Abstract
A novel series of hybrid molecules 4a–i and 5a–i were prepared by condensation of 4-(trimethylsilylethynyl)benzaldehyde 1 with substituted o-phenylenediamines. These in turn were reacted with 2-(azidomethoxy)ethyl acetate in a Cu alkyne–azide cycloaddition (CuAAC) to generate the 1,2,3-triazole pharmacophore under microwave assistance. The newly synthesized compounds were examined for their in vitro antimicrobial activities against Gram-positive and Gram-negative bacteria and the phytopathogenic fungi Verticillium dahliae and Fusarium oxysporum f. sp. albedinis. 2-((4-(4-(5-Trifluoromethyl benzimidazol-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethanol 5e showed a moderate inhibition of 30% in the Foa sporulation test.
Keywords: Antibacterial activity, Antifungal activity, Hybrid molecules
Introduction
Infectious diseases have been a serious and growing threat to human health during the past few decades. The decrease of sensitivity to anti-microbial agents in current use has also been increasing for a great variety of pathogens and the resistance to multiple drugs is more and more prevalent for several microorganisms, especially for Gram-positive bacteria and some intractable fungi. The benzimidazole ring is an important nitrogen-containing heterocyclic compound, found in several pharmaceutical, synthetic, and natural products. Benzimidazole-based compounds can exhibit a broad range of biological activities; i.e., they are efficient structures against the human cytomegalovirus (HCMV) 1, they have also been used as antihistaminic 2, antimicrobial 3, antifungal 4, and anti-herpes (HSV-1) 5 agents.
The principal way to prepare benzimidazole derivatives is the condensation of 1,2-phenylenediamines and carbonyl compounds such as aldehydes 6–11 or acid derivatives 12–15. Quite often this reaction is run using the aldehyde route under mild oxidative conditions.
The 1,2,3-triazole core has been applied in many synthetic organic chemistry approaches. This heterocycle exhibited a broad range of efficient biological activities. Moreover, 1,2,3-triazole derivatives are shown to possess a diversity of interesting biological activities, including anti-HIV and hepatitis C 16–18, anti-allergic 19, antifungal 20–23, anti-tubercular 24,25, anti-inflammatory agents 26, and antimicrobial 27.
One of the most popular methods to prepare 1,2,3-triazoles is the cycloaddition of alkyl azides with terminal alkynes via 1,3-dipolar cycloaddition reaction – the Huisgen reaction – as a regioisomeric mixture of the 1,4- and 1,5-disubstituted ones 28. This reaction was further developed by Meldal and Sharpless. They discovered that the use of copper(I) as catalyst guarantees a high regioselectivity and excellent yield allowing exclusively the 1,4-regioisomer. The copper-catalyzed azide–alkyne cycloaddition (CuAAC) has thus attracted attention for use in a variety of applications 29–32.
Syntheses of heterocycles have seen impressive improvements in using different synthetic methods. Especially, microwave ovens as the heating source have become a very helpful procedure in their preparation. Microwave irradiation in general is a powerful tool for fast and efficient synthesis of a diversity of organic products 33.
Combining preferred heterocyclic entities to construct hybrid molecules have emerged as an interesting exploratory concept for developing new pharmaceutical compounds. They may provide scaffolds on which pharmacophores can be arranged to yield potent and hopefully selective drugs. The increase in bacterial resistance has attracted considerable interest in the discovery and development of new classes of anti-bacterial agents. The new agents should preferably have chemical characteristics that clearly differ from those of existing agents. The search for efficient antibiotics is growing for the reason that most of antibiotic agents develop resistance in clinical applications. Hybrid molecules remain an active area of research despite their extensive investigation.
Following this concept, we report the synthesis, antibacterial and antifungal evaluation of novel hybrid molecules that covalently connect 1,2,3-triazolide and benzimidazoles via a benzene connector.
Results and discussion
Chemistry
In this report, the hybrid molecules were prepared as illustrated in the schemes below. In the first step, the 4-(trimethylsilylethynyl)benzaldehyde 1 was condensed with o-phenylenediamine derivatives in the presence of Na2S2O5 34,35 in dioxane under microwave irradiation to get 2-(4-(2-(trimethylsilyl)ethynyl)phenyl)benzimidazole derivatives 2a–i in good yields (Scheme 1).
Scheme 1.
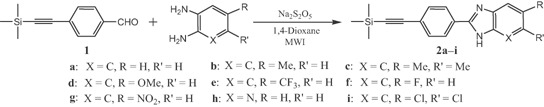
Condensation reaction to 2a–i of 4-(trimethylsilylethynyl)benzaldehyde 1 with substituted o-phenylenediamines.
In the second step, the trimethylsilyl group was removed by reacting the products 2a–i with tetrabutylammonium fluoride in tetrahydrofuran at room temperature 36,37 to obtain the terminal acetylene linked to the benzimidazole core 3a–i (Scheme 2).
Scheme 2.
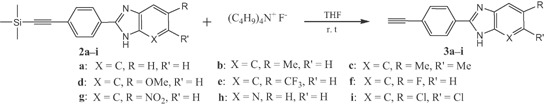
Deprotection of 2a–i to obtain the free acetylenic compounds 3a–i.
Next, the resulting compounds 3a–i with terminal acetylene and the azido substrate were condensed using the Cu alkyne–azide cycloaddition (CuAAC) and triethylamine under microwave irradiation to achieve the new ring 1-substituted 1,2,3-triazole connected via benzene to the benzimidazole nucleus 4a–i with excellent yields (Table 1, Scheme 3) and in very short reaction time. The next step is cleavage of the acetyl group using potassium carbonate (K2CO3) in methanol 38,39 in order to liberate the hydroxy group of the corresponding hybrid triazolides 5a–h. The latter were obtained in almost quantitative yields (Table 1, Scheme 3).
Table 1.
Results of 1,2,3-triazole-benzimidazole hybrids
| Entry | Product | Yielda) (%) | Product | Yielda) (%) |
|---|---|---|---|---|
| 1 | 4a | 93 | 5a | 99 |
| 2 | 4b | 82 | 5b | 98 |
| 3 | 4c | 75 | 5c | 98 |
| 4 | 4d | 86 | 5d | 99 |
| 5 | 4e | 70 | 5e | 95 |
| 6 | 4f | 80 | 5f | 98 |
| 7 | 4g | 60 | 5g | 98 |
| 8 | 4h | 65 | 5h | 95 |
| 9 | 4i | 67 | – | – |
Isolated yields.
Scheme 3.
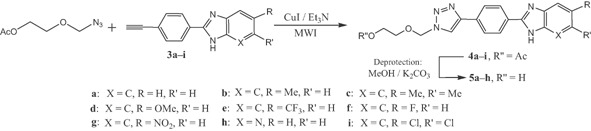
Microwave assisted Cu alkyne–azide cycloaddition to 4a–i followed by deprotection to 5a–h.
The structures of all compounds were characterized by 1H NMR, 13C NMR, mass spectrometry, and HRMS spectra. The structure of 5a was also confirmed by X-ray diffraction analysis. This structure contains three planar parts: the benzimidazole, the benzene, and the triazole ring. Furthermore, various intermolecular hydrogen bond interactions between the molecules form a three-dimensional network (the hydrogen at N1 with the nitrogens at N4 and N5 as well as the hydrogen at O2 with the nitrogen at N2). The molecular structure of single crystal 5a is shown in Fig. 1 40.
Figure 1.
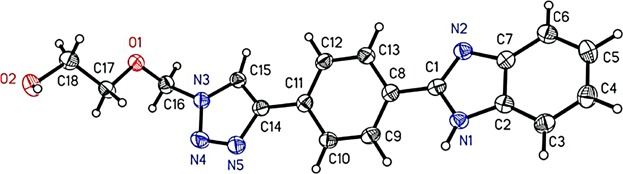
X-ray crystal structure of compound 5a; displacement ellipsoids are drawn at the 50% probability level.
Antibacterial in vitro screening of compounds 4a–i and 5a–h
All described compounds (4a–i, 5a–h) were evaluated in vitro for their antibacterial activity against the following bacterial strains: Staphylococcus aureus (ATCC 13709 in vivo, ATCC 25923, Oxford and MRSA in vivo), Enterococcus faecalis (ATCC 29212 VanS), Enterococcus faecium (Van A), Streptococcus pneumoniae (VanA, ATCC49619, PenR and Blood effect), Haemophilus influenzae (ATCC 31517 MMSA), Escherichia coli (ATCC 25922), and Pseudomonas aeruginosa (ATCC 27853), using standard techniques and the minimum inhibitory concentration values (MICs) 41. All products were dissolved in DMSO to have a concentration of 2.56 µg/mL. The MIC of synthesized compounds against Gram-negative and Gram-positive bacteria were tested using ciprofloxacin and linezolid as standard drugs for comparison. All compounds lacked antibacterial activity with MICs greater than 64 µg/mL, only 5d showed an activity against H. influenza Hi4 at 32 µg/mL.
Screening of antifungal activity in vitro
The newly synthesized compounds 5a–h were screened for antifungal activities in vitro against two phytopathogenic fungi Verticillium dahliae Kleb (VD) and Fusarium oxysporum f. sp. albedinis (Foa) by using the mycelia linear growth rate method followed by sporulation test (Table 2). The two tests were carried out as described 42,43. All compounds were tested at a concentration of 20 µg/mL (H. B. Lazrek, unpublished results). A positive control is Pelt, which is a systemic fungicide, a benzimidazole precursor (70% of methyl thiophanate). The trial was established as a completely randomized experimental design with five replicates. Data were subjected to analysis of variance using SPSS software V17.0. The mean values among treatments were compared by Duncan's multiple range tests at a 5% (p < 0.05) level to determine significant difference between the inhibition rates of various compounds at the same concentration.
Table 2.
Antifungal activities of compounds 5a–h at 20 µg/mL
| Linear growth inhibitory rates (%)a) | ||
|---|---|---|
| Compounds | VD | Foa |
| PELT | 100 ± 0.1 | 100 ± 0.14 |
| 5a | 11.29 ± 0.3 | 3.02 ± 0.96 |
| 5b | 13.76 ± 0.6 | −1.59 ± 0.05 |
| 5c | 10.64 ± 0.41 | 2.7 ± 0.16 |
| 5d | 7.21 ± 0.84 | −0.16 ± 0.02 |
| 5e | 29.76 ± 0.2 | 17.01 ± 0.96 |
| 5f | −1.69 ± 0.03 | 2.3 ± 0.29 |
| 5g | −7.72 ± 1.03 | −1.41 ± 0.3 |
| 5h | 1.56 ± 0.07 | −1.14 ± 0.05 |
Linear growth inhibitory rates, showing as mean ± standard error.
The result of the mycelia linear growth rate indicates that some of the compounds show a weak inhibition against the two fungi, the only compound that shows a significantly increased rate is compound 5e with (29.76%) (p < 0.05) against Verticillium dahliae ( Fig. 2). On the other hand, the sporulation was evaluated in the aim to know their fungicidal or fungistatic effect (Table 2, 3). The structure of this molecule uniquely holds a CF3 group fixed to the benzimidazole core. Since the trifluoromethyl group due to their lipophilicity is known to modulate absorption and metabolism, it may explain the enhanced activity.
Figure 2.
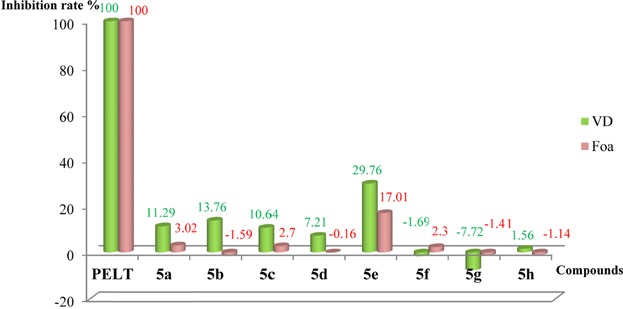
Comparison of the inhibition rate (%) of compounds 5a–h at the 8th day against VD and Foa at 20 µg/mL.
Table 3.
Title compounds and their sporulation medium
| Sporulation inhibitory rates (means %)a) | ||
|---|---|---|
| Compounds | VD | Foa |
| PELT | 100 ± 0.02 | 100 ± 0.2 |
| 5a | −1.88 ± 0.03 | −5.85 ± 0.04 |
| 5b | −18.87 ± 1.9 | 16.36 ± 0.2 |
| 5c | −2.13 ± 0.83 | −34.79 ± 0.72 |
| 5d | −14.82 ± 0.97 | 21.94 ± 0.26 |
| 5e | 22.04 ± 1.02 | 30.62 ± 0.5 |
| 5f | −0.31 ± 0.4 | −77.59 ± 2.64 |
| 5g | −12.92 ± 0.6 | −61.05 ± 1.34 |
| 5h | 5.59 ± 0.4 | −48.72 ± 2.35 |
Shown as mean ± standard error.
As shown in Table 3, benzimidazole derivatives 5b, 5d, and 5e exhibited a weak inhibition effect on the growth of Foa sporulation.
Conclusion
In summary, we have described the synthesis of a series of hybrid molecules, which combined two “privileged pharmacophore” heterocycles (benzimidazole and 1,2,3-triazole) in excellent yields using simple, efficient, and fast routes. These benzimidazole/triazoles 5a–h were tested against Gram-negative and Gram-positive bacteria and as antifungal agents, only very modest inhibition against sporulation of F. oxysporum f. sp. albedinis (Foa) was observed for some of these analogs and a modest inhibition was found for the CF3 substituted 5e.
Experimental
General methods
All starting materials for synthesis were purchased from Alfa Aesar, Fluka, or Sigma–Aldrich; thin-layer chromatography (TLC): aluminum sheets, silica gel 60 F254. Melting points were measured using a Stuart Scientific melting point (SMP1) apparatus. NMR spectra were recorded with a Bruker AC-300 MHz and AC-400 MHz in CDCl3 and DMSO-d6 with SiMe4 as an internal standard. Chemical shifts are given in ppm and the spin–spin coupling constants, J, are given in Hz (s, singlet; d, doublet; t, triplet; m, multiplet, and br, broad); Bzm = benzimidazole and Imzp = imidazo[4,5-b]pyridine.
All microwave-assisted syntheses were carried out in a dedicated CEM-Discover mono-mode microwave apparatus. ESI-mass spectrometry was performed on a Fisons instrument equipped with a VG platform II with quadrupole analyzer. High-resolution mass spectrometry (HRMS) was performed with a MALDI Orbitrap LTQ XL instrument (Thermo Fisher).
General procedure for the synthesis of compounds 4a–h
A mixture of 4-(trimethylsilylethynyl)benzaldehyde (150 mg, 0.74 mmol), 2 equivalents of the appropriate ortho-phenylenediamine and 1.01 equivalents of sodium metabisulfite were dissolved in 1 mL of dioxane and reacted under microwave irradiation for 2 min. The residue was poured in water and extracted with ethyl acetate (3 × 20 mL). The organic extract was dried over Na2SO4 and evaporated under reduced pressure. The crude products were purified by column chromatography using a methylene chloride and methanol mixture in 98:2 ratio. The benzimidazole compounds 2a–i were obtained in good yields (84–95%).
The trimethylsilyl group was cleaved by reaction of tetrabutylammonium fluoride (1 equivalent) with the compounds 2a–i in tetrahydrofuran (2.5 mL), the reaction was monitored by TLC analysis until complete consumption of the protected product (20–30 min). The resulting products 3a–i were purified by column chromatography eluting with methylene chloride/methanol (95:5). This reaction is characterized by giving excellent yields.
The terminal alkynes 3a–i, ethanol 2-(azidomethoxy)acetate (2.5 equivalents), triethylamine (1 equivalent), and copper(I) iodide (0.1 equivalents) were irradiated in a microwave oven using 300 W as power level for 2 min. The crude products 4a–i were then purified on silica gel column using methylene chloride/methanol (95:5) as eluent to afford the expected compounds.
Spectral data for selected compounds 4a–h
2-((4-(4-(Benzimidazol-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethyl acetate (4a)
Yield: 93%; Rf: 0.63; eluent: CH2Cl2/MeOH, 95:5 v/v; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.90 (s, 3H, –CH3), 3.75 (m, 4H, –CH2–), 5.78 (s, 2H, –CH2–), 7.26 (m, 2H, Ar–H), 7.64 (m, 3H, Ar–H, CH-triazole), 8.02 (d, 2H, Ar–H, J = 7.6 Hz), 8.19 (d, 2H, Ar–H, J = 7.2 Hz), 8.71 (s, 1H, NH). 13C NMR (100 MHz, CDCl3) δ (ppm): 59.71, 67.28 (CH2), 78.35 (CH2), 114.96, 122.38 (Bzm-CH), 122.94, 125.94 (phenyl-CH), 127.15 (phenyl-C), 128.79 (triazole-CH), 131.57 (Bzm-C), 146.18 (triazole-C), 151.77 (Bzm-C), 171.12 (CO). ESI-MS m/z calcd. for C20H19N5O3 (M+H): 378.40, found: 378.50.
2-((4-(4-(5-Methyl benzimidazol-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethyl acetate (4b)
Yield: 82%; Rf: 0.63; eluent: CH2Cl2/MeOH, 95:5 v/v; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.89 (s, 3H, –CH3), 2.38 (s, 3H, Ar–CH3), 3.74 (m, 4H, –CH2–), 5.77 (s, 2H, –CH2–), 7.07 (d, 2H, Ar–H, J = 8.0 Hz), 7.43 (s, 1H, CH-triazole), 7.54 (d, 1H, Ar–H, J = 7.2 Hz), 7.99 (d, 2H, Ar–H, J = 8.00 Hz), 8.17 (d, 2H, Ar–H, J = 7.6 Hz), 8.69 (s, 1H, NH). 13C NMR (100 MHz, CDCl3) δ (ppm): 20.36 (CH3), 21.08 (Bzm-CH3), 62.74, 67.33 (CH2), 78.46 (CH2), 114.17, 114.97, 122.29 (Bzm-CH), 124.44, 125.82 (phenyl-CH), 126.97 (phenyl-C), 127.16 (triazole-CH), 131.57, 132.67 (Bzm-C), 146.13 (triazole-C), 153.91 (Bzm-C), 171.09 (CO). ESI-MS m/z calcd. for C21H21N5O3 (M+H): 392.42, found: 392.40.
2-((4-(4-(5,6-Dimethyl benzimidazol-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethyl acetate (4c)
Yield: 75%; Rf: 0.64; eluent: CH2Cl2/MeOH, 95:5 v/v; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.89 (s, 3H, –CH3), 2.27 (s, 6H, Ar–CH3), 3.74 (m, 4H, –CH2–), 5.77 (s, 2H, –CH2–), 7.38 (s, 1H, CH-triazole), 7.99 (m, 3H, Ar–H), 8.13 (m, 3H, Ar–H), 8.68 (s, 1H, NH). 13C NMR (100 MHz, CDCl3) δ (ppm): 19.83 (Bzm-CH3), 20.37 (CH3), 62.70, 67.27 (CH2), 78.33 (CH2), 114.94 (Bzm-CH), 125.84, 126.71 (phenyl-CH), 129.09 (triazole-CH), 131.12 (phenyl-C), 131.61 (Bzm-C), 146.25 (triazole-C), 151.92 (Bzm-C), 171.16 (CO). ESI-MS m/z calcd. for C22H23N5O3 (M−H): 404.45, found: 404.27.
2-((4-(4-(5-Methoxy benzimidazol-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethyl acetate (4d)
Yield: 86%; Rf: 0.60; eluent: CH2Cl2/MeOH, 95:5 v/v; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.88 (s, 3H, –CH3), 3.72 (m, 4H, –CH2–), 3.74 (s, 3H, –OCH3), 5.75 (s, 2H, –CH2–), 6.83 (dd, 2H, Ar–H, J = 8.0 Hz), 7.12 (s, 1H, CH-triazole), 7.54 (d, 1H, Ar–H, J = 8.0 Hz), 7.90 (d, 2H, Ar–H, J = 8.4 Hz), 8.12 (d, 2H, Ar–H, J = 8.4 Hz), 8.61 (s, 1H, NH). 13C NMR (75 MHz, CDCl3) δ (ppm): 20.35 (CH3), 55.42 (–OCH3), 62.74, 67.28 (CH2), 78.33 (CH2), 97.22, 112.52, 116.15 (Bzm-CH), 125.76, 125.90 (phenyl-CH), 126.82 (triazole-CH), 128.67 (phenyl-C), 131.29 (Bzm-C), 146.14 (triazole-C), 156.13 (Bzm-C), 171.16 (CO). ESI-MS m/z calcd. for C21H21N5O4 (M+H): 408.42, found: 408.40.
2-((4-(4-(5-Trifluoromethyl benzimidazol-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethyl acetate (4e)
Yield: 70%; Rf: 0.50; eluent: CH2Cl2/MeOH, 95:5 v/v; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.90 (s, 3H, –CH3), 3.74 (m, 4H, –CH2–), 5.77 (s, 2H, –CH2–), 7.51 (d, 1H, Ar–H, J = 8.4 Hz), 7.76 (d, 1H, Ar–H, J = 8.4 Hz), 7.94 (s, 1H, CH-triazole), 8.01 (d, 2H, Ar–H, J = 8.4 Hz), 8.17 (m, 3H, Ar–H), 8.70 (s, 1H, NH). 13C NMR (75 MHz, CDCl3) δ (ppm): 20.35 (CH3), 62.73, 67.25 (CH2), 78.32 (CH2), 119.20, 122.40, 123.43 (Bzm-CH), 123.01 (–CF3), 125.70, 125.92 (phenyl-CH), 127.57 (triazole-CH), 128.30 (phenyl-C), 132.05 (Bzm-C), 146.05 (triazole-C), 153.45 (Bzm-C), 171.09 (CO). ESI-MS m/z calcd. for C21H18F3N5O3 (M+H): 446.39, found: 446.35.
2-((4-(4-(5-Fluoro benzimidazol-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethyl acetate (4f)
Yield : 80%; Rf: 0.50; eluent: CH2Cl2/MeOH, 95:5 v/v; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.89 (s, 3H, –CH3), 3.74 (m, 4H, –CH2–), 5.76 (s, 2H, –CH2–), 7.02 (m, 1H, Ar–H), 7.36 (d, 1H, Ar–H, J = 11.6 Hz), 7.58 (s, 1H, CH-triazole), 7.98 (d, 2H, Ar–H, J = 8.4 Hz), 8.12 (m, 3H, Ar–H, J = 8.4 Hz), 8.67 (s, 1H, NH). 13C NMR (75 MHz, CDCl3) δ (ppm): 20.34 (CH3), 62.73, 67.24 (CH2), 78.31 (CH2), 115.64, 122.34 (Bzm-CH), 125.71, 125.99 (phenyl-CH), 127.14 (triazole-CH), 128.55 (phenyl-C), 131.60 (Bzm-C), 146.11 (triazole-C), 157.22, 160.35 (Bzm-C), 171.16 (CO). ESI-MS m/z calcd. for C20H18FN5O3 (M−H): 394.39, found: 394.45.
2-((4-(4-(6-Nitrobenzimidazol-2-yl)phenyl)-1H-1,2,3-triazol-1-yl)methoxy)ethyl acetate (4g)
Yield: 60%; Rf: 0.50; eluent: CH2Cl2/MeOH, 95:5 v/v; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.90 (s, 3H, –CH3), 3.73 (m, 4H, –CH2–), 5.73 (s, 2H, –CH2–), 7.60 (d, 1H, Ar–H, J = 8.4 Hz), 7.86–7.89 (m, 2H, Ar–H, 8.2 Hz), 7.95–8.03 (m, 4H, Ar–H, J = 8.0 Hz), 8.30 (s, 1H, CH-triazole), 8.62 (s, 1H, NH). 13C NMR (75 MHz, CDCl3) δ (ppm): 20.38 (CH3), 62.75, 67.29 (CH2), 78.39 (CH2), 104.51, 114.13, 118.22 (Bzm-CH), 125.82, 127.52 (phenyl-CH), 128.63 (triazole-CH), 132.25, 133.15 (phenyl-C), 145.99, 142.71 (Bzm-C), 150.68 (triazole-C), 155.25 (Bzm-C), 171.12 (CO). ESI-MS m/z calcd. for C20H18N6O5 (M−H): 421.39, found: 421.44.
2-((4-(4-(Imidazo[4,5-b]pyridin-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethyl acetate (4h)
Yield: 65%; Rf: 0.60; eluent: CH2Cl2/MeOH, 95:5 v/v; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.90 (s, 3H, –CH3), 3.75 (m, 4H, –CH2–), 5.78 (s, 2H, –CH2–), 7.29 (dd, 1H, Ar–H, J = 12 Hz), 8.00–8.05 (m, 4H, Ar–H), 8.23 (d, 2H, Ar–H, J = 12.4 Hz), 8.33 (s, 1H, CH-triazole), 8.71 (s, 1H, NH). 13C NMR (75 MHz, CDCl3) δ (ppm): 20.37 (CH3), 62.74, 67.27 (CH2), 78.35 (CH2), 118.70, 122.49 (Imzp-CH), 125.92, 126.02 (phenyl-CH), 127.58 (phenyl-C), 128.49 (triazole-CH), 132.08 (Imzp-CH), 144.07 (Imzp-C), 146.09 (triazole-C), 152.65 (Imzp-C), 171.11 (CO). ESI-MS m/z calcd. for C19H18N6O3 (M+H): 379.38, found: 379.50.
2-((4-(4-(5,6-Dichlorobenzimidazol-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethyl acetate (4i)
Yield: 67%; Rf: 0.65; eluent: CH2Cl2/MeOH, 95:5 v/v; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.89 (s, 3H, –CH3), 3.74 (m, 4H, –CH2–), 5.76 (s, 2H, –CH2–), 7.77 (m, 2H, Ar–H), 7.95 (s, 1H, CH-triazole), 8.07 (m, 2H, Ar–H, 8.4 Hz), 8.13 (d, 2H, Ar–H, J = 8.0 Hz), 8.67 (s, 1H, NH). 13C NMR (75 MHz, CDCl3) δ (ppm): 20.36 (CH3), 62.73, 67.24 (CH2), 78.30 (CH2), 122.29, 124.86 (Bzm-CH), 125.70, 127.08 (phenyl-CH), 128.10 (Bzm-C), 128.89 (triazole-CH), 131.80, 133.35 (phenyl-C), 137.48 (Bzm-C), 146.03 (triazole-C), 153.15 (Bzm-C), 171.07 (CO). ESI-MS (M−H), m/z calcd. for C20H18N6O5: 446.29, found: 445.40.
General procedure for the synthesis of compounds 5a–h
The compounds 4a–h were reacted at room temperature with K2CO3 (2 equivalents) in methanol 2.5 mL. The mixture was stirred for 30 min. The reaction was monitored by TLC analysis. The solvent was evaporated under reduced pressure. Then, the crude compounds 5a–h were purified by passing through a flash chromatography column using methylene chloride/methanol (95:5) as eluent.
Spectral data for selected compounds 5a–h
2-((4-(4-(Benzimidazol-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethanol (5a)
M.p. 229–230°C; yield: 99%; Rf: 0.40; eluent: CH2Cl2/MeOH, 9:1 v/v; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 3.52 (t, 2H, –CH2–, J = 4.0 Hz), 3.55 (t, 2H, –CH2–, J = 4.0 Hz), 5.19 (bs, 1H, –OH), 5.76 (s, 2H, –CH2–), 7.21–7.33 (m, 2H, Ar–H), 7.60–7.69 (m, 3H, Ar–H, CH-triazole), 8.01 (d, 2H, Ar–H, J = 8.4 Hz), 8.17 (d, 2H, Ar–H, J = 8.4 Hz), 8.64 (s, 1H, NH). 13C NMR (100 MHz, CDCl3) δ (ppm): 59.71, 70.90 (CH2), 78.56 (CH2), 122.25, 122.77 (Bzm-CH), 125.90 (phenyl-CH), 127.07 (phenyl-C), 128.94 (triazole-CH), 131.46 (Bzm-C), 146.15 (triazole-C), 151.73 (Bzm-C). ESI-MS m/z calcd. for C18H17N5O2 (M+H): 336.36, found: 336.4. HRMS (M+H): calcd. for C18H17N5O2 : 336.14550, found: 336.14574.
2-((4-(4-(5-Methyl benzimidazol-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethanol (5b)
M.p. 203–204°C; yield: 98%; Rf: 0.40; eluent: CH2Cl2/MeOH, 9:1 v/v; 1H NMR (400 MHz,DMSO-d6) δ (ppm): 2.34 (s, 3H, Ar–CH3), 3.52 (t, 2H, –CH2–, J = 3.6 Hz), 3.54 (t, 2H, –CH2–, J = 3.6 Hz), 5.26 (bs, 1H, –OH), 5.75 (s, 2H, –CH2–), 7.00 (d, 2H, Ar–H, J = 8.0 Hz), 7.37 (s, 1H, CH-triazole), 7.49 (d, 1H, Ar–H, J = 7.6 Hz), 7.95 (d, 2H, Ar–H, J = 8.00 Hz), 8.14 (d, 2H, Ar–H, J = 6.4 Hz), 8.64 (s, 1H, NH). 13C NMR (100 MHz, CDCl3) δ (ppm): 21.08 (Bzm-CH3), 59.71, 70.89 (CH2), 78.54 (CH2), 114.21, 122.15, 124.17 (Bzm-CH), 125.83 (phenyl-CH), 126.88 (phenyl-C), 129.04 (triazole-CH), 131.25, 132.21 (Bzm-C), 146.18 (triazole-C), 150.44 (Bzm-C). ESI-MS m/z calcd. for C19H19N5O2 (M+H): 350.39, found: 350.1. HRMS (M+H): calcd. for C19H19N5O2: 350.16115, found: 350.16114.
2-((4-(4-(5,6-Dimethyl benzimidazol-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethanol (5c)
M.p. 233–234°C; yield: 98%; Rf: 0.45; eluent: CH2Cl2/MeOH, 9:1 v/v; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.26 (s, 6H, Ar–CH3), 3.51 (t, 2H, –CH2–, J = 3.6 Hz), 3.55 (t, 2H, –CH2–, J = 3.6 Hz), 5.19 (bs, 1H, –OH), 5.76 (s, 2H, –CH2–), 7.36 (s, 1H, CH-triazole), 7.96–7.99 (m, 3H, Ar–H), 8.11–8.14 (m, 3H, Ar–H), 8.66 (s, 1H, NH). 13C NMR (100 MHz, CDCl3) δ (ppm): 19.83 (Bzm-CH3), 59.70, 70.89 (CH2), 78.55 (CH2), 122.17 (Bzm-CH), 126.79 (phenyl-CH), 129.18 (phenyl-C), 131.09 (triazole-CH), 131.50 (Bzm-C), 146.21 (triazole-C), 149.87 (Bzm-C). ESI-MS m/z calcd. for C20H21N5O2 (M+H): 364.41, found: 364.5. HRMS (M+H): calcd. for C20H21N5O2: 364.17680, found: 364.17704.
2-((4-(4-(5-Methoxy benzimidazol-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethanol (5d)
M.p. 172–173°C; yield: 99%; Rf: 0.40; eluent: CH2Cl2/MeOH, 9:1 v/v; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 3.48 (t, 2H, –CH2–, J = 3.6 Hz), 3.52 (t, 2H, –CH2–, J = 4 Hz), 3.74 (s, 3H, –OCH3), 5.16 (bs, 1H, –OH), 5.74 (s, 2H, –CH2–), 6.81–6.84 (dd, 2H, Ar–H, J = 11.6 Hz), 7.09 (s, 1H, CH-triazole), 7.47 (d, 1H, Ar–H, J = 11.6 Hz), 7.96 (d, 2H, Ar–H, J = 11.2 Hz), 8.08 (d, 2H, Ar–H, J = 10.8 Hz), 8.64 (s, 1H, NH). 13C NMR (75 MHz, CDCl3) δ (ppm): 55.39 (–OCH3), 59.68, 70.86 (CH2), 78.33 (CH2), 112.29, 122.16 (Bzm-CH), 125.84, 125.91 (phenyl-CH), 126.70 (phenyl-C), 129.05 (triazole-CH), 131.14 (Bzm-C), 146.16 (triazole-C), 155.99 (Bzm-C). ESI-MS m/z calcd. for C19H19N5O3 (M+H): 366.39, found: 366.3. HRMS (M+H): calcd. for C19H19N5O3: 366.15607, found: 366.15583.
2-((4-(4-(5-Trifluoromethyl benzimidazol-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethanol (5e)
M.p. 150–151°C; yield: 95%; Rf: 0.30; eluent: CH2Cl2/MeOH, 9:1 v/v; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 3.52 (m, 4H, –CH2–), 5.16 (bs, 1H, –OH), 5.72 (s, 2H, –CH2–), 7.41 (d, 1H, Ar–H, J = 11.2 Hz), 7.70 (d, 1H, Ar–H, J = 11.2 Hz), 7.86 (s, 1H, CH-triazole), 7.94 (d, 2H, Ar–H, J = 10.0 Hz), 8.10 (m, 3H, Ar–H), 8.63 (s, 1H, NH). 13C NMR (75 MHz, CDCl3) δ (ppm): 59.68, 62.46 (CH2), 78.52 (CH2), 119.08, 122.26, 122.90 (Bzm-CH), 123.32 (–CF3) 125.64, 125.84 (phenyl-CH), 127.30 (triazole-CH), 128.16 (phenyl-C), 131.96 (Bzm-C), 145.99 (triazole-C), 153.33 (Bzm-C). ESI-MS m/z calcd. for C19H16F3N5O2 (M+H): 404.36, found: 404.5. HRMS (M+H): calcd. for C19H16F3N5O2: 404.13289, found: 404.13232.
2-((4-(4-(5-Fluorobenzimidazol-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethanol (5f)
M.p. 220–222°C; yield: 98%; Rf: 0.30; eluent: CH2Cl2/MeOH, 9:1 v/v; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 3.52 (m, 4H, –CH2–), 5.17 (bs, 1H, –OH), 5.73 (s, 2H, –CH2–), 6.99 (m, 1H, Ar–H, J = 11.2 Hz), 7.30 (d, 1H, Ar–H, J = 12 Hz), 7.52 (s, 1H, CH-triazole), 7.94 (d, 2H, Ar–H, J = 11.6 Hz), 8.07 (m, 3H, Ar–H, J = 11.2 Hz), 8.62 (s, 1H, NH). 13C NMR (100 MHz, CDCl3) δ (ppm): 59.69, 70.87 (CH2), 78.52 (CH2), 110.54, 110.86, 122.19 (Bzm-CH), 125.80, 125.81 (phenyl-CH), 126.94 (triazole-CH), 128.50 (phenyl-C), 131.50 (Bzm-C), 146.07 (triazole-C), 157.18, 160.36 (Bzm-C). ESI-MS m/z calcd. for C18H16FN5O2 (M+H): 353.35, found: 354.4. HRMS (M+H): calcd. for C18H16FN5O2: 354.13608, found: 354.13594.
2-((4-(4-(6-Nitrobenzimidazol-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethanol (5g)
M.p. 223–225°C; yield: 98%; Rf: 0.30; eluent: CH2Cl2/MeOH, 9:1 v/v; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 3.52 (t, 2H, –CH2–, J = 3.2 Hz), 3.55 (t, 2H, –CH2–, J = 3.6 Hz), 5.19 (bs, 1H, –OH), 5.77 (s, 2H, –CH2–), 7.66 (d, 2H, Ar–H, J = 8.8 Hz), 7.94–7.98 (m, 1H, Ar–H), 8.03 (d, 2H, Ar–H, J = 8.0 Hz), 8.10 (d, 2H, Ar–H, J = 8.0 Hz), 8.39 (s, 1H, CH-triazole), 8.64 (s, 1H, NH). 13C NMR (75 MHz, CDCl3) δ (ppm): 59.70, 70.90 (CH2), 78.57 (CH2), 104.51, 118.20, 122.44 (Bzm-CH), 125.88, 127.53 (phenyl-CH), 128.64 (triazole-CH), 132.28, 133.13 (phenyl-C), 142.71 (Bzm-C), 145.97 (triazole-C), 150.72 (Bzm-C). ESI-MS m/z calcd. for C18H16N6O4 (M−H): 379.36, found: 379.4. HRMS (M+H): calcd. for C18H16N6O4: 381.13058, found: 381.13046.
2-((4-(4-(Imidazo[4,5-b]pyridin-2-yl)phenyl)-1,2,3-triazol-1-yl)methoxy)ethanol (5h)
M.p. 274–276°C; yield: 95%; Rf: 0.40; eluent: CH2Cl2/MeOH, 9:1 v/v; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 3.52 (t, 2H, –CH2–, J = 4.0 Hz), 3.56 (t, 2H, –CH2–, J = 4.0 Hz), 5.18 (bs, 1H, –OH), 5.77 (s, 2H, –CH2–), 7.29 (dd, 1H, Ar–H, J = 8.0 Hz), 8.00–8.10 (m, 4H, Ar–H), 8.23 (d, 2H, Ar–H, J = 8.4 Hz), 8.33 (s, 1H, CH-triazole), 8.71 (s, 1H, NH). 13C NMR (100 MHz, CDCl3) δ (ppm): 59.70, 70.90 (CH2), 78.58 (CH2), 122.35, 122.54 (Imzp-CH), 125.84, 126.05 (phenyl-CH), 127.63 (phenyl-C), 128.44 (triazole-CH), 132.12 (Imzp-CH), 146.04 (triazole-C), 146.02, 152.67 (Imzp-C). ESI-MS m/z calcd. for C17H16N6O2 (M+H): 337.35, found: 337.3. HRMS (M+H): calcd. for C17H16N6O2 : 337.14075, found: 337.14084.
Acknowledgments
The Centre National de Recherche Scientifique et Technique (CNRST, Rabat, Morocco): Research programme RS 2011/01 supported this project. We would like to thank all members of the instrumental laboratory (Goethe-University Frankfurt am Main) for their help (NMR and mass analysis). We gratefully acknowledge the experimental help in testing the compounds by Phil Dudfield VP, Alliances and Informatics Galapagos NV, 102 avenue Gaston Roussel, 93230 Romainville.
The authors have declared no conflict of interest.
References
- 1.Zhu Z, Lippa B, Drach JC, Townsend LB. J. Med. Chem. 2000;43:2430–2437. doi: 10.1021/jm990290y. [DOI] [PubMed] [Google Scholar]
- 2.Terzioglu N, van Rijn R, Bakker RA, De Esch IJP, Leurs R. Bioorg. Med. Chem. Lett. 2004;14:5251–5256. doi: 10.1016/j.bmcl.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 3.Forseca T, Gigante B, Gilchrist TL. Tetrahedron. 2001;57:1793–1799. [Google Scholar]
- 4.Garcia-Domenech R, Rios-Santamarina I, Catala A, Calabuig C, del Castillo L, Galvez J. J. Mol. Struct. (Theochem) 2003;624:97–107. [Google Scholar]
- 5.Migawa MT, Girardet JL, Walker JA, Koszalka GW, Chamberlain SD, Drach JC, Townsend LB. J. Med. Chem. 1998;41:1242–1251. doi: 10.1021/jm970545c. [DOI] [PubMed] [Google Scholar]
- 6.Perumal S, Mariappan S, Selvaraj S. Arkivoc. 2004;8:46–51. [Google Scholar]
- 7.Coban G, Zencir S, Zupkó I, Réthy B, Gunes HS, Topcu Z. Eur. J. Med. Chem. 2009;44:2280–2285. doi: 10.1016/j.ejmech.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Bahrami K, Khodaei M, Naali F. J. Org. Chem. 2008;73:6835–6837. doi: 10.1021/jo8010232. [DOI] [PubMed] [Google Scholar]
- 9.Varala R, Nasreen A, Enugala R, Adapa SR. Tetrahedron Lett. 2007;48:69–72. [Google Scholar]
- 10.Zheng N, Anderson KW, Huang X, Nguyen HN, Buchwald SL. Angew. Chem. Int. Ed. 2007;46:7509–7512. doi: 10.1002/anie.200702542. [DOI] [PubMed] [Google Scholar]
- 11.Reddy GV, Rao NSR, Narsaiah B, Rao PS. Synth. Commun. 2002;32:2467–2476. [Google Scholar]
- 12.Dettmann S, Szymanowitz K, Wellner A, Schiedel A, Müller CE, Gust R. Bioorg. Med. Chem. 2010;18:4905–4916. doi: 10.1016/j.bmc.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Kumar BVS, Vaidya SD, Kumar RV, Bhirud SB, Mane RB. Eur. J. Med. Chem. 2006;41:599–604. doi: 10.1016/j.ejmech.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Yun H, Baogen W, Jun Y, Dale R, Lisa R, Ray R, Lawrence B, Suzie S, Eric ES. Bioorg. Med. Chem. Lett. 2003;13:3253–3256. [Google Scholar]
- 15.Reinhardt BB, Horace F, John PY, Margaret MZ, Donna RB, Rene PT, Dianne MK, Alan HK, John AM, Magid AG. J. Med. Chem. 2001;44:1516–1529. [Google Scholar]
- 16.Alvarez R, Velazquez S, San-Felix A, Aquaro S, De Clercq E, Perno CF, Karlsson A, Balzarini J, Camarasa MJ. J. Med. Chem. 1994;37:4185–4194. doi: 10.1021/jm00050a015. [DOI] [PubMed] [Google Scholar]
- 17.De Clercq E. Clin. Microbiol. Rev. 1997;10:674–693. doi: 10.1128/cmr.10.4.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Clercq E. Nat. Rev. Drug Discov. 2002;1:13–25. doi: 10.1038/nrd703. [DOI] [PubMed] [Google Scholar]
- 19.Buckle DR, Rockell CJM, Smith H, Spicer BA. J. Med. Chem. 1986;29:2262–2267. doi: 10.1021/jm00161a022. [DOI] [PubMed] [Google Scholar]
- 20.Vicentini CB, Brandolini V, Guarneri M, Giori P. Farmaco. 1992;47:1021–1034. [PubMed] [Google Scholar]
- 21.Joan CFT, Elizabeth H, Beatrice M, Daniel PB. Antimicrob. Agents Chemother. 1998;42:313–318. [Google Scholar]
- 22.Aher NG, Pore VS, Mishra NN, Kumar A, Shukla PK, Sharma A, Bhat MK. Bioorg. Med. Chem. Lett. 2009;19:759–763. doi: 10.1016/j.bmcl.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Guangyou C, Yiwan Z, Chonglin C, Jia L, Xing Z. Molecules. 2014;19:5674–5691. [Google Scholar]
- 24.Costa MS, Boechat N, Rangel EA, Silva FDCD, Souza AMTD, Rodrigues CR, Castro HC, Junior IN, Lourenc MCS, Wardell SMSV, Ferreirab VF. Bioorg. Med. Chem. 2006;14:8644–8653. doi: 10.1016/j.bmc.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Patpi SR, Pulipati L, Yogeeswari P, Sriram D, Jain N, Sridhar B, Murthy R, Anjana DT, Kalivendi SV, Kantevar S. J. Med. Chem. 2012;55:3911–3922. doi: 10.1021/jm300125e. [DOI] [PubMed] [Google Scholar]
- 26.De Simone R, Chini MG, Bruno I, Riccio R, Mueller D, Werz O, Bifulco G. J. Med. Chem. 2011;54:1565–1575. doi: 10.1021/jm101238d. [DOI] [PubMed] [Google Scholar]
- 27.Genin MJ, Allwine DA, Anderson DJ, Barbachyn MR, Emmert DE, Garmon SA, Graber DR, Grega KC, Hester JB, Hutchinson DK, Morris J, Reischer RJ, Ford CW, Zurenko GE, Hamel JC, Schaadt RD, Stapert D, Yagi BH. J. Med. Chem. 2000;43:953–970. doi: 10.1021/jm990373e. [DOI] [PubMed] [Google Scholar]
- 28.Huisgen R. 1,3-Dipolar Cycloaddition Chemistry. Vol. 1. New York: Wiley; 1984. pp. 1–176. [Google Scholar]
- 29.Tornøe CW, Christensen C, Meldal M. J. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 30.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem. Int. Ed. 2002;41:2565–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Meldal M, Tornøe CW. Chem. Rev. 2008;108(8):2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 32.Pradere U, Roy V, Mc Brayer TR, Schinazi RF, Agrofoglio LA. Tetrahedron. 2008;64:9044–9051. doi: 10.1016/j.tet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kappe CO, Stadler A. Microwaves in Organic and Medicinal Chemistry. Weinheim: Wiley-VCH; 2005. [Google Scholar]
- 34.Göker H, Kus C, Boykin DW, Yildizc S, Altanlarc N. Bioorg. Med. Chem. 2002;10:2589–2596. doi: 10.1016/s0968-0896(02)00103-7. [DOI] [PubMed] [Google Scholar]
- 35.Navarrete-Vázquez G, Moreno-Diaz H, Estrada-Soto S, Torres-Piedra M, León-Rivera I, Tlahuext H, Muñoz-Muñiz O, Torres-Gómez H. Synth. Commun. 2007;37:2815–2825. [Google Scholar]
- 36.Romanazzi G, Dell'Aquila A, Suranna GP, Marinelli F, Cotrone S, Altamura D, Giannini C, Torsi L, Mastrorilli P. J. Mater. Chem. 2011;21:15186–15189. [Google Scholar]
- 37.Ji H, Gao G. Arkivoc. 2009;7:261–280. [Google Scholar]
- 38.Krim J, Sillahi B, Taourirte M, Rakib EM, Engels JW. Arkivoc. 2009;8:142–152. [Google Scholar]
- 39.Lazrek HB, Taourirte M, Oulih T, Barascut JL, Imbach JL, Pannecouque C, Witrouw M, De Clercq E. Nucleosides Nucleotides Nucleic Acids. 2001;20:1949–1960. doi: 10.1081/NCN-100108325. [DOI] [PubMed] [Google Scholar]
- 40.Ouahrouch A, Taourirte M, Lazrek HB, Bats JW, Engels JW. Acta Crystallogr. E. 2012;68(Pt 6):o1908. doi: 10.1107/S1600536812023410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard. 7th ed. 2006. M7-A7 (ISBN 1-56238-587-9), 940 West Valley Road, suite 1400, Wayne, Pennsylvania 19087–1898 USA. [Google Scholar]
- 42.Bouslim F. 1996. Ph. D. Thesis, Contribution à l'étude de l'helminthosporiose du riz au Maroc due à H. Oriza, University. Ibn Tofaïl, Faculty of Sciences, Kénitra, Morocco.
- 43.Leroux P, Credet A. Document sur l'étude de l'activité des fongicides. Versailles: INRA; 1978. [Google Scholar]


