Abstract
Inconsistent information exists in the relationship between obstructive sleep apnea (OSA) and perinatal outcomes. This study was intended to investigate whether OSA in pregnant women has a potential to elevate the incidence of the maternal and neonatal outcomes by performing a meta-analysis of all available cohort studies. Five cohort studies including 977 participants were eligible for inclusion. The association between OSA and the risk of perinatal outcomes was expressed as relative risks (RR), with 95% confidence interval (CI). Our results revealed that OSA group was associated with more frequent preeclampsia (RR 1.96; 95% CI 1.34 to 2.86), preterm birth (RR 1.90; 95%CI 1.24 to 2.91), cesarean delivery (RR 1.87; 95% CI 1.52 to 2.29) and neonatal intensive care unit (NICU) (RR 2.65; 95% CI 1.86 to 3.76). On analyzing data for the prevalence of gestational diabetes and small gestational age (SGA) < 10th percentile (RR 1.40; 95% CI 0.62 to 3.19, and RR 0.64; 95%CI 0.33 to1.24, respectively), there were no significant differences in both group. Findings from this meta-analysis indicate that OSA in pregnant women significantly increases the incidence of maternal and neonatal outcomes, which is associated with more frequent preeclampsia, preterm birth, cesarean delivery and NICU admission.
Obstructive sleep apnea (OSA), a common sleep-related breathing disorder, is characterized by recurrent episodes of complete or partial upper airway collapse and obstruction during sleep and is associated with recurrent oxygen desaturations and sleep fragmentation1. The repeated episodes of hypoxia and reoxygenation are associated with significant endocrine and metabolic disturbance, which are responsible for the increase in hypertension, metabolic syndrome and cardiovascular risk observed among patients with OSA2,3. The clinical features of patients with OSA include loud frequent snoring, excessive daytime somnolence, personality changes, and nocturia. There is general agreement among investigators that snoring is more prevalent in pregnant women compared with non-pregnant women4. The prevalence of OSA is estimated to be 5% to 6% among women of reproductive age, however, the incidence of OSA in pregnant women is unknown5. Although the true prevalence rate in pregnancy is still unknown, many physiologic changes contribute to increased risk for OSA.
OSA occurs when the upper airway collapses during sleep, resulting in cessation of breathing, and is accompanied by episodic hypoxia and hypercapnia. Furthermore, OSA activates the sympathetic nervous system and inflammatory pathways6. Given these mechanisms, investigators have been trying to speculate the effect of OSA in pregnancy concerning for both maternal and neonatal outcomes. Increasing evidence now shows that OSA in pregnancy is associated with adverse pregnancy outcomes, including increased risks of preeclampsia, gestational diabetes and fetal growth restriction7,8. Preeclampsia belongs to the category of hypertensive disorders, which are the most common medical complications of pregnancy and a very important cause of maternal and perinatal morbidity and mortality worldwide9. It has been suggested that there is a recognized association between OSA and type 2 diabetes, with an incidence at around 40% of patients with OSA suffering from diabetes10,11, and at least partially this relationship is independent of adiposity12. However, due to these data mainly obtaining from general population, the association between OSA in pregnant women and diabetes is still unclear. We then speculate that the presence of OSA in pregnancy may predispose to the development of gestational diabetes. Meanwhile, the relationship between OSA and fetal outcomes is also receiving escalated attention. Studies showed that women with severe snoring in the third trimester of pregnancy had a higher risk for fetal-grown-restricted neonates, and women with sleep deprivation had a higher risk for preterm births, although the mechanisms underlying these associations remain unclear13,14. On the contrary, it's also reported that OSA in pregnant women does not elevate the risk of adverse fetal outcomes15. Taken together, controversies regarding to the association between OSA and adverse pregnancy outcomes still exist and are yet unanswered.
To address these concerns and to update the state of knowledge in this area, we performed a meta-analysis of cohort studies16,17,18,19,20 to examine the risk of perinatal outcomes, including preeclampsia, preterm birth, gestational diabetes, cesarean delivery, neonatal intensive care unit (NICU), small for gestational age (SGA) between pregnant women with and without OSA.
Methods
Literature search
A systematic literature search was performed to identify all cohort studies published before April 2014 that investigated the association between OSA and the perinatal outcomes. Electronic databases, including PubMed, EMBASE, CINAHL, Cochrane databases, and Google Scholar were searched, using a combination of the following terms: “obstructive sleep apnea” or “OSA” or “sleep-disordered breathing” or “SDB” and “perinatal outcomes” or “adverse maternal outcome” or “gestational diabetes” or “preeclampsia” and “cohort study” or “observational study”. The reference lists from relevant publications were also checked for additional publications that might be appropriate for inclusion in the meta-analysis. If there was a question of duplicative data, authors were contacted to determine whether there was an overlap of patients.
Inclusion and exclusion criteria
Two authors independently screened the searches; and disagreements were resolved by discussion or by seeking an independent third opinion. Studies were selected on the basis of inclusion and exclusion criteria. Inclusion Criteria: (1) the study population was limited to pregnant women with or without OSA; (2) we only selected cohort study or observational study; (3) for strong definition we included only studies that provide data for at least one of the following variables: preeclampsia, preterm birth, gestational diabetes, cesarean delivery and SGA; (4) only full-length original articles were considered. Reports containing overlapping data, cross-sectional studies, literature reviews and studies that used self-reported surrogate parameters such as snoring to assess OSA were excluded.
Data extraction
Data extraction was performed by two investigators independently, and disagreement was resolved by discussion. The following information were extracted from each study: first author's name, publication year, number of subjects, age, study design, method used to assess OSA, the maternal and neonatal outcomes, method for selecting participant, inclusion, exclusion and confounding variables.
Quality assessment
Quality assessment tool was based on six different types of bias common in cohort studies namely, selection, exposure, outcome, analytic, attrition and confounding. We classified study bias on the basis of minimal, low, moderate, and high or not reported21. Studies with: (1) high risk of bias or “not reported” in three or more domains or (2) an overall assessment of bias as “high” were excluded using a sensitivity analysis. Selection bias and confounding were given predominance in the overall assessment of bias due to their importance in this meta-analysis.
Maternal and neonatal outcome data
Maternal outcome data included preeclampsia, gestational diabetes, preterm birth. The diagnosis of preeclampsia required a systolic blood pressure more than 140 mmHg or a diastolic blood pressure more than 90 mmHg, on two occasions 4 hours to 14 days apart, occurring within 4 hours to 14 days of evident significant (>300 mg/dL) proteinuri22; the diagnosis of gestational diabetes required at least one abnormal result on a 2-hour 75 g oral glucose tolerance test or at least two abnormal values on a 3-hour 100 g oral glucose tolerance test during pregnanc23; a diagnosis of preterm birth was made for the interval 20 to 36 weeks of completed gestation24. Neonatal outcome data included mode of delivery (vaginal or cesarean), NICU admission and SGA. SGA was defined as <10th percentile adjusting for fetal gender and gestational age25.
Statistical analysis
RR with 95% confidence intervals (CI) was used to assess the relationship between OSA and perinatal outcomes. Tests of heterogeneity across studies were performed using the Cochrane Q-test and the I2 test26. The DerSimonian and Laird random effect model was adopted as the pooling method if substantial heterogeneity is present (I2 > 50%)27; otherwise, the fixed effect model was used as the pooling method.
Sensitivity analysis was used to determine the robustness of the results to assess uncertain decisions or assumptions about the data and to assess the methods that were used. The pooled estimates were reappraised when suspicious studies were excluded, and the reappraised results were compared with the original results to assess stability and reliability of our meta-analysis. Two sensitivity analyses were used in our meta-analysis. First, we estimated the pooled RR by study design. This was considered important as various types of study designs may differ in methodological quality. For example, prospective cohort studies were considered to be associated with higher in quality than retrospective cohort studies. Second, sensitivity analyses by the overall assessment of bias were performed. These studies of high risk of bias were excluded. They may weaken the conclusions.
STATA version 12.0 software (Stata Corporation, College Station, TX, USA) was used to perform all statistical analyses and to construct funnel plots. The overall effect was calculated with the Z test. A p value of < 0.05 was considered significant.
Results
Characteristics of the studies
A flow chart indicating the procedure for identifying the studies is presented in Figure 1. Based on the penetrating criteria, five cohort studies were selected, which included 977 participants16,17,18,19,20. Summary of association of studied cohort studies are mentioned in Table 1. The number of participants in each study ranged between 35 and 285. We selected studies published in the last 5 year. Among five selected studies four were prospective cohorts and one was a retrospective cohort19. To delineate the influence of obesity, three studies16,17,18 were stratified by BMI < 30 and ≥30, with the exception two studies19,20. As shown in Table 1, full PSG was used to diagnosis OSA in two studied19,20, portable PSG was used in one study17, Berlin questionnaire and Epworth score were used to evaluate OSA in two studies16,18. The quality assessment of the included studies was based on a bias classification tool estimating six types of bias in Table 2. Overall, the risk of bias for the studies included in the meta-analysis was considered “minimal” in 4 studies16,17,18,20 and “low” in one study19.
Figure 1. Flow diagram of included and excluded studies.
Table 1. Characteristics of studies included in the meta-analysis.
| First author Year | Study size | age | Study type | Measure for OSA diagnosis | Maternal outcome | Neonatal outcome | Method for selecting participant | Inclusion | Exclusion | Confounding Variable |
|---|---|---|---|---|---|---|---|---|---|---|
| KoHS16 2013 | 276 | 20–45 | Prospective cohort study | Berlin questionnaire | Preeclampsia, gestational diabetes, Preterm birth | Cesarean delivery, NICU admission, SGA < 10th percentile | Random survey by means of a self-administered close-ended questionnaire | 1. The questionnaire were properly filled in; | The questionnaire were not completed or the data of obstetric outcome were not fully available | age, obesity |
| 2. The data about obstetric outcomes were fully available. | BMI classification and the logistic regression was use to adjust the confounders | |||||||||
| Louis J17 2012 | 161 | 20–44 | Prospective cohort study | Good quality: Portable PSG (AHI > 5) | Preeclampsia, gestational diabetes Preterm birth | Cesarean delivery, NICU admission | Randomly selected | 1. Participants were obese, age 18 years or older; | 1. Subjects with chronic use of narcotic or other drugs affecting the central nevous system and inability to maintain sleep beyond 2 hours; | Age, race BMI |
| 2. Participants received obstetric care by their physicians or nurse practitioners. | 2.Women with a documented history of nonadherence. | Multivariable logistic regression model was performed after adjusting for the effect of confounders | ||||||||
| Olivarea18 SA2011 | 220 | 18–50 | Prospective cohort study | Berlin and Epworth scale | Preeclampsia, gestational diabetes | SGA < 10th percentile | NA | Gravidae of 18–50, with confirmed viable singleton gestations | Subjects with known sleep-disordered breathing and patients with significant underlying pulmonary or cardiac comorbidities, or with known multiple gestations | Age, obesity |
| BMI classification and the logistic regression was use to adjust the confounders | ||||||||||
| Louis JM19 2010 | 285 | 20–40 | retrospective cohort study | Good quality: full PSG (AHI > 5) | Preeclampsia, Preterm birth | Cesarean delivery, NICU admission, SGA < 10th percentile | Random number table in a masked fashion | 1.confirmed diagnosis of OSA | Multiple gestations and subjects with OSA without documentation of PSG confirmed OSA | Age, race, obesity |
| 2.Receive the prenatal care | Use the normal weight and obese as control group and the multivariable logistic regression analysis was performed | |||||||||
| Sahin FK20 2008 | 35 | 22–42 | Prospective observation study | Good quality: full PSG (AHI > 5) | Preeclampsia, gestational diabetes | NICU admission | NA | Pregnant women who agreed to participate in the study were scheduled for PSG and NST recording for one night after 34weeks of gestation. | Subjects who suffered from cardiac decompensation, or respiratory insufficiency or malignancy | Obesity |
| Use the non-obese women with OSA as control |
OSA: obstructive sleep apnea; BMI: body mass index; PSG: polysomnogram; SGA: small for gestational age; NICU: neonatal intensive care unit.
Table 2. Quality assessment based on evaluation of bias.
| study | Selection bias | Exposure bias | Outcome Assessment bias | Confounding factor bias | Analytical bias | Attrition bias | Overall likelihood of bias |
|---|---|---|---|---|---|---|---|
| KoHS16 2013 | Minimal (Random survey by questionnaire) | Minimal (direct completion of survey) | Minimal (hospital records and specific definition used) | Minimal (adjusting for maternal age, BMI) | Minimal (Multivariable logistic regression model for the confounders) | Minimal (all subjects from initiation to final outcome accounted for) | minimal |
| Louis J17 2012 | Minimal (Randomly selected) | Minimal (direct measurement of exposure) | Minimal (hospital records and specific definition used) | Minimal (adjusting for maternal age, race BMI) | Minimal (Multivariable logistic regression model for the confounders) | Minimal (<10% attrition and reasons for loss of follow up explained) | minimal |
| Olivarea18 SA 2011 | Minimal (all gravidae in the Ben Taub General Hospital obstetrics clinic) | Minimal (direct completion of survey) | Minimal (hospital records and specific definition used) | Minimal (adjusting for age and BMI) | Minimal (Multivariable logistic regression model for adjusting confounders) | Minimal (no loss to follow up) | minimal |
| Louis JM19 2010 | Minimal (randomly selected) | Low (validated prenatal database between January 2000-December 2008) | Low (assessment from administrative database) | Minimal (adjusting for maternal age, race BMI) | Minimal (Multivariable logistic regression model for the confounders) | Minimal (all subjects accounted for) | low |
| Sahin FK20 2008 | Minimal (all pregnant women between May 2006 and June 2007 in Afyonkarahisar Kocatepe university hospital) | Minimal (direct measurement of exposure) | Minimal (hospital records and specific definition used) | Low (adjusting for BMI) | Low (Mann-Whitney U test and the chi-square or Fisher exact test were applied) | Minimal (no loss to follow up) | minimal |
Meta-analysis
Maternal outcome: Preeclampsia
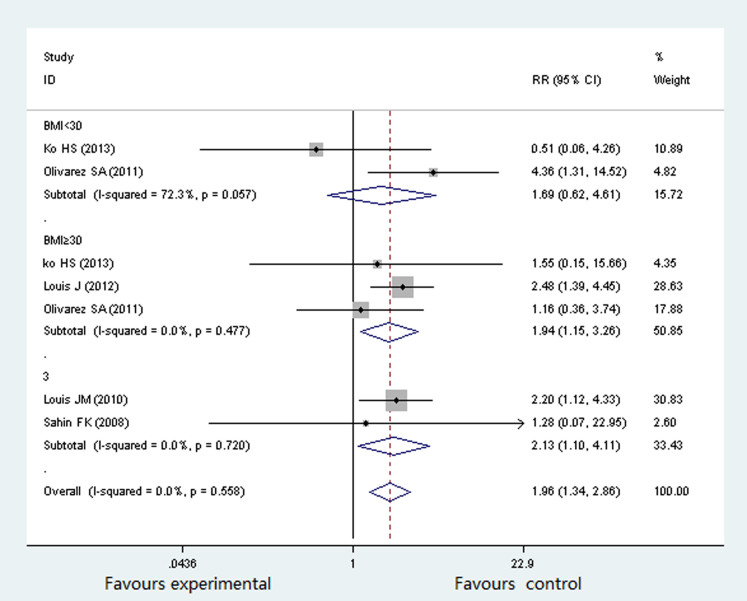
Five studies16,17,18,19,20 involving 977 participants evaluated the association between OSA and preeclampsia. The percentage of pregnant women with OSA suffering from preeclampsia was 3.37%16, 42.3%17, 14.3%18, 19.3%19 and 25%20 respectively. There was no significant heterogeneity (P = 0.558, I2 = 0%) across the overall analysis, and thus a fixed effects model was used. Three studies were stratified by BMI < 30 and ≥30. As shown in Figure 2, the rate of preeclampsia was significantly higher in OSA group than in non-OSA group (RR 1.96; 95% CI 1.34 to 2.86; P = 0.000). In the subgroup of BMI < 30, there was no statistical significant difference in both group (RR 1.69; 95% CI 0.62 to 4.61; P = 0.304); while in the subgroup of BMI ≥ 30, the prevalence of preeclampsia was significantly higher in participants with OSA (RR 1.94; 95% CI 1.15 to 3.26; P = 0.013).
Figure 2. Forest plots of the association between OSA and Preeclampsia.
Results are expressed as relative risk (RR) and 95% CI.
Maternal outcome: gestational diabetes
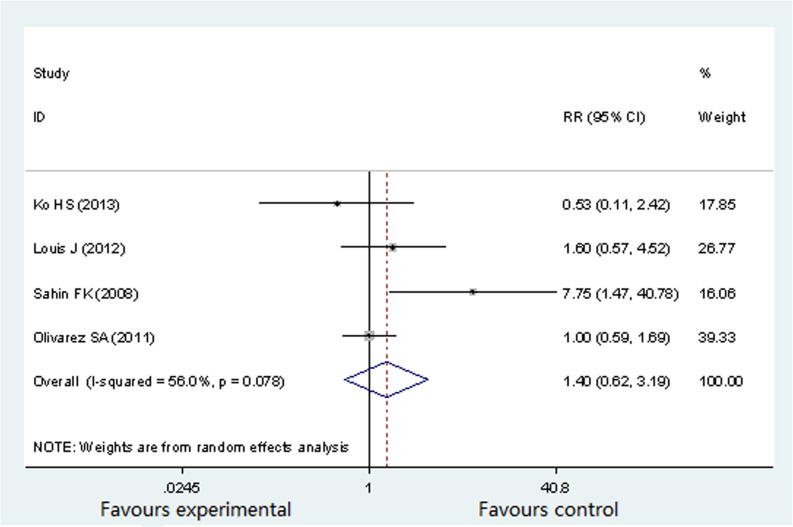
Four studies (692 participants) reported gestational diabetes16,17,18,20, with an incidence of gestational diabetes at 2.25%16, 19%17, 25%18 and 50%20 respectively in pregnant women with OSA. Significant heterogeneity was present among all selected studies (P = 0.078, I2 = 56%). Therefore, a random effects model was selected for this analysis. As shown in Figure 3, there was no significant difference in the prevalence of the gestational diabetes between OSA group and non-OSA group (RR 1.40; 95%CI 0.62 to 3.19; P = 0.418).
Figure 3. Forest plots of the association between OSA and gestational diabetes.
Results are expressed as RR and 95% CI.
Maternal outcome: preterm birth
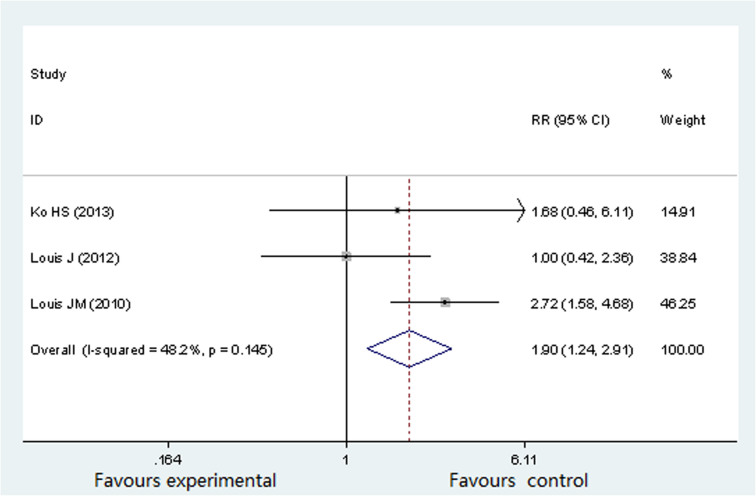
Three studies (722 participants) evaluated the associated between OSA and preterm birth16,17,19. The occurrence rates of preterm birth in pregnant women with OSA were as follows: 4.49%16, 17.6%17 and 29.8%19 separately. There was no significant heterogeneity (P = 0.145, I2 = 48.2%) across the analysis, and thus a fixed effects model was used. Figure 4 showed that the prevalence of preterm birth was significantly higher in pregnant women with OSA (RR 1.90; 95%CI 1.24 to 2.91; P = 0.003).
Figure 4. Forest plots of the association between OSA and preterm birth.
Results are expressed as RR and 95% CI.
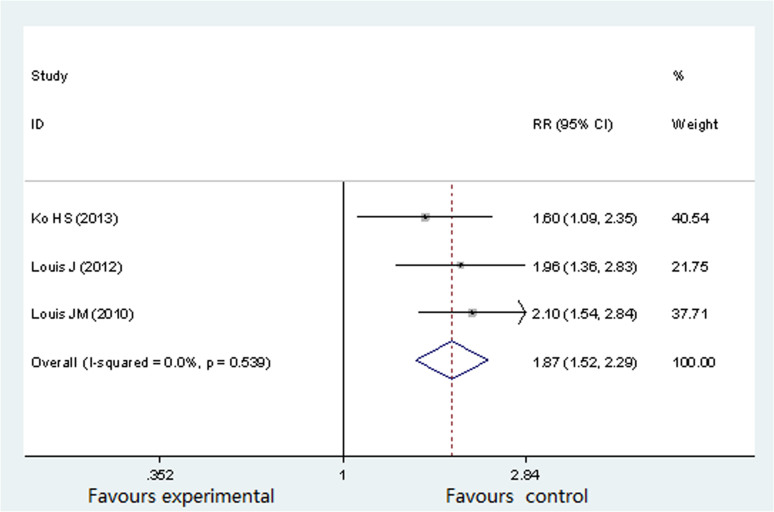
Neonatal outcome: cesarean delivery
Three studies (722 participants) reported difference in cesarean delivery16,17,19. The percentage of cesarean delivery was 36%16, 65.4%17 and 57.9%19 separately, in pregnant women suffering from OSA. There was no significant heterogeneity (P = 0.539, I2 = 0%) across the analysis, and thus a fixed effects model was use. As showed in Figure 5, cesarean delivery rate was significantly higher in pregnant women with OSA (RR 1.87; 95% CI 1.52 to 2.29; P = 0.000).
Figure 5. Forest plots of the association between OSA and cesarean delivery.
Results are expressed as RR and 95% CI.
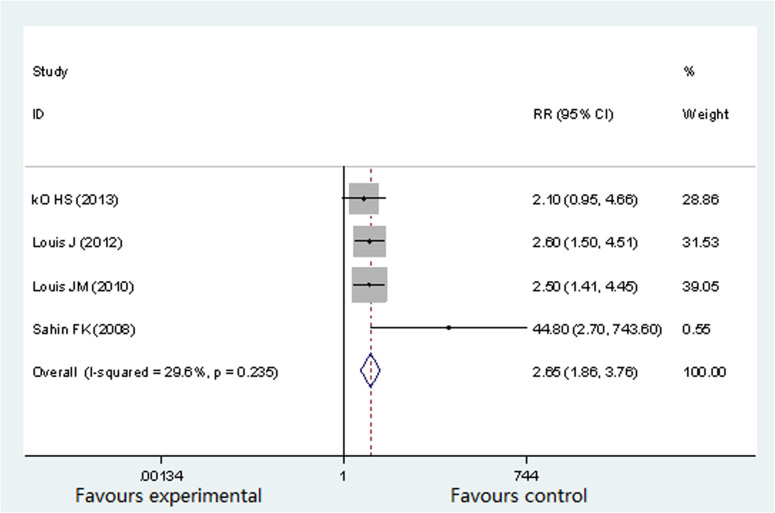
Neonatal outcome: NICU admission
Four studies (757 participants) reported the difference in NICU16,17,19,20. The occurrence rates of NICU admission in pregnant women with OSA were as follows: 12.36%16, 46.1%17, 26.3%19 and 41.9%20. There was no significant heterogeneity (P = 0.235, I2 = 29.6%) across the analysis, and thus a fixed effects model was use. Figure 6 showed that the prevalence of NICU admission in OSA group was significantly higher than in non-OSA group (RR 2.65; 95% CI 1.86 to 3.76; P = 0.000).
Figure 6. Forest plots of the association between OSA and NICU admission.
Results are expressed as RR and 95% CI.
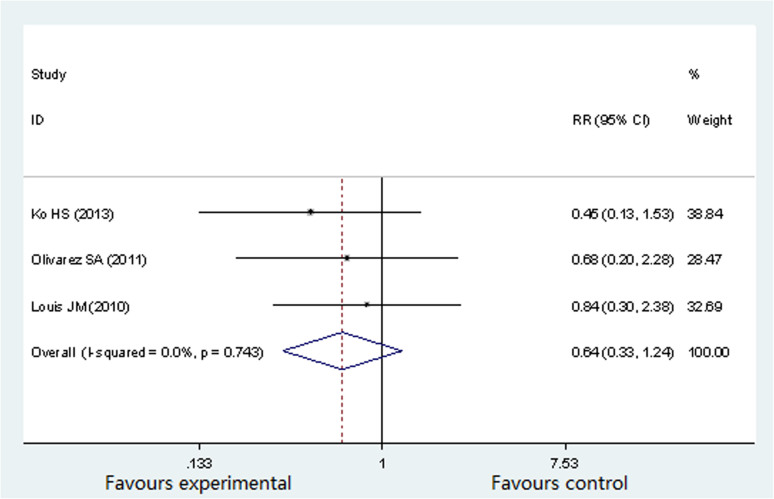
Neonatal outcome: SAG < 10th percentile
Three studies involving 787 participants evaluated the associated between OSA and SGA16,18,19. SGA was defined as <10th percentile adjusting for fetal gender and gestational age. The SGA incidence in pregnant women with OSA occupied 3.37%16, 5.4%18 and 7.0%19 separately. There was no significant heterogeneity (P = 0.743, I2 = 0%) across the analysis, and thus a fixed effects model was use. As showed in Figure 7, there was no significant difference in the prevalence of SAG < 10th between OSA group and non-OSA group (RR 0.64; 95% CI 0.33 to1.24; P = 0.189).
Figure 7. Forest plots of the association between OSA and SGA.
Results are expressed as RR and 95% CI.
Sensitivity analysis
There was no study of high risk of bias in our meta-analysis, so the subgroup analyses by study design were performed. When the retrospective cohort study19 or Berlin questionnaire studies16,18 were excluded, the summary RR, 95% CI, and P value for preeclampsia and NICU admission (as these were the outcomes with most studies included in the meta-analysis) were still similar to the results before they were excluded (Table 3), indicating that the results of our study were reliable and believable.
Table 3. Results of sensitivity analysis.
| Result of the study | |||||
|---|---|---|---|---|---|
| Excluded | Outcomes | No | Study size | RR (95% CI) | P |
| No study excluded | Preeclampsia | 5 | 977 | 1.96(1.34, 2.86) | 0.000 |
| NICU admission | 4 | 757 | 2.64 (1.85, 3.78) | 0.000 | |
| Retrospective cohort Study19 excluded | Preeclampsia | 4 | 692 | 1.86 (1.78, 2.93) | 0.008 |
| NICU admission | 3 | 472 | 2.74 (1.73, 4.33) | 0.000 | |
| Berlin questionnaire Studies16,18 excluded | Preeclampsia | 3 | 481 | 2.31 (1.83, 3.23) | 0.037 |
Discussion
This is the first report evaluating the relationship between OSA and perinatal outcomes by using cohort studies for analysis. Cohort study is to measure the effect of potential causes on certain outcomes, which can help determine risk factors for a disease because it is a longitudinal observation of the individual through time. The merit of cohort study lies in, before running a cohort study, it has been set which is cause and which is effect28. In this present meta-analysis of cohort studies, we aim to investigate whether OSA in pregnant women has a potential to elevate the incidence of the maternal and neonatal outcomes. And the results revealed that compared to non-OSA group, OSA group was associated with more frequent preeclampsia, preterm birth, cesarean delivery and NICU admission, while no significant difference were viewed in the relationship between gestational diabetes and SGA < 10 th percentile in both groups.
Obstructive sleep apnea is characterized by periodic apnea and hypopnea during sleep that results in asphyxia and waking from sleep. It is estimated to affect nearly 5% of the general population and snoring affects 6.7% of women. However, the prevalence of the OSA in the pregnant population has not been adequately characterized29. It's reported that when pregnant women suffering from OSA simultaneously, increased small airway closure at lung volumes, especially in the late pregnancy, would result in ventilation perfusion mismatch30, which would lead to physiologic dyspnea, higher risk of maternal hypoxemia and reduced oxygen delivery to the fetus for up to 75% of pregnant women.
The results of maternal outcome data in our meta-analysis showed that OSA group was associated with more frequent preeclampsia and preterm birth. OSA may contribute to the development of preeclampsia via recurrent episodes of placental hypoxia, increased hypertension and by inducing endothelial dysfunction. Due to the complexity of the relationship between OSA and preeclampsia, some confounding factors have been identified such as obesity, increasing maternal age, ethnicity et ac. However, Louis J et al.17 concluded that OSA may have independent association with preeclampsia (OR 3.55; 95% CI 1.12–11.3) even after adjusting for BMI, maternal age, and diabetes. And Olivarez SA, et al.18 also revealed that among non-obese gravidae, frequency of preeclampsia was significantly higher among women with OSA (OR 6.58; 95% CI 1.04–38.51). Regarding to the relationship between OSA and gestational diabetes, there was a discrepancy between our study and a recently published systematic review and meta-analysis31. The possible underlying causes might be: Firstly, the data in our paper was extracted from pregnant women with or without OSA, whereas the data of the previous review was obtained from general population; Secondly, we conducted quality assessment for each eligible study included in our meta-analysis; Finally, we just included cohort studies whereas RCT, case-control and cross sectional studies were all included in the previous review. Thereby their conclusion is just to say there is a positive correlation between OSA and gestational diabetes, but not demonstrating which is cause and which is effect.
The relationship between OSA and fetal outcomes is receiving increasing attention. It is plausible that OSA with the repeated episodes of hypoxia and hypercapnia, systemic inflammatory response and endothelial dysfunction may be an important intermediary. With respect to neonatal outcomes, in our meta-analysis, the results showed that OSA group was associated with high prevalence of cesarean delivery and neonatal intensive care unit admission. Louis J et al.17 concluded that within a cohort of obese pregnant patients, OSA was significantly associated with more frequent cesarean deliveries and NICU admission. Many of these admissions were secondary to respiratory morbidity in the neonate. Epidemiologic and observation studies have demonstrated that term neonates with transient tachypnea of the newborn have a fourfold increased odd of being delivered by cesarean32. In addition, we observed no significant difference in the rate of small for gestational age in our study. A retrospective cohort study of women with PSG-confirmed OSA also found no difference in SGA babies among affected women compared with obese and non-obese controls19. However, a retrospective cross-sectional study of 502 women by Franklin et al.33 reported an increase in SGA infant among women who snored. One possible explanation for such different findings is confounding by BMI, as increasing BMI will generally be associated with excessive fetal size. Thus, the number of women with SGA infant will thus be small34.
Our study has several limitations that require consideration. Firstly, the total number of prospective cohort studies relating to OSA and perinatal outcomes was limited. We were unable to compare studies on different populations by subgroup analysis. Secondly, the uniform definitions of OSA might be needed to diagnose OSA and to evaluate the relationship between OSA and the occurrence of maternal and neonatal outcomes.
Conclusion
Our study findings suggest that OSA among pregnant women is associated with more frequent preeclampsia, preterm birth, cesarean delivery and NICU admission. Limitations remain and the role of other potential confounders such as smoking, alcohol, sleeping pills and comorbidities are still unclear and need to be more emphasized. Our findings raise the need for adequately powered studies with appropriate adjusting for confounding variables and with polysomnography to truly ascertain the attributable risk of OSA with respect to adverse perinatal outcomes.
Author Contributions
T.X. and T.P.L. conceived and designed the study and helped to draft the manuscript. T.X. and Y.F. carried out the search of Embase and Pubmed database and performed the statistical analysis. H.P. and D.Y.G. performed the data collection and extraction and arrangement. All authors reviewed the manuscript.
Acknowledgments
This work was supported by National Science-technology Support Plan Projects of China (2012BAI05B03).The author is grateful to Dr. Zhuang Cui for his thoughtful comments and guidance.
References
- Young T. et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328, 1230–1235 (1993). [DOI] [PubMed] [Google Scholar]
- Bradley T. D. & Floras J. S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 373, 82–93 (2009). [DOI] [PubMed] [Google Scholar]
- Becker H. F. et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 107, 68–73 (2003). [DOI] [PubMed] [Google Scholar]
- Sahota P. K., Jain S. S. & Dhand R. Sleep disorders in pregnancy. Cur Opin Pulm Med 9, 477–483 (2003). [DOI] [PubMed] [Google Scholar]
- Bixler E. O. et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 163, 608–613 (2001). [DOI] [PubMed] [Google Scholar]
- Garcia-Rio F. et al. Sleep apnea and hypertension: the role of peripheral and the sympathetic system. Chest 117, 1417–1425 (2000). [DOI] [PubMed] [Google Scholar]
- Bourjeily G., Raker C. A., Chalhoub M. & Miller M. A. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J 36, 849–855 (2010). [DOI] [PubMed] [Google Scholar]
- Facco F. L. et al. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obster Gynecol 203, 142.e1–5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J. et al. Pregnant women with gestational hypertension may have a high frequency of sleep disordered breathing. Sleep 34, 1033–1038 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meslier N. et al. Impaired glucose-insulin metabolism in males with obstructive sleep apnoea syndrome. Eur Respir J 22, 156–160 (2003). [DOI] [PubMed] [Google Scholar]
- Elmasry A. et al. Sleep-disordered breathing and glucose metabolism in hypertensive men: a population-based study. J Intern Med 249, 153–161 (2001). [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Mawdsley L., Mugarza J. A., Calverley P. M. & Wilding J. P. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J 25, 735–741 (2004). [DOI] [PubMed] [Google Scholar]
- Micheli K. et al. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology 22, 738–744 (2001). [DOI] [PubMed] [Google Scholar]
- Chen Y. H. et al. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obster Gynecol 206, 136.e1–5 (2012). [DOI] [PubMed] [Google Scholar]
- Loube D. I. et al. Self-reported snoring in pregnancy. Association with fetal outcome. Chest 109, 885–889 (1996). [DOI] [PubMed] [Google Scholar]
- Ko H. S. et al. Obstructive sleep apnea screening and perinatal outcomes in Korean Pregant women. Arch Gynecol Obstet 287, 429–433 (2013). [DOI] [PubMed] [Google Scholar]
- Louis J. et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol 120, 1285–1292 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivarea S. A. et al. Obstructive sleep apnea screening in pregnancy, perinatal outcomes, and impact of maternal obesity. Am J Perinatol 28, 651–658 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis J. M., Auckley D., Sokol R. J. & Mercer B. M. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol 202, 261.e1–5 (2011). [DOI] [PubMed] [Google Scholar]
- Sahin F. K. et al. Obstructive sleep apnea in pregnancy and fetal outcome. Int J Gynaecol Obstet 100, 141–146 (2008). [DOI] [PubMed] [Google Scholar]
- McDonald S. D. et al. Preterm birth and low birth weight among in vitro fertilization singletons: A systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol 146, 138–148 (2009). [DOI] [PubMed] [Google Scholar]
- ACOG Practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33. Obstet Gynecol 99, 159–167 (2002). [DOI] [PubMed] [Google Scholar]
- Carpenter M. W. & Coustan D. R. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 144, 768–773 (1982). [DOI] [PubMed] [Google Scholar]
- Kramer M. S. et al. The contribution of mild and moderate preterm birth to infant mortality. Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. 2000;. JAMA 284, 843–849 (2000). [DOI] [PubMed] [Google Scholar]
- Saigal S. & Doyle L. W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371, 261–269 (2008). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558 (2002). [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann C. J. Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J 20, 54–60 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roush S. F. & Bell L. Obstructive sleep apnea in pregnancy. J Am Board Fam Pract 17, 292–294 (2004). [DOI] [PubMed] [Google Scholar]
- Bevan D. R. et al. Closing volume and pregnancy. BrMed J 1, 13–15 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamidi S. et al. Maternal sleep-disordered breathing and adverse pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol 210, 52.e1–52.e14 (2014). [DOI] [PubMed] [Google Scholar]
- Tutdibi E. et al. Impact of labor on outcomes in transient tachypnea of the newborn: population-based study. Pediatrics 125, e577–83 (2010). [DOI] [PubMed] [Google Scholar]
- Franklin K. A. et al. Snoring, pregnancy-induced hypertension, and growth retardation of the fetus. Chest 117, 137–141 (2000). [DOI] [PubMed] [Google Scholar]
- Fung A. M., Wilson D. L., Barnes M. & Walker S. P. Obstructive sleep apnea and pregnancy: the effect on perinatal outcomes. J Perinatol 32, 399–406 (2012). [DOI] [PubMed] [Google Scholar]