Abstract
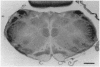
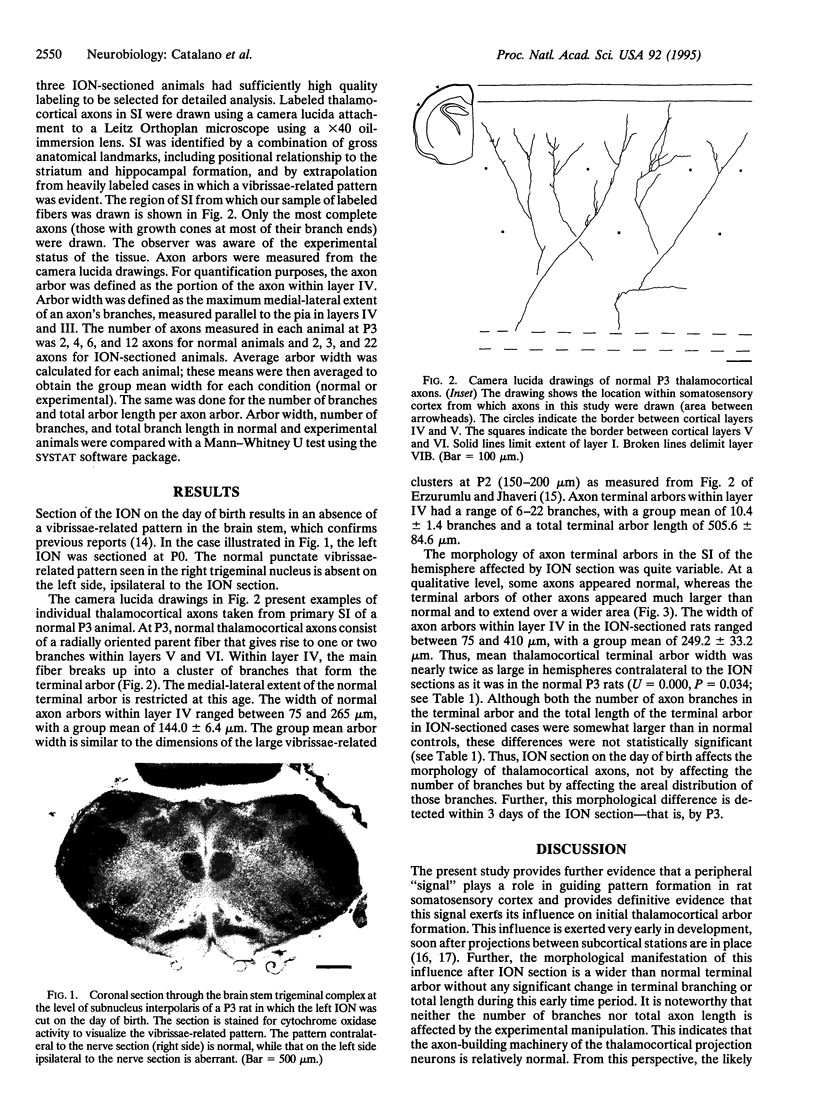
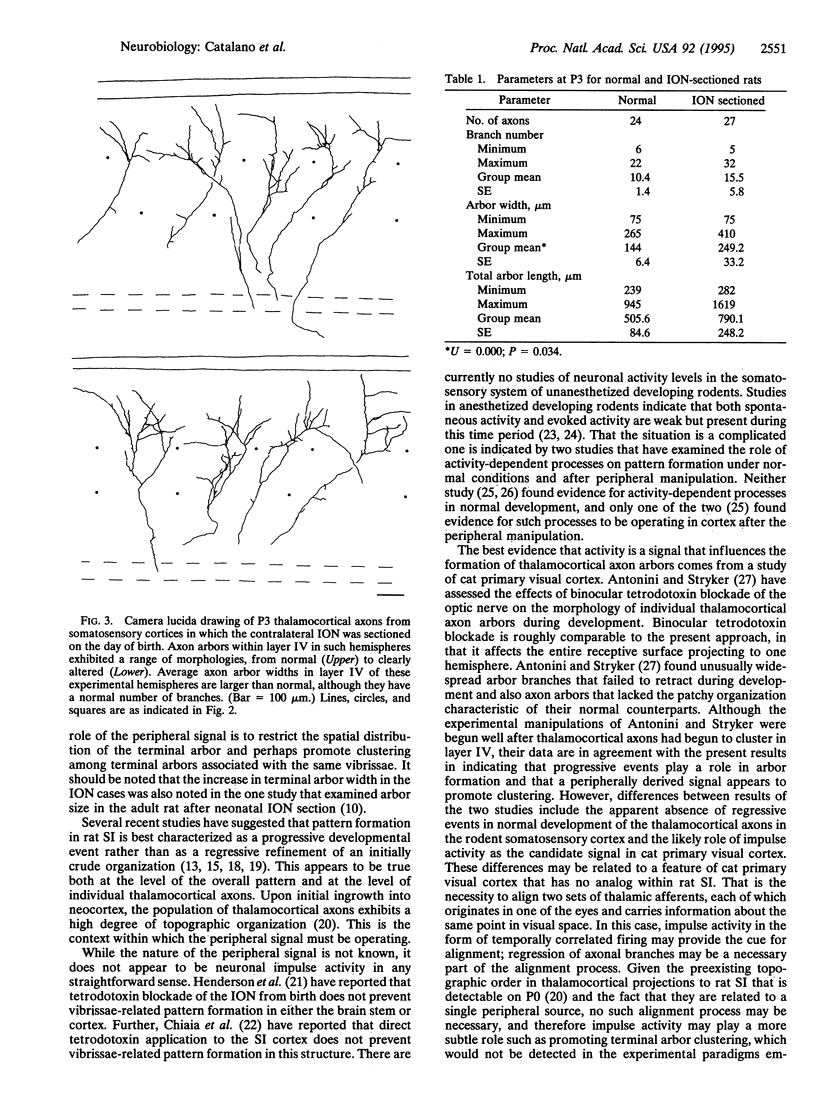
The effect of day of birth (postnatal day 0; P0) infraorbital nerve section on the morphology of individual thalamocortical axons in rat somatosensory cortex was examined on P3. Thalamic fibers were labeled in fixed brains with the carbocyanine dye 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate, and individual photo-converted thalamocortical fibers were reconstructed. In normal animals on P3, axon arbor terminal formation within layer IV has commenced and terminal arbor width is comparable to that of a cortical "barrel." After infraorbital nerve section, the average width of thalamocortical terminal arbors is significantly greater than is the average arbor width of normal rats of the same age; however, neither the number of branches per terminal arbor nor total arbor length differs between groups. These observations suggest that the role of the periphery in guiding terminal arbor formation is exerted both very rapidly and at the level of the single thalamic axon. Further, these results indicate a close association between individual axon terminal arbor morphology and pattern formation in the rat somatosensory cortex.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agmon A., Yang L. T., O'Dowd D. K., Jones E. G. Organized growth of thalamocortical axons from the deep tier of terminations into layer IV of developing mouse barrel cortex. J Neurosci. 1993 Dec;13(12):5365–5382. doi: 10.1523/JNEUROSCI.13-12-05365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A., Stryker M. P. Development of individual geniculocortical arbors in cat striate cortex and effects of binocular impulse blockade. J Neurosci. 1993 Aug;13(8):3549–3573. doi: 10.1523/JNEUROSCI.13-08-03549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-James M. The functional status and columnar organization of single cells responding to cutaneous stimulation in neonatal rat somatosensory cortex S1. J Physiol. 1975 Apr;246(3):501–538. doi: 10.1113/jphysiol.1975.sp010902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belford G. R., Killackey H. P. The development of vibrissae representation in subcortical trigeminal centers of the neonatal rat. J Comp Neurol. 1979 Nov 1;188(1):63–74. doi: 10.1002/cne.901880106. [DOI] [PubMed] [Google Scholar]
- Catalano S. M., Robertson R. T., Killackey H. P. Early ingrowth of thalamocortical afferents to the neocortex of the prenatal rat. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):2999–3003. doi: 10.1073/pnas.88.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaia N. L., Bauer W. R., Rhoades R. W. Prenatal development of the receptive fields of individual trigeminal ganglion cells in the rat. J Neurophysiol. 1993 Apr;69(4):1171–1180. doi: 10.1152/jn.1993.69.4.1171. [DOI] [PubMed] [Google Scholar]
- Chiaia N. L., Fish S. E., Bauer W. R., Bennett-Clarke C. A., Rhoades R. W. Postnatal blockade of cortical activity by tetrodotoxin does not disrupt the formation of vibrissa-related patterns in the rat's somatosensory cortex. Brain Res Dev Brain Res. 1992 Apr 24;66(2):244–250. doi: 10.1016/0165-3806(92)90086-c. [DOI] [PubMed] [Google Scholar]
- Chiaia N. L., Fish S. E., Bauer W. R., Figley B. A., Eck M., Bennett-Clarke C. A., Rhoades R. W. Effects of postnatal blockage of cortical activity with tetrodotoxin upon lesion-induced reorganization of vibrissae-related patterns in the somatosensory cortex of rat. Brain Res Dev Brain Res. 1994 Jun 17;79(2):301–306. doi: 10.1016/0165-3806(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Dawson D. R., Killackey H. P. Distinguishing topography and somatotopy in the thalamocortical projections of the developing rat. Brain Res. 1985 Jan;349(1-2):309–313. doi: 10.1016/0165-3806(85)90162-2. [DOI] [PubMed] [Google Scholar]
- Erzurumlu R. S., Jhaveri S. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somatosensory cortex. Brain Res Dev Brain Res. 1990 Nov 1;56(2):229–234. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- Erzurumlu R. S., Killackey H. P. Development of order in the rat trigeminal system. J Comp Neurol. 1983 Feb 1;213(4):365–380. doi: 10.1002/cne.902130402. [DOI] [PubMed] [Google Scholar]
- Friedlander M. J., Martin K. A. Development of Y-axon innervation of cortical area 18 in the cat. J Physiol. 1989 Sep;416:183–213. doi: 10.1113/jphysiol.1989.sp017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson T. A., Woolsey T. A., Jacquin M. F. Infraorbital nerve blockade from birth does not disrupt central trigeminal pattern formation in the rat. Brain Res Dev Brain Res. 1992 Mar 20;66(1):146–152. doi: 10.1016/0165-3806(92)90152-m. [DOI] [PubMed] [Google Scholar]
- Jensen K. F., Killackey H. P. Terminal arbors of axons projecting to the somatosensory cortex of the adult rat. I. The normal morphology of specific thalamocortical afferents. J Neurosci. 1987 Nov;7(11):3529–3543. doi: 10.1523/JNEUROSCI.07-11-03529.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. F., Killackey H. P. Terminal arbors of axons projecting to the somatosensory cortex of the adult rat. II. The altered morphology of thalamocortical afferents following neonatal infraorbital nerve cut. J Neurosci. 1987 Nov;7(11):3544–3553. doi: 10.1523/JNEUROSCI.07-11-03544.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killackey H. P. Anatomical evidence for cortical subdivisions based on vertically discrete thalamic projections from the ventral posterior nucleus to cortical barrels in the rat. Brain Res. 1973 Mar 15;51:326–331. doi: 10.1016/0006-8993(73)90383-1. [DOI] [PubMed] [Google Scholar]
- Killackey H. P., Belford G. R. The formation of afferent patterns in the somatosensory cortex of the neonatal rat. J Comp Neurol. 1979 Jan 15;183(2):285–303. doi: 10.1002/cne.901830206. [DOI] [PubMed] [Google Scholar]
- Killackey H. P., Belford G., Ryugo R., Ryugo D. K. Anomalous organization of thalamocortical projections consequent to vibrissae removal in the newborn rat and mouse. Brain Res. 1976 Mar 12;104(2):309–315. doi: 10.1016/0006-8993(76)90623-5. [DOI] [PubMed] [Google Scholar]
- Sandell J. H., Masland R. H. Photoconversion of some fluorescent markers to a diaminobenzidine product. J Histochem Cytochem. 1988 May;36(5):555–559. doi: 10.1177/36.5.3356898. [DOI] [PubMed] [Google Scholar]
- Schlaggar B. L., Fox K., O'Leary D. D. Postsynaptic control of plasticity in developing somatosensory cortex. Nature. 1993 Aug 12;364(6438):623–626. doi: 10.1038/364623a0. [DOI] [PubMed] [Google Scholar]
- Schlaggar B. L., O'Leary D. D. Early development of the somatotopic map and barrel patterning in rat somatosensory cortex. J Comp Neurol. 1994 Aug 1;346(1):80–96. doi: 10.1002/cne.903460106. [DOI] [PubMed] [Google Scholar]
- Van der Loos H., Woolsey T. A. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973 Jan 26;179(4071):395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979 Jul 27;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Woolsey T. A., Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970 Jan 20;17(2):205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]