM 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium chloride (INT), and 200 mM Tris (pH 8.2). The changes in absorbance were read kinetically at 492 nm for 15 min (kinetic LDH assay) on a monochromator-based reader (Powerwave HT, Biotek) at 37 °C. LDH activity values are shown as Vmax for kinetic assays in mOD/min.
Measurement of mitochondrial and nuclear DNA integrity
To measure DNA integrity (damage) we used gene-specific semi-quantitative PCR assays using LongAmp Taq DNA Polymerase (New England BioLabs, Ipswich, MA) as described [29]. Briefly, total DNA was isolated using DNase Blood and Tissue Kit (QIAGEN, Hilden, Germany). Damage to nuclear DNA was estimated by quantification of the PCR amplification of the 9kb nuclear-specific DNA fragment using PicoGreen fluorescent dye to detect double-stranded DNA (Quant-iT™ PicoGreen, Molecular Probe). Damage to the mitochondrial DNA was estimated by quantification of the PCR amplification of the 10kb mitochondrial-specific DNA fragment using PicoGreen staining. Obtained data was normalized by the PCR amplification of 117bp mitochondrial genome-specific fragment for correction of the multiple copies of the mitochondrial genome. Preliminary assays were carried out to ensure the linearity of PCR amplification with respect to the number of cycles and DNA concentration.
Measurement of protein oxidation
Protein oxidation was measured in whole extracts and mitochondrial preparations as described [28]. Cells were lysed using buffer containing 150 mM NaCl, 50mM Tris-HCl, pH 8.0 and 1% NP-40 supplemented with protease inhibitors (Complete mini, Roche Applied Science, Indianapolis, IN). Cells were lysed on ice for 30 min followed by centrifugation 20,000 × g, 15 min at 4 °C. Protein concentration in supernatant was measured by DC protein assay (Bio-Rad, Hercules, CA). Carbonyl groups of the proteins, as a result of their reaction with oxygen free radicals reaction, is considered a hallmark of protein oxidation status. Therefore, the level of carbonyl groups was quantified using the Oxyblot Protein Oxidation detection kit according to the manufacturer's instructions (Millipore, Billerica, MA). Briefly, 2.5 µg of the total or mitochondrial proteins were denatured with 6% SDS (final concentration), followed by derivatization of exposed carbonyl groups with 2,4-dinitrophenylhydrazine (DNPH) to form 2,4-dinitrophenylhydrazone (DNP-hydrazones) by adding 10 µl of 1 × DNPH solution. After incubation at 37°C for 15 min the reaction was stopped by addition of 7.5 µl of the Neutralization solution. The DNP-derivatized samples were transferred to a nitrocellulose membrane using MINIFOL Slot Blot System (Whatman) followed by Western blot analysis using primary antibody specific to DNP-moiety of the oxidatively modified proteins. Preliminary assays were carried out to ensure the linearity of obtained signal with respect to the protein concentration. Pierce enhanced chemiluminescent substrate (Pierce ECL, Thermo Fisher Scientific Inc., USA) was used to detect the chemiluminescent signal in a CCD-camera based detection system (GBox, Syngene USA, Frederick, MD).
Measurement of cellular oxidant production
Cellular oxidant production was measured as described [28]. 40,000 of bEnd.3 cells were seeded in the Lab-Tek II chamber coverglass system (Nalgen Nunc International) and incubated at 37°C and 10% CO2 humidified incubator overnight. The cells were loaded with 5 µM of MitoSOX Red dye (Invitrogen M36008) for 10 min. Various concentrations of AP39 were added with fresh media and cells were further incubated for 1h. Cells were then washed three times with PBS and the specific fluorescence of the dye was visualized using Nikon eclipse 80i inverted microscope with the Photometric CoolSNAP HQ2 camera and the NIS-Elements BR 3.10 software.
Generation of oxidative stress and selective mitochondrial DNA damage with glucose oxidase
To generate oxidative stress, we used glucose oxidase (GOx) (Sigma-Aldrich, St. Louis, MO), which, in the presence of glucose in the cell culture medium, generates hydrogen peroxide (H2O2) [30]. The production of reactive oxygen species (ROS) from GOx was measured by CM-H2DCF DA (Invitrogen) Various concentrations of GOx were added to tissue culture medium containing 5 µM CM-H2DCF DA and fluorescence (Ex494/Em520) was measured using SpectraMax M2 (Molecular Devices) microplate reader after 1h. A ROS standard curve was constructed with H2O2. Glucose oxidase (GOx) was added to the cells at various concentrations, in the presence or absence of various concentrations of AP39 (or control compounds AP219 or ADT-OH), followed by a washout and further incubation in the presence of tissue culture medium for up to 24 hours.
Statistics
Data are shown as means ± standard error of the mean (SEM). Student’s t-test, one-way and two-way ANOVA with Tukey’s post hoc test were used to detect differences between groups; p<0.05 was considered statistically significantly different. All statistical calculations were performed using Graphpad Prism 6 analysis software.
Results
AP39 increases the abundance of mitochondrial H2S in bEnd.3 endothelial cells
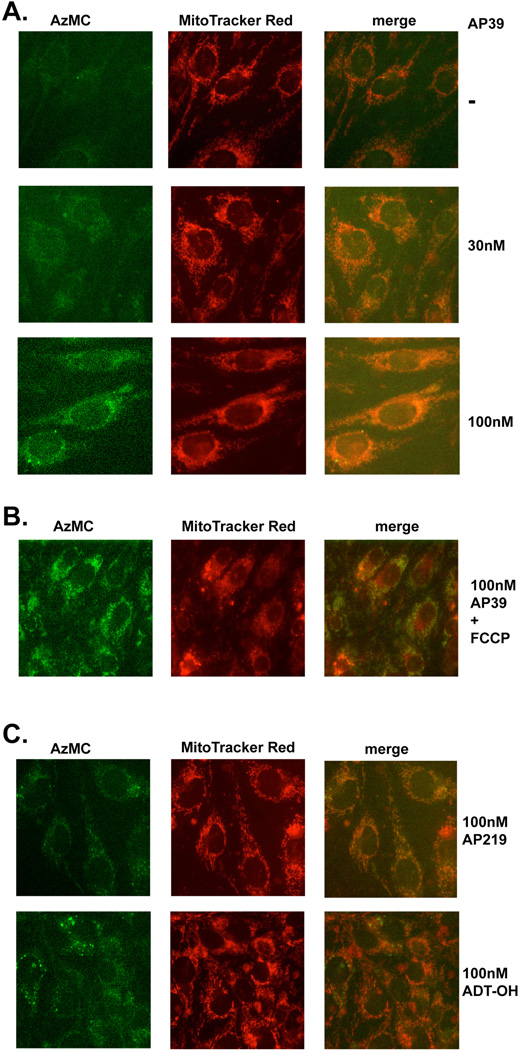
Exposure of the cells to AP39 (30–300 nM) for 1 hour resulted in a slight increase in the fluorescence of the H2S-detecting dye, 7-azido-4-methylcoumarin, with significant co-localization of the signal to mitochondria (Figure 2). Please also note that there was already a detectable baseline fluorescent signal in the cells. Consistently with the fact that CBS and CSE under physiological conditions are cytosolic, while 3-MST has both mitochondrial and cytosolic localization [10,13], the low levels of basal H2S detected did not show a mitochondrial preference (Figure 2). In the presence of the mitochondrial uncoupler FCCP, AP39 no longer showed induced a preferentially mitochondrial increase H2S fluorescence (Figure 2B), consistent with the fact [20] that the mitochondrial uptake of TPP+ is dependent on mitochondrial membrane potential. The compound AP219 (which contains the AP39 scaffold without the H2S releasing moiety) did not induce an increase in H2S fluorescence in the cells, while the H2S donor moiety ADT-OH, on its own, showed a diffuse, slight increase in the H2S fluorescence signal (Figure 2C).
Fig. 2. AP39 generates H2S mainly in mitochondria.
(A) Endothelial cells (bEnd.3) were treated with various concentration of AP39 (as indicated) for 1 hour and intracellular H2S was detected using AzMC fluorescent probe as described in Materials and Methods. Mitochondrial localization was monitored by MitoTracker Red. Note the concentration-dependent increase in H2S signal in response to AP39 treatment, which, at least in part, co-localized within the mitochondrial regions. (B) Lack of increase in mitochondrial H2S signal in response to AP39, when cells were pretreated with the mitochondrial uncoupling agent FCCP. (C) Lack of increase in H2S fluorescence with AP219 and lack of increase in mitochondrial H2S fluorescence with ADT-OH. The figures are representative of three independent experiments that were run in duplicates for each end-point.
Effect of AP39 on mitochondrial function/cellular bioenergetics under resting conditions
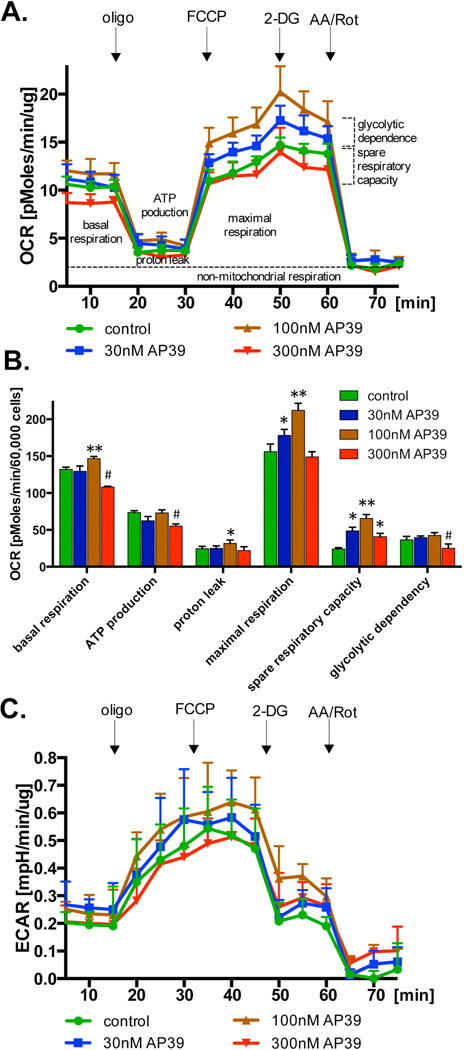
Similar to the responses previously noted with authentic H2S [4,14–17], incubation of bEnd.3 cells with AP39 initially caused an increase in basal OCR at 100 nM, while an inhibition at 300 nM (Figure 3A,B). AP39 also induced a concentration-dependent increase in FCCP-stimulated OCR at 30 and 100 nM, while at 300 nM, the response switched into an inhibitory effect (Figure 3B). The FCCP-induced increase in OCR represents the 'respiratory reserve capacity', a key bioenergetic parameter, which corresponds to a spare respiratory that is available to the cells in response to increases in energy demand. The pattern of the response to AP39 is consistent with the previously known bell-shaped pharmacological character [31] of H2S. Although the effects are fairly small, it may be noted that the increase in basal OCR due to 100 nM AP39 is higher than the sum of an increase in ATP-linked OCR and proton leak (Figure 3B), perhaps due to an additional, AP39-induced increase in non-mitochondrial oxygen consumption. AP39 failed to affect glycolytic activity, as assessed by the quantification of extracellular acidification rate (ECAR) (Figure 3C).
Fig. 3. Effect of AP39 on cellular bioenergetics in resting cells.
Endothelial cells (bEnd.3) were incubated with various concentration of AP39 for 1 hour and bioenergetics parameters were determined using Extracellular Flux Analyzer as described in the Materials and Methods. Part (A) shows representative tracings; part (B) shows calculated bioenergetic parameters. * and ** show significant enhancement of a bioenergetic parameter, compared to control (no AP39) (p<0.05 and p<0.01, respectively); # shows significant inhibition of a bioenergetic parameter, compared to control (p<0.05). The results shown in part (B) show mean±SEM values from three independent experiments with 5 replicates for each end-point.
AP39 exerts cytoprotective effects in cells subjected to oxidative stress
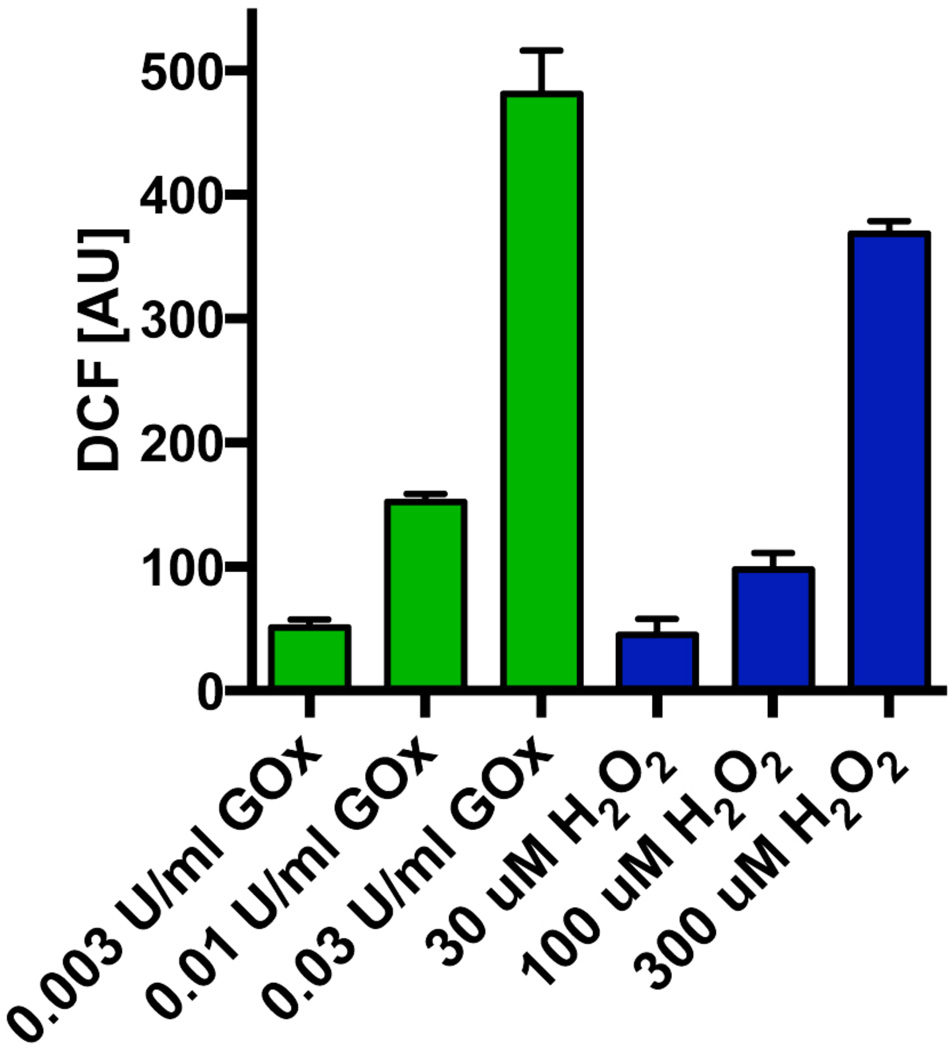
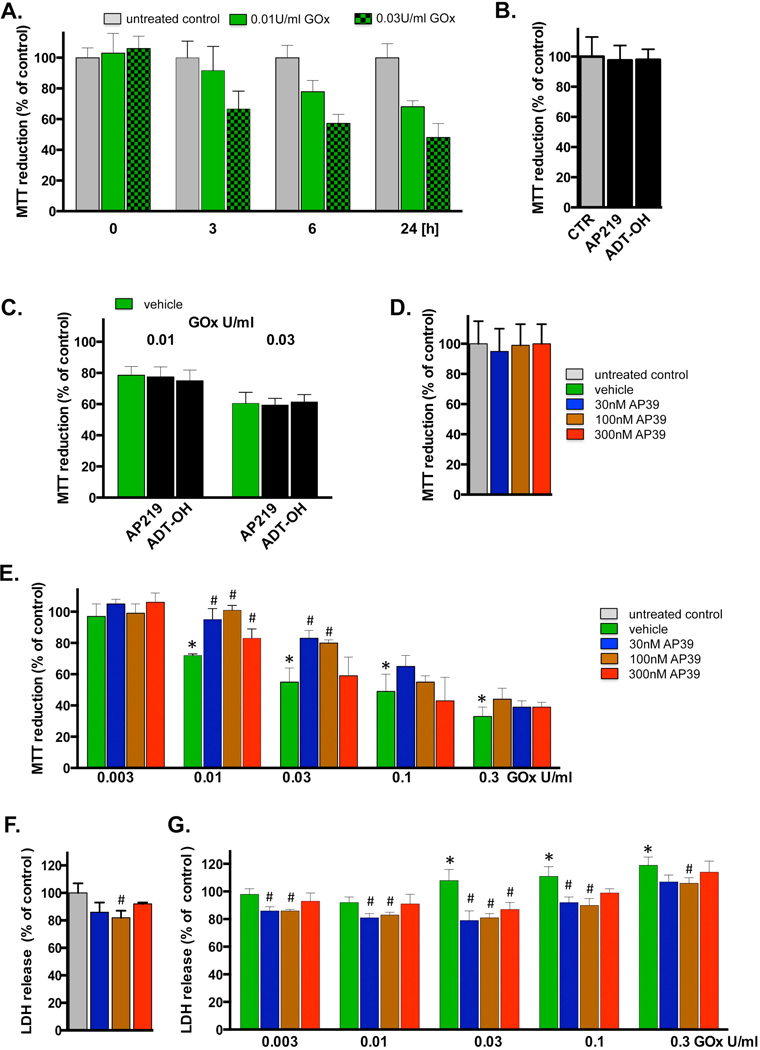
As expected, the exposure of the cells to glucose oxidase resulted in a concentration-dependent generation of ROS. As shown in Figure 4, ROS production in response to 0.03, 0.01 and 0.03 U/ml resulted in ROS fluorescence values comparable to the fluorescence induced by H2O2 concentrations of 30, 100 and 300 µM, respectively. Exposure of the cells to glucose oxidase for 1h, followed by a washout, resulted in a time-dependent decrease in mitochondrial activity/cellular viability, evidenced by suppressed MTT conversion (Figure 5A,E). At the highest glucose oxidase concentrations used there was also a statistically significant increase in the amount of LDH in the culture medium, indicating a loss of cell membrane integrity and cell necrosis (Figure 5F). Co-treatment with AP39, particularly at 100 nM, partially attenuated the cytotoxic effect of glucose oxidase treatment (Figure 5E,F). AP39 caused a slight reduction in basal LDH release in cells not exposed to glucose oxidase, perhaps due to inhibition of a low degree of on-going cell injury under baseline conditions in the cell cultures (Figure 5F). AP219 or ADT-OH, at 300 nM, failed to affect mitochondrial activity/cell viability under basal conditions or in the presence of oxidative stress (Figure 5B–D).
Fig. 4. Generation of H2O2 by glucose oxidase in culture medium.
Comparison of the increase in DCF fluorescence in response to glucose oxidase and H2O2 at 1 hour after incubation in tissue culture medium. The figure shows mean±SEM values from three independent experiments that were run in duplicates.
Fig. 5. Cytoprotective effects of AP39 in oxidatively stressed cells.
(A) Time-course of the change in MTT reduction at various time points after glucose oxidase exposure in bEnd.3 endothelial cells. Cells were exposed to glucose oxidase (0.01 or 0.03 U/ml) for 1h, followed by a washout and replacement of the medium with fresh tissue culture medium. Cells were incubated for a subsequent 23h period, followed by the measurement of MTT conversion. (B) Lack of effect of AP219 or ADT-OH (100 nM) on MTT reduction in bEnd.3 endothelial cells, as measured at 24 hours. (C) Lack of protective effect of AP219 or ADT-OH (100 nM) on the glucose oxidase-induced decrease in MTT reduction, as measured in bEnd.3 endothelial cells at 24 hours. (D) Lack of effect of AP39 (30–300 nM) on the mitochondrial conversion of MTT to formazan, an index of mitochondrial function/cell viability in bEnd.3 endothelial cells at 24 hours. Note that AP39 alone does not affect MTT conversion. (E) Effect of various concentrations of AP39 on MTT conversion in cells exposed to various concentration of oxidative stress induced by increasing concentration of glucose oxidase (GOx). Note that there is a decrease in MTT conversion in GOx treated cells; these effects are attenuated by AP39. Please also note that AP39-mediated protection was only observed at intermediate concentrations of GOx; the protective effects were no longer observed at the highest concentrations (0.1 – 0.3 U/ml) of GOx used. (F) Effect of AP39 on the breakdown of the integrity of the plasma membrane, as measured by LDH release into the extracellular medium. Please note that an intermediate concentration of AP39 attenuates basal LDH release in cells not treated with oxidants, perhaps indicative of improved viability or protection against a small degree of baseline cell dysfunction/cell death. (G) Effect of AP39 on LDH release in cells exposed to various concentration of GOx. Please note that AP39 decreased the release of LDH at intermediate concentrations of GOx; the protective effects were no longer observed at the highest concentration (0.3 U/ml) of GOx used. * p<0.05 shows a significant decrease in MTT or a significant increase in LDH in response to GOx treatment, when compared to baseline control (in the absence of GOx or AP39). # p<0.05 shows a significant enhancement of MTT or a significant reduction of LDH by AP39, when compared to its corresponding control at the same concentration of GOx, or in the absence of GOx. Results shown in parts A–G show mean±SEM values from three independent experiments with 4–8 replicates for each end-point.
AP39 attenuates the loss of cellular bioenergetics during oxidative stress
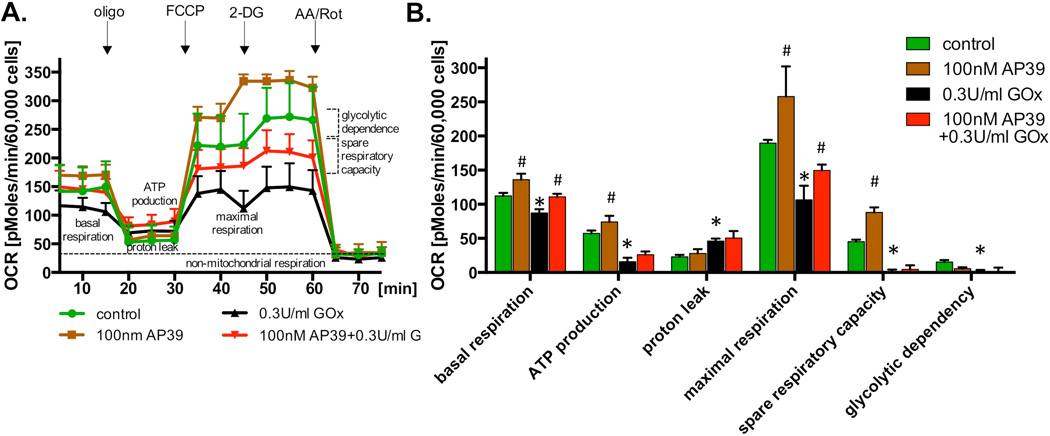
We have selected 0.3 U/ml of glucose oxidase for the next set of experiments. This concentration of glucose oxidase was found to attenuate MTT conversion, but did not yet induce significant increase in LDH release (Figure 5). Incubation of the endothelial cells with this concentration of glucose oxidase suppressed most of the cellular bioenergetic parameters. Some of these alterations (such as the decrease in basal respiratory rate and the decrease in maximal oxygen consumption), were smaller in the presence of 100nM of AP39 (Figure 6). However, it should also be noted that the recovery of OCR by AP39 treatment might be independent of the oxidative insult used, as the absolute value of an increase in OCR is comparable in the non-treated and the glucose-oxidase-treated cells.
Fig. 6. Protection by AP39 against the oxidative stress-induced loss of cellular bioenergetics.
(A) Endothelial cells (bEnd.3) were incubated with 100 nM AP39, 0.3 U/ml GOx or their combination for 1 hour and bioenergetics parameters were determined using Extracellular Flux Analysis. Various calculated bioenergetics parameters are shown in panel (B). Please note, that similarly to the results shown in Figure 3, 100 nM AP39 increased basal respiration, maximal respiration and spare respiratory capacity. The bioenergetic parameters were markedly reduced by oxidative stress. Treatment of oxidatively stressed endothelial cells with AP39 attenuated partially maintained basal and maximal respiration, but did not affect the adverse effect of GOx on the other bioenergetic parameters evaluated. * and ** shows significant enhancement of a bioenergetic parameter, compared to control (no AP39) (p<0.05 and p<0.01, respectively); # shows significant inhibition of a bioenergetic parameter, compared to control (p<0.05). The results show mean±SEM values from three independent experiments with 5 replicates for each end-point.
AP39 protects against oxidative mitochondrial DNA damage
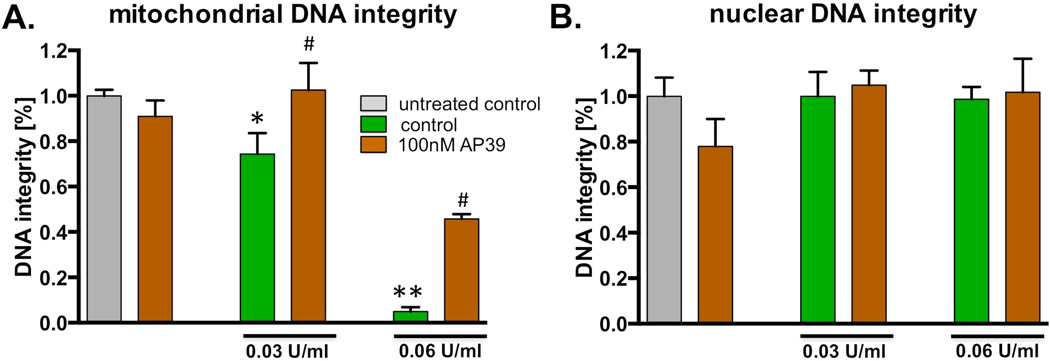
The intermediate concentrations (0.03 and 0.06 U/ml) of glucose oxidase were used for studies of mitochondrial and nuclear DNA integrity. In line with prior studies in various cell types ([32,33], the intermediate concentration of glucose oxidase preferentially caused a loss of DNA mitochondrial integrity, while no significant changes in nuclear DNA integrity were noted (Figure 7). AP39 (100 nM) exerted a protective effect against mitochondrial DNA damage by partially restoring its integrity (Figure 7).
Fig. 7. AP39 protects mitochondrial DNA from oxidatively induced damage.
The bEnd.3 cells were exposed to two different concentrations of glucose oxidase (as indicated) in the presence or absence of 100 nM AP39 for 1 hour. DNA integrity (A) mitochondrial and (B) nuclear genome was estimated using PCR of long DNA fragments. Note, that 100nM of AP39 does not decrease integrity of the mitochondrial or nuclear DNA. The concentration of GOx used in this study induces only reduction of mitochondrial DNA integrity, in dose-dependent manner. AP39 significantly protects mitochondrial DNA from GOx challenged. * and ** shows significant enhancement of a mitochondrial DNA integrity, compared to GOx treated samples (p<0.05 and p<0.01, respectively). The results show mean±SEM values from two independent experiments that were run in triplicates for each end-point.
AP39 reduces the degree of intracellular oxidative stress
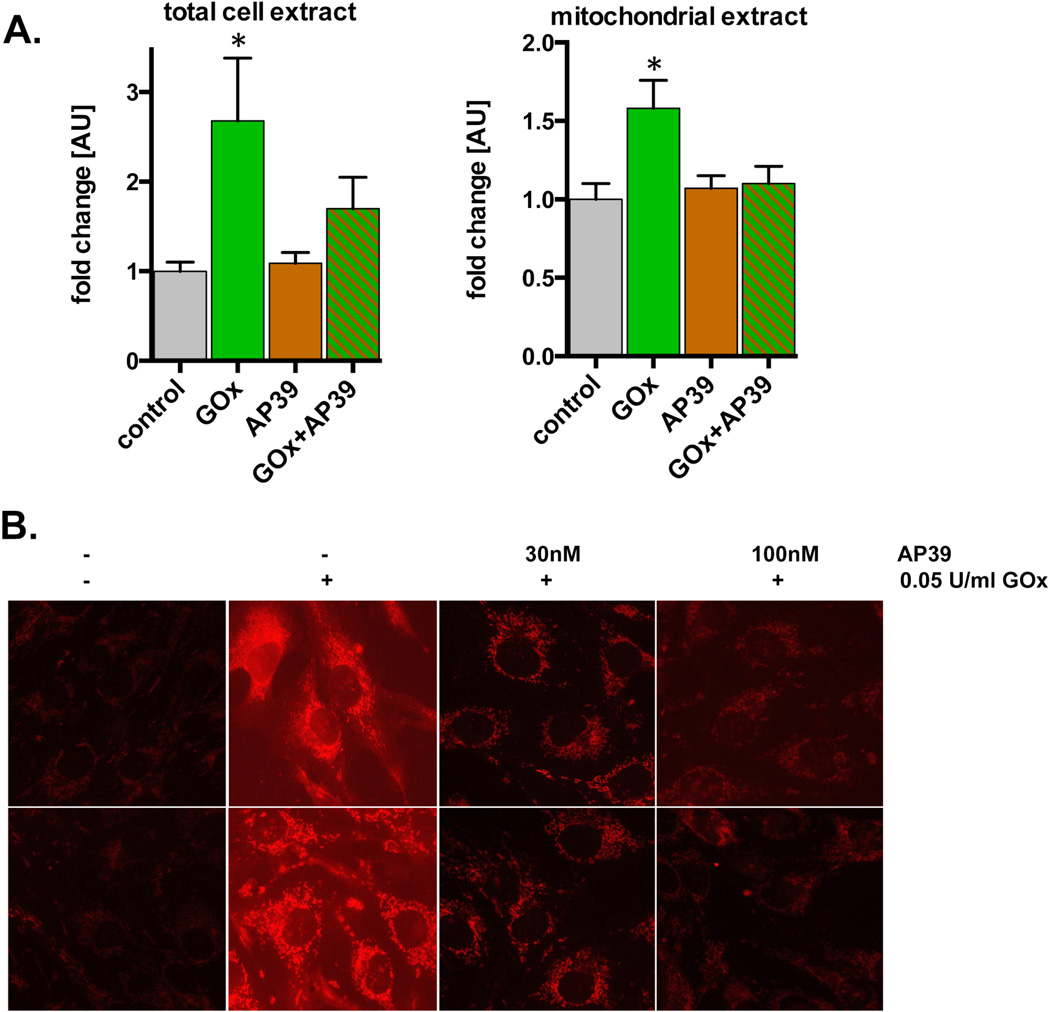
AP39 treatment of the cells resulted in a reduction in cellular and mitochondrial fluorescence in cells treated with glucose oxidase (0.03 – 0.06 U/ml), as evidenced by the measurement of oxidatively modified proteins (Oxyblot assay) of whole cell homogenates and mitochondrial extracts (Figure 8A) and by reduction of the fluorescence of the MitoSOX dye (Figure 8B).
Fig. 8. Reduction of oxidative stress by AP39 in endothelial cells exposed to glucose oxidase.
(A) The effect of AP39 on the level of oxidized carbonyl groups induced by 0.03 U/ml GOx was measured in total cell extracts or mitochondrial preparations of bEnd.3 cells (oxyblot assay). Note that there was increase in oxidative carbonylation in response to GOx, and this was attenuated in the AP39 treated samples. *shows a significant increase in protein oxidation compared to the baseline control; p<0.05. (B) The effect of AP39 is shown on the level of MitoSOX fluorescence. Note the concentration-dependent reduction of superoxide generation in AP39 treated cells. The results show mean±SEM values from two independent experiments that were run in triplicates for each end-point.
Discussion
The current results show that the recently synthesized mitochondrially-targeted H2S donor, AP39, exerts characteristic effects on mitochondrial electron transport and cellular bioenergetics. These effects tend to be stimulatory at lower concentrations (30 and 100 nM), but tend to diminish or convert into inhibitory effects at a higher concentration (300 nM). An appropriately selected, intermediate concentration of AP39 (100 nM) reduced intracellular oxidative stress and in the meantime it consequently sustained the cell viability, mitochondrial respiration and mitochondrial DNA integrity.
The effects of AP39 on mitochondrial electron transport are consistent with previous studies demonstrating that low concentrations of H2S can act as electron donors and stimulators of mitochondrial electron transport and ATP production in various cell types including colonocytes, hepatocytes, and colon cancer cells [4,13–17]. This effect has been initially attributed to a direct electron donation, whereby H2S donates electrons to the mitochondrial enzyme SQR, which, in turn, "feeds them" into the mitochondrial electron transport chain [14,17]. However, additional work has identified a secondary site of regulation, which relates to inhibition of mitochondrial phosphodiesterases and a consequent elevation of intramitochondrial cAMP levels [25]. The stimulatory effects of H2S at higher concentrations are diminished, as a secondary effect of H2S becomes more and more prominent. In many cell types, this effect has been attributed to a direct inhibition of cytochrome c oxidase (Complex IV) [13,14,31]. However, given the fact that in endothelial cells Complex IV does not exert a significant control over cellular respiration [34], additional mechanisms may also be conceivable, perhaps on other mitochondrial complexes, such as the ATP synthase. We hypothesize that, similar to authentic H2S or other H2S donors, the net balance of activating and inhibitory mitochondrial effect determines the ultimate effect of various concentrations of AP39 in the experimental conditions used in the current study.
It is interesting to note that the concentrations of AP39 (nanomolar concentration range, most significantly 100 nM) that resulted in either mitochondrial stimulation or in mitochondrial inhibition were lower than what was previously reported with H2S (low micromolar concentrations; typically 1–10 µM). We interpret this to be the result of the fact that AP39 is accumulating in the mitochondria, and, therefore, its local concentrations are likely to be substantially higher than the 'nominal' concentrations that were applied to the culture medium. We have, indeed, confirmed the selective increase of H2S after AP39 administration in our fluorescent microscopy studies (Figure 2). The selective accumulation of AP39 is likely due to the presence of its triphenylphosphonium (TPP+) group, which has been previously used to selectively target various molecules (e.g. antioxidants, mitochondrial channel modulators, cytotoxic agents, molecular probes, etc.) [35–41]. A similar approach has also been previously used to generate mitochondrially targeted nitric oxide [42,43], but, to our knowledge, AP39 is the first successful attempt for the selective mitochondrial delivery of H2S. Based on prior examples in the literature, the lipophilic triphenylphosphonium cation (as well as the biologically active 'payload' that is covalently attached to it) is accumulated 5- to 10-fold into the cytoplasm from the extracellular space by the plasma membrane potential and then further accumulated 100- to 500-fold into the mitochondrial matrix by the mitochondrial membrane potential [20]. These lipophilic cations pass directly through the lipid bilayer and do not utilize specific uptake systems [20]. The current findings are consistent with the hypothesis that the triphenylphosphonium can be useful for the mitochondrial delivery of H2S. The control experiments with the "H2S-donor-less" parent compound AP219 and with the H2S donor ADT-OH show that the parent molecule does not affect cell viability or cellular H2S fluorescence, while the "TPP-less" H2S donor ADT-OH does not accumulate in the mitochondria (Figure 2). The findings also indicate, however, that the amount of H2S to be delivered must meet a specific concentration range, in order to exert positive bioenergetic effects; exceeding this range will start to exert inhibitory effects, consistently with the bell-shaped mitochondrial concentration-response curve of H2S itself.
To induce oxidative stress, we have used glucose oxidase, which generates low, but steady-state levels of H2O2, in the culture medium. We have used this approach because we believe that such delivery of H2O2 is more relevant from a pathophysiological standpoint, than sudden 'bursts' of authentic extracellular H2O2 [44,45]. According to prior studies, low concentrations of glucose oxidase induce secondary alterations in intracellular pathways, including depletion of intracellular antioxidants, deterioration of mitochondrial membrane potential, and the impairment of mitochondrial electron transport, culminating in a secondary production of ROS from endogenous sources [46–49]. We hypothesize that in the current study H2S, released from AP39, protected against mitochondrial dysfunction and cell injury, in part by attenuating the primary attack of the H2O2 generated by extracellular glucose oxidase, and in part by 'stabilizing' the mitochondria, and protecting against the secondary, mitochondrion-dependent phase of cell injury. The reduction of mitochondrial protein oxidation (Oxyblot assay) and the reduced signal detected with the mitoSOX assay in AP39-treated cells is consistent with this hypothesis. (With respect to the MitoSOX assay, we would also like to point out that this assay, although frequently used in the literature to estimate mitochondrial oxidative stress, is not fully specific to mitochondrial ROS species. Additional measurements, for example the quantification of the superoxide-specific product, 2-hydroxy-mitoethidium would need to be conducted to strengthen the mitochondrial specificity of our conclusions.)
While AP39 (but not the inactive scaffold AP219) exerted the above-listed antioxidant and cytoprotective effects in oxidatively stressed endothelial cells, the non-targeted H2S donor ADT-OH was functionally inactive. We hypothesize that, when ADT-OH is added to the cell culture media at 300 nM, the amount of H2S that reaches the mitochondrial compartment is too low to counteract oxidative stress.
It must be noted that in the Extracellular Flux Analysis studies, AP39 already enhanced baseline bioenergetic parameters, and some of the protection in oxidant-treated cells may be simply due to the fact that the cells started out from a higher 'baseline' rate of oxygen consumption, electron transport and/or cellular bioenergetic status. Thus, some of the protection seen here may not be specific to the particular mode of oxidant stress or cellular energetic dysfunction employed in our experimental conditions.
The exact molecular mode of the protective effect of AP39 remains to be investigated in further studies. It is nevertheless interesting to note that the deleterious effects of glucose oxidase on cell viability/mitochondrial function progressed for 24 hours, although the glucose oxidase was subjected to a washout after 1h of exposure. (This may also explain why the glucose oxidase concentrations used in the Seahorse Extracellular Flux analysis, that was conducted at 1h after glucose oxidase exposure, needed to be higher than the glucose oxidase concentrations used to generate mitochondrial dysfunction/cell death, as measured by the MTT/LDH assays at 24h.) Based on prior studies, we hypothesize that the progressive onset of cell dysfunction after glucose oxidase may be, at least in part, related to an initial mitochondrial DNA injury, which is an independent actor in the development of the subsequent cell death [29,32]. We further hypothesize that the antioxidant effect of H2S donation, by protecting against this early mitochondrial DNA injury (as shown in Figure 7) is part of the reason why the cell viability is maintained at 24 hours in the cells that received AP39 co-treatment together with glucose oxidase during the initial 1h period.
Another issue that remains to be resolved is the cell-based antioxidant effect of H2S (reported here, as well as in multiple prior studies, as reviewed in [1,3,50–57]), when compared to the very slow in vitro biochemical reaction constant of H2S with all reactive oxygen species studied so far (superoxide, hydroxyl radical, peroxynitrite, etc.) [58,59]. Although these explicitly detailed reaction kinetics are useful in determining the mode of action of H2S, it must be emphasized, however, that in living cells the situation is infinitely more complex than in the studies where two species are reacted in a stopped flow chamber; in the presence of various antioxidants (enzymatic and non-enzymatic) and multiple additional reactive species (e.g. nitric oxide), several additional reactions may take place. For example, the reaction of H2S with peroxynitrite may yield NO donors [60,61], which may then exert cytoprotective effects on their own. Likewise, it is conceivable that H2S may affect (e.g. activate or upregulate) various endogenous antioxidant systems in the cell.
The protection by H2S against oxidant-mediated cytotoxicity noted in the current report is consistent with many previous reports, but not with all of them. For example, Eghbal and colleagues have previously reported in hepatocytes that low (non-toxic) concentrations of H2S enhanced the cytotoxic effect of H2O2, generated by glucose/glucose oxidase [62]. However, in the Eghbal study, a general H2S donor (as opposed to a mitochondrial-specific one) was used, which may account for the difference.
It is interesting to note that intermediate concentrations of glucose oxidase induced a marked damage to mitochondrial DNA, but not to nuclear DNA. This is in line with prior studies in various cell types [29,32], and it is also in line with the generally accepted view [63–66] that mitochondrial DNA (which does not contain histones or introns and is situated close to the mitochondrial electron transport chain, a source of ROS) is more susceptible to oxidative damage than nuclear DNA. As discussed elsewhere [63–68], the damage of mitochondrial DNA, which encodes crucial components of each of the four mitochondrial electron transport chain complexes, may lead to a loss of the local regulation of the electron transport chain, culminating in mitochondrial oxidant generation, deterioration of cellular bioenergetics, and initiation of mitochondrial cell death pathways (such as the release of cytochrome c and apoptosis-inducing factor from the mitochondria). In this context, protection by H2S against oxidative mitochondrial DNA injury may constitute a novel mode of cellular protection. The mechanism of this effect may involve direct and indirect antioxidative effects (as discussed above), but we cannot exclude that H2S may also affect the integrity or activity of various mitochondrial DNA repair proteins. This possibility remains to be explored in further experiments.
In summary, the current report demonstrates various mitochondrial effects of the mitochondrial H2S donor AP39 that are consistent with the role of H2S in the regulation of mitochondrial function under normal conditions and during oxidative stress. It is interesting to note that recent studies observed the mitochondrial translocation of H2S-producing enzymes, as a form of endogenous protective response to various insults including hypoxia [69]. In this context, AP39 may be conceptually viewed as a pharmacological tool or potential therapeutic agent that 'mimics' this type of endogenous protective mechanism.
Given the importance of oxidant-mediated endothelial mitochondrial dysfunction in cardiovascular diseases, diabetic complications, inflammatory diseases, and various forms of critical illness [29, 70–76], which are often also associated with impairments in H2S homeostasis [8, 77–85], we hypothesize that appropriately chosen, mitochondrially selective H2S donors may exert potential therapeutic effects in some pathophysiological conditions. The most appropriate conditions and the exact mode of the donor's application (especially in light of the bell-shaped pharmacological character of H2S) remain to be defined in future in vitro and in vivo studies.
Highlights.
-
-
Addition of AP39 targets H2S release to the mitochondria of endothelial cells.
-
-
AP39 exerts a bell-shaped effect on mitochondrial activity.
-
-
AP39 does not affect mitochondrial or nuclear DNA integrity under basal conditions.
-
-
Under oxidative stress conditions, AP39 exerts cytoprotective effects.
-
-
Under oxidative stress conditions, AP39 maintains mitochondrial DNA integrity.
Acknowledgement
This work has been supported by the National Institutes of Health and the American Diabetes Association to C.S and by the Medical Research Council UK to M.W. and S.L.T. M.K. is supported by the James W. McLaughlin Fellowship Fund of the University of Texas Medical Branch, Galveston.
Footnotes
Conflict of Interest Statement
MW, MEW and AP have filed for patent protection for AP39 WO2013045951 on 01/10/12.
References
- 1.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 2.Szabo C, Papapetropoulos A. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br. J. Pharmacol. 2011;164:853–865. doi: 10.1111/j.1476-5381.2010.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whiteman M, Le Trionnaire S, Chopra M, Fox B, Whatmore J. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin. Sci. (Lond) 2011;121:459–488. doi: 10.1042/CS20110267. [DOI] [PubMed] [Google Scholar]
- 4.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 5.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu LF, Lu M, Wu ZY, Wong PT, Bian JS. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol. Pharmacol. 2009;75:27–34. doi: 10.1124/mol.108.047985. [DOI] [PubMed] [Google Scholar]
- 7.Yao LL, Huang XW, Wang YG, Cao YX, Zhang CC, Zhu YC. Hydrogen sulfide protects cardiomyocytes from hypoxia/reoxygenation-induced apoptosis by preventing GSK-3beta-dependent opening of mPTP. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H1310–H1319. doi: 10.1152/ajpheart.00339.2009. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gerö D, Szoleczky P, Chang T, Zhou Z, Wu L, Wang R, Papapetropoulos A, Szabo C. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc. Natl. Acad. Sci. USA. 2011;108:13829–13834. doi: 10.1073/pnas.1105121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun WH, Liu F, Chen Y, Zhu YC. Hydrogen sulfide decreases the levels of ROS by inhibiting mitochondrial complex IV and increasing SOD activities in cardiomyocytes under ischemia/reperfusion. Biochem. Biophys. Res. Commun. 2012;421:164–169. doi: 10.1016/j.bbrc.2012.03.121. [DOI] [PubMed] [Google Scholar]
- 10.Guo W, Kan JT, Cheng ZY, Chen JF, Shen YQ, Xu J, Wu D, Zhu YZ. Hydrogen sulfide as an endogenous modulator in mitochondria and mitochondria dysfunction. Oxid. Med. Cell Longev. 2012;2012:878052. doi: 10.1155/2012/878052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen YD, Wang H, Kho SH, Rinkiko S, Sheng X, Shen HM, Zhu YZ. Hydrogen sulfide protects HUVECs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PLoS One. 2013;8:e53147. doi: 10.1371/journal.pone.0053147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Y, Yang X, Zhao S, Wei C, Yin Y, Liu T, Jiang S, Xie J, Wan X, Mao M, Wu J. Hydrogen sulfide prevents OGD/R-induced apoptosis via improving mitochondrial dysfunction and suppressing an ROS-mediated caspase-3 pathway in cortical neurons. Neurochem. Int. 2013;63:826–831. doi: 10.1016/j.neuint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Módis K, Coletta C, Erdélyi K, Papapetropoulos A, Szabo C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013;27:601–611. doi: 10.1096/fj.12-216507. [DOI] [PubMed] [Google Scholar]
- 14.Goubern M, Andriamihaja M, Nübel T, Blachier F, Bouillaud F. Sulfide, the first inorganic substrate for human cells. FASEB J. 2007;21:1699–1706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- 15.Bouillaud F, Blachier F. Mitochondria and sulfide: a very old story of poisoning, feeding, and signaling? Antioxid. Redox. Signal. 2011;15:379–391. doi: 10.1089/ars.2010.3678. [DOI] [PubMed] [Google Scholar]
- 16.Szabo C, Coletta C, Chao C, Módis K, Szczesny B, Papapetropoulos A, Hellmich MR. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA. 2013;110:12474–12479. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabo C, Ransy C, Módis K, Andriamihaja M, Murghes B, Coletta C, Olah G, Yanagi K, Bouillaud F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br. J. Pharmacol. 2013 doi: 10.1111/bph.12369. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, Moore PK. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox. Signal. 2010;12:1147–1154. doi: 10.1089/ars.2009.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiteman M, Le Trionnaire S, Chopra M, Fox B, Whatmore J. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin. Sci. (Lond) 2011;121:459–488. doi: 10.1042/CS20110267. [DOI] [PubMed] [Google Scholar]
- 20.Smith RA, Hartley RC, Murphy MP. Mitochondria-targeted small molecule therapeutics and probes. Antioxid. Redox. Signal. 2011;15:3021–3038. doi: 10.1089/ars.2011.3969. [DOI] [PubMed] [Google Scholar]
- 21.Wood ME, Whiteman M, Perry A. Hydrogen sulfide releasing compounds and their use. WO2013045951 Patent application.
- 22.Thurnhofer S, Vetter W. Synthesis of (S)-(+)-enantiomers of food-relevant (n-5)-monoenoic acid saturated anteiso-fatty acids by a Wittig reaction. Tetrahedron. 2007;63:1140–1145. [Google Scholar]
- 23.D'Araio E, Shaw N, Millward A, Demaine A, Whiteman M, Hodgkinson A A. Hydrogen sulfide induces heme oxygenase-1 in human kidney cells. Acta Diabetol. 2014;51:155–157. doi: 10.1007/s00592-013-0501-y. [DOI] [PubMed] [Google Scholar]
- 24.Thorson MK, Majtan T, Kraus JP, Barrios AM. Identification of cystathionine β-synthase inhibitors using a hydrogen sulfide selective probe. Angew. Chem. Int. Ed. Engl. 2013;52:4641–4644. doi: 10.1002/anie.201300841. [DOI] [PubMed] [Google Scholar]
- 24.Módis K, Coletta C, Asimakopoulou A, Szczesny B, Celia C, Papapetropoulos A, Hellmich MR, Szabo C. Effect of S-adenosyl-L-methionine (SAM), an allosteric activator of cystathionine-β-synthase (CBS) on colorectal cancer cell proliferation and bioenergetics in vitro. Nitric Oxide Biol. Chem. 2014 doi: 10.1016/j.niox.2014.03.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov. Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal. Chem. 2009;81:6868–6878. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerö D, Szoleczky P, Suzuki K, Módis K, Oláh G, Coletta C, Szabo C. Cell-based screening identifies paroxetine as an inhibitor of diabetic endothelial dysfunction. Diabetes. 2013;62:953–964. doi: 10.2337/db12-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szczesny B, Olah G, Walker DK, Volpi E, Rasmussen BB, Szabo C, Mitra S. Deficiency in repair of the mitochondrial genome sensitizes proliferating myoblasts to oxidative damage. PLoS One. 2013;8:e75201. doi: 10.1371/journal.pone.0075201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salazar JJ, Van Houten B. Preferential mitochondrial DNA injury caused by glucose oxidase as a steady generator of hydrogen peroxide in human fibroblasts. Mutat. Res. 1997;385:139–149. doi: 10.1016/s0921-8777(97)00047-5. [DOI] [PubMed] [Google Scholar]
- 31.Szabo C, Ransy C, Módis K, Andriamihaja M, Murghes B, Coletta C, Olah G, Yanagi K, Bouillaud F. Regulation of Mitochondrial Bioenergetic Function by Hydrogen Sulfide. Part I. Biochemical and Physiological Mechanisms. Br. J. Pharmacol. 2014 doi: 10.1111/bph.12369. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tann AW, Boldogh I, Meiss G, Qian W, Van Houten B, Mitra S, Szczesny B. Apoptosis induced by persistent single-strand breaks in mitochondrial genome: critical role of EXOG (5'-EXO/endonuclease) in their repair. J. Biol. Chem. 2011;286:31975–31983. doi: 10.1074/jbc.M110.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Módis K, Panopoulos P, Coletta C, Papapetropoulos A, Szabo C. Hydrogen sulfide-mediated stimulation of mitochondrial electron transport involves inhibition of the mitochondrial phosphodiesterase 2A, elevation of cAMP and activation of protein kinase A. Biochem Pharmacol. 2013;86:1311–1319. doi: 10.1016/j.bcp.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 34.Dranka BP, Hill BG, Darley-Usmar VM. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. 2010;48:905–914. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith RA, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc. Natl. Acad. Sci. USA. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. NY. Acad. Sci. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 37.Kelso GF, Maroz A, Cochemé HM, Logan A, Prime TA, Peskin AV, Winterbourn CC, James AM, Ross MF, Brooker S, Porteous CM, Anderson RF, Murphy MP, Smith RA. A mitochondria-targeted macrocyclic Mn(II) superoxide dismutase mimetic. Chem. Biol. 2012;19:1237–1246. doi: 10.1016/j.chembiol.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Solesio ME, Prime TA, Logan A, Murphy MP, Del Mar Arroyo-Jimenez M, Jordán J, Galindo MF. The mitochondria-targeted anti-oxidant MitoQ reduces aspects of mitochondrial fission in the 6-OHDA cell model of Parkinson's disease. Biochim. Biophys. Acta. 2013;1832:174–182. doi: 10.1016/j.bbadis.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Constant-Urban C, Charif M, Goffin E, Van Heugen JC, Elmoualij B, Chiap P, Mouithys-Mickalad A, Serteyn D, Lebrun P, Pirotte B, De Tullio P. Triphenylphosphonium salts of 1,2,4-benzothiadiazine 1,1-dioxides related to diazoxide targeting mitochondrial ATP-sensitive potassium channels. Bioorg. Med. Chem. Lett. 2013;23:5878–5881. doi: 10.1016/j.bmcl.2013.08.091. [DOI] [PubMed] [Google Scholar]
- 40.Yuan H, Cho H, Chen HH, Panagia M, Sosnovik DE, Josephson L. Fluorescent and radiolabeled triphenylphosphonium probes for imaging mitochondria. Chem. Commun. (Camb) 2013;49:10361–10363. doi: 10.1039/c3cc45802d. [DOI] [PubMed] [Google Scholar]
- 41.Millard M, Gallagher JD, Olenyuk BZ, Neamati N. A selective mitochondrialtargeted chlorambucil with remarkable cytotoxicity in breast and pancreatic cancers. J. Med. Chem. 2013;56:9170–9179. doi: 10.1021/jm4012438. [DOI] [PubMed] [Google Scholar]
- 42.Kumar S, Sitasawad SL. N-acetylcysteine prevents glucose/glucose oxidase-induced oxidative stress, mitochondrial damage and apoptosis in H9c2 cells. Life Sci. 2009;84:328–336. doi: 10.1016/j.lfs.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Horinouchi T, Nakagawa H, Suzuki T, Fukuhara K, Miyata N. A novel mitochondria-localizing nitrobenzene derivative as a donor for photo-uncaging of nitric oxide. Bioorg. Med. Chem. Lett. 2011;21:2000–2002. doi: 10.1016/j.bmcl.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 44.Sobotta MC, Barata AG, Schmidt U, Mueller S, Millonig G, Dick TP. Exposing cells to H2O2: a quantitative comparison between continuous low-dose and one-time high-dose treatments. Free Radic. Biol. Med. 2013;60:325–335. doi: 10.1016/j.freeradbiomed.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 45.Marinho HS, Cyrne L, Cadenas E, Antunes F. H2O2 delivery to cells: steady-state versus bolus addition. Methods. Enzymol. 2013;526:159–173. doi: 10.1016/B978-0-12-405883-5.00010-7. [DOI] [PubMed] [Google Scholar]
- 46.Lee JC, Son YO, Choi KC, Jang YS. Hydrogen peroxide induces apoptosis of BJAB cells due to formation of hydroxyl radicals via intracellular iron-mediated Fenton chemistry in glucose oxidase-mediated oxidative stress. Mol. Cells. 2006;22:21–29. [PubMed] [Google Scholar]
- 47.Belikova NA, Jiang J, Stoyanovsky DA, Glumac A, Bayir H, Greenberger JS, Kagan VE. Mitochondria-targeted (2-hydroxyamino-vinyl)-triphenyl-phosphonium releases NO· and protects mouse embryonic cells against irradiation-induced apoptosis. FEBS Lett. 2009;583:1945–1950. doi: 10.1016/j.febslet.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaczara P, Sarna T, Burke JM. Dynamics of H2O2 availability to ARPE-19 cultures in models of oxidative stress. Free Radic. Biol. Med. 2010;48:1064–1070. doi: 10.1016/j.freeradbiomed.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baek JY, Han SH, Sung SH, Lee HE, Kim YM, Noh YH, Bae SH, Rhee SG, Chang TS. Sulfiredoxin protein is critical for redox balance and survival of cells exposed to low steady-state levels of H2O2 . J. Biol. Chem. 2012;287:81–89. doi: 10.1074/jbc.M111.316711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whiteman M, Moore PK. Hydrogen sulfide and the vasculature: a novel vasculoprotective entity and regulator of nitric oxide bioavailability? J. Cell Mol. Med. 2009;13:488–507. doi: 10.1111/j.1582-4934.2009.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calvert JW, Coetzee WA, Lefer DJ. Novel insights into hydrogen sulfide--mediated cytoprotection. Antioxid, Redox Signal. 2010;12:1203–1217. doi: 10.1089/ars.2009.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu LF, Lu M, Hon Wong PT, Bian JS. Hydrogen sulfide: neurophysiology and neuropathology. Antioxid. Redox. Signal. 2011;15:405–419. doi: 10.1089/ars.2010.3517. [DOI] [PubMed] [Google Scholar]
- 53.Noda M, Fujita K, Lee CH, Yoshioka T T. The principle and the potential approach to ROS-dependent cytotoxicity by non-pharmaceutical therapies: optimal use of medical gases with antioxidant properties. Curr. Pharm. Des. 2011;17:2253–2263. doi: 10.2174/138161211797052600. [DOI] [PubMed] [Google Scholar]
- 54.Predmore BL, Lefer DJ, Gojon G. Hydrogen sulfide in biochemistry and medicine. Antioxid, Redox. Signal. 2012;17:119–140. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martelli A, Testai L, Marino A, Breschi MC, Da Settimo F, Calderone V. Hydrogen sulphide: biopharmacological roles in the cardiovascular system and pharmaceutical perspectives. Curr. Med. Chem. 2012;19:3325–3336. doi: 10.2174/092986712801215928. [DOI] [PubMed] [Google Scholar]
- 56.Fujita K, Yamafuji M, Nakabeppu Y, Noda M. Therapeutic approach to neurodegenerative diseases by medical gases: focusing on redox signaling and related antioxidant enzymes. Oxid. Med. Cell. Longev. 2012;2012:324256. doi: 10.1155/2012/324256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carballal S, Trujillo M, Cuevasanta E, Bartesaghi S, Möller MN, Folkes LK, García-Bereguiaín MA, Gutiérrez-Merino C, Wardman P, Denicola A, Radi R, Alvarez B. Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radic. Biol. Med. 2011;50:196–205. doi: 10.1016/j.freeradbiomed.2010.10.705. [DOI] [PubMed] [Google Scholar]
- 59.Praschberger M, Hermann M, Laggner C, Jirovetz L, Exner M, Kapiotis S, Gmeiner BM, Laggner H. Carbamoylation abrogates the antioxidant potential of hydrogen sulfide. Biochimie. 2013;95:2069–2075. doi: 10.1016/j.biochi.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 60.Filipovic MR, Miljkovic J, Allgäuer A, Chaurio R, Shubina T, Herrmann M, Ivanovic-Burmazovic I. Biochemical insight into physiological effects of H2S: reaction with peroxynitrite and formation of a new nitric oxide donor, sulfinyl nitrite. Biochem. J. 2012;441:609–621. doi: 10.1042/BJ20111389. [DOI] [PubMed] [Google Scholar]
- 61.Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem. Biophys. Res. Commun. 2006;343:303–310. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- 62.Eghbal MA, Pennefather PS, O'Brien PJ. H2S cytotoxicity mechanism involves reactive oxygen species formation and mitochondrial depolarisation. Toxicology. 2004;203:69–76. doi: 10.1016/j.tox.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 63.Hill BG, Benavides GA, Lancaster JR, Jr, Ballinger S, Dell'Italia L, Jianhua Z, Darley-Usmar VM. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hegde ML, Mantha AK, Hazra TK, Bhakat KK, Mitra S, Szczesny B. Oxidative genome damage and its repair: implications in aging and neurodegenerative diseases. Mech. Ageing Dev. 2012;133:157–168. doi: 10.1016/j.mad.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang CH, Wu SB, Wu YT, Wei YH. Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Exp. Biol. Med. (Maywood) 2013;238:450–460. doi: 10.1177/1535370213493069. [DOI] [PubMed] [Google Scholar]
- 66.Lagouge M, Larsson NG. The role of mitochondrial DNA mutations and free radicals in disease and ageing. J. Intern. Med. 2013;273:529–543. doi: 10.1111/joim.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morán M, Moreno-Lastres D, Marín-Buera L, Arenas J, Martín MA, Ugalde C. Mitochondrial respiratory chain dysfunction: implications in neurodegeneration. Free Radic. Biol. Med. 2012;53:595–609. doi: 10.1016/j.freeradbiomed.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 68.Gomez-Cabrera MC, Sanchis-Gomar F, Garcia-Valles R, Pareja-Galeano H, Gambini J, Borras C, Viña J. Mitochondria as sources and targets of damage in cellular aging. Clin. Chem. Lab. Med. 2012;50:1287–1295. doi: 10.1515/cclm-2011-0795. [DOI] [PubMed] [Google Scholar]
- 69.Teng H, Wu B, Zhao K, Yang G, Wu L, Wang R. Oxygen-sensitive mitochondrial accumulation of cystathionine β-synthase mediated by Lon protease. Proc. Natl. Acad. Sci. USA. 2013;110:12679–12684. doi: 10.1073/pnas.1308487110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blake R, Trounce IA. Mitochondrial dysfunction and complications associated with diabetes. Biochim. Biophys. Acta. 2013 doi: 10.1016/j.bbagen.2013.11.007. In press. [DOI] [PubMed] [Google Scholar]
- 71.Pangare M, Makino A. Mitochondrial function in vascular endothelial cell in diabetes. J. Smooth Muscle Res. 2012;48:1–26. doi: 10.1540/jsmr.48.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hulsmans M, Van Dooren E, Holvoet P. Mitochondrial reactive oxygen species and risk of atherosclerosis. Curr. Atheroscler. Rep. 2012;14:264–276. doi: 10.1007/s11883-012-0237-0. [DOI] [PubMed] [Google Scholar]
- 73.Huet O, Dupic L, Harrois A, Duranteau J. Oxidative stress and endothelial dysfunction during sepsis. Front. Biosci. (Landmark Ed) 2011;16:1986–1995. doi: 10.2741/3835. [DOI] [PubMed] [Google Scholar]
- 74.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox. Signal. 2010;12:537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Szabo C, Módis K. Pathophysiological roles of peroxynitrite in circulatory shock. Shock. 2010;34:s4–s14. doi: 10.1097/SHK.0b013e3181e7e9ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Víctor VM, Espulgues JV, Hernández-Mijares A, Rocha M. Oxidative stress and mitochondrial dysfunction in sepsis: a potential therapy with mitochondria-targeted antioxidants. Infect. Disord. Drug Targets. 2009;9:376–389. doi: 10.2174/187152609788922519. [DOI] [PubMed] [Google Scholar]
- 77.Wang K, Ahmad S, Cai M, Rennie J, Fujisawa T, Crispi F, Baily J, Miller MR, Cudmore M, Hadoke PW, Wang R, Gratacós E, Buhimschi IA, Buhimschi CS, Ahmed A. Dysregulation of hydrogen sulfide producing enzyme cystathionine γ-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation. 2013;127:2514–2522. doi: 10.1161/CIRCULATIONAHA.113.001631. [DOI] [PubMed] [Google Scholar]
- 78.Szabo C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox. Signal. 2012;17:68–80. doi: 10.1089/ars.2011.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mani S, Li H, Untereiner A, Wu L, Yang G, Austin RC, Dickhout JG, Lhoták Š, Meng QH, Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127:2523–2534. doi: 10.1161/CIRCULATIONAHA.113.002208. [DOI] [PubMed] [Google Scholar]
- 80.Li XH, Zhang CY, Wu JX, Zhang T. Changes in plasma hydrogen sulfide and nitric oxide levels and their clinical significance in children with Kawasaki disease. Chin. Med. J. (Engl) 2011;124:3445–3449. [PubMed] [Google Scholar]
- 81.Whiteman M, Gooding KM, Whatmore JL, Ball CI, Mawson D, Skinner K, Tooke JE, Shore AC. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010;53:1722–1726. doi: 10.1007/s00125-010-1761-5. [DOI] [PubMed] [Google Scholar]
- 82.Módis K, Asimakopoulou A, Coletta C, Papapetropoulos A, Szabo C. Oxidative stress suppresses the cellular bioenergetic effect of the 3-mercaptopyruvate sulfurtransferase/hydrogen sulfide pathway. Biochem. Biophys. Res. Commun. 2013;433:401–407. doi: 10.1016/j.bbrc.2013.02.131. [DOI] [PubMed] [Google Scholar]
- 83.Perna AF, Luciano MG, Ingrosso D, Pulzella P, Sepe I, Lanza D, Violetti E, Capasso R, Lombardi C, De Santo NG. Hydrogen sulphide-generating pathways in haemodialysis patients: a study on relevant metabolites and transcriptional regulation of genes encoding for key enzymes. Nephrol. Dial. Transplant. 2009;24:3756–3763. doi: 10.1093/ndt/gfp378. [DOI] [PubMed] [Google Scholar]
- 84.Srilatha B, Muthulakshmi P, Adaikan PG, Moore PK. Endogenous hydrogen sulfide insufficiency as a predictor of sexual dysfunction in aging rats. Aging Male. 2012;15:153–158. doi: 10.3109/13685538.2012.668722. [DOI] [PubMed] [Google Scholar]
- 85.Wang P, Zhang G, Wondimu T, Ross B, Wang R. Hydrogen sulfide and asthma. Exp. Physiol. 2011;96:847–852. doi: 10.1113/expphysiol.2011.057448. [DOI] [PubMed] [Google Scholar]