Abstract
Background
Early exposure to cow’s milk (CM) proteins have been implicated in the pathogenesis of type 1 diabetes (T1D).
Objective
We analyzed the development of the humoral immune response to dietary CM proteins in early childhood and its relation to later T1D.
Subjects and methods
We studied a subgroup of 94 children randomized to be weaned to a CM-based infant formula in the trial to reduce insulin-dependent diabetes mellitus in the genetically at risk (TRIGR) pilot study. All subjects carried human leukocyte antigen-conferred T1D susceptibility and had an affected first-degree relative. After 7 years of follow-up, 8 subjects had progressed to T1D, 15 had at least one disease-associated autoantibody, and 71 remained autoantibody negative (controls). Immunoglobulin (Ig) G and IgA class antibodies to whole CM formula, beta-lactoglobulin (BLG), bovine serum albumin, and alpha-casein and IgG antibodies to bovine insulin (BI) were measured with enzyme-linked immunosorbent assays from sequential samples.
Results
The children with later T1D showed increased IgG levels to BLG from 3 to 18 months of age (p = 0.028) and enhanced IgA levels to CM formula at the age of 9 months (p = 0.022) compared with controls. In the children with an affected father or sibling, IgG antibodies to BI were higher in autoantibody-positive subjects than in autoantibody-negative subjects at 18 months of age (p = 0.022).
Conclusion
An enhanced humoral immune response to various CM proteins in infancy is seen in a subgroup of those children who later progress to T1D. Accordingly, a dysregulated immune response to oral antigens is an early event in the pathogenesis of T1D.
Keywords: autoantibodies, BLG antibodies, BI, CM proteins, T1D
The role of cow’s milk (CM) as a trigger of the autoimmune process leading to type 1 diabetes (T1D) is supported by epidemiological data showing that early dietary exposure to CM proteins increases the risk of beta-cell autoimmunity and T1D (1–4). Experimental studies in animals have demonstrated that diet modifies the development of autoimmune diabetes, that is, avoidance of CM proteins decreases the risk of diabetes in biobreeding (BB) rats (5) and non-obese diabetic mice (6).
In humans, earlier studies have shown enhanced humoral immune responses to CM proteins, such as beta-lactoglobulin (BLG), casein (CAS), and bovine serum albumin (BSA) in patients with newly diagnosed T1D (7–10). Also, enhanced cellular responses to BLG and dietary wheat gluten have been detected in patients with newly diagnosed T1D (11, 12). These findings suggest that activation of the gut immune system is related to the development of T1D (13). However, the mechanisms as to how CM proteins may be linked to the pathogenic processes are unknown.
Little is known about the natural development of antibody responses to oral antigens in early life. Previously we have observed in healthy children that the oral introduction of CM proteins in early infancy induces both cellular and humoral immune responses against BLG in infants who were exposed to BLG orally in CM formulas. The cellular immune response later decreases supporting the development of oral tolerance (14). Also, exposure to bovine insulin (BI) present in CM formula induces production of immunoglobulin (Ig) G antibodies to BI in infants (15, 16).
In this study, we examined the association between the early development of humoral immune responses to dietary CM proteins and later progression to T1D in children who took part in the trial to reduce insulin-dependent diabetes mellitus in the genetically at risk (TRIGR) pilot study. We measured IgG and IgA class antibodies to whole CM formula, BLG, BSA, and alpha-CAS and IgG antibodies to BI and tetanus toxoid (TT) by using specific enzyme-linked immunosorbent assays (ELISA) in 8 children, who later progressed to clinical T1D, 15 children who developed at least one disease-associated autoantibody, and in 71 children remaining autoantibody negative (controls). We set out to assess whether enhanced humoral immune responses to CM proteins were detectable already in infancy as a marker of an aberrant gut immune system among children who later presented with overt T1D.
Subjects and methods
Subjects
The subjects were derived from the second pilot study of the TRIGR project in Finland, which has been described in detail earlier (17). Briefly, newborn infants with at least one first-degree relative (mother, father, or sibling) with T1D were invited to the study between April 1995 and November 1997, but only individuals at increased genetic risk [human leukocyte antigen (HLA)-DQB1*02/*0302, *0302/x, or *02/y, where x stands for alleles other than *02, *0301,*0602, or *0603, and y stands for alleles other than *0301,*0302, *0602, or *0603] entered the intervention study. The study design was double blind, and infants were randomized to receive, whenever breast milk was not available, either a conventional whey-based adapted CM protein formula (Mead Johnson, Evansville, IL, USA) or an extensively hydrolyzed casein-based intervention formula (Nutramigen; Mead Johnson) until the age of 6–8 months, depending on when the formula was started. The minimum exposure time to the study formula was 2 months according to the study protocol. Exclusive breast-feeding was encouraged. The control formula was supplemented with 20% Nutramigen to mask the flavor and smell distinctions between the two study formulas. During the intervention period, all infant food products containing CM or beef were excluded in the diet of the infants, but the diet of the lactating mothers was not modified.
Because we focused our interest on the early development of an antibody response to CM proteins, we included in the present work those children who were randomized to be weaned to the CM-based infant formula (n = 118). In addition to the cord blood sample, serum samples were obtained from the participants at study center visits at the age of 3, 6, 9, 12, 18 and 24 months and subsequently at the age of 3, 5 and 7 years. In the present series of 118 children, 14 were excluded from the analyses because of incomplete feeding data and 10 because of no exposure of the study formula at all, leaving 94 children in the study.
These 94 participants were split into three groups: subjects diagnosed with T1D by the age of 7 years (n = 8), subjects who developed at least one disease-associated autoantibody reactivity detectable during the observation period without progression to T1D (n = 15), and autoantibody-negative subjects, that is, the control group (n = 71). The median age at diagnosis of T1D was 57 months (range 20–88 months) in the group of children who presented with clinical T1D. Among the 15 autoantibody-positive children, the median age of the appearance of the first autoantibody specificity was 36 months (range 9–84 months). Twelve children tested positive for islet cell antibodies, four for insulin autoantibodies (IAA), three for anti-glutamic acid decarboxylase antibodies, and three for insulinoma-associated antigen-2 antibodies (IA-2A) at least once during the observation period of 84 months. Three subjects tested positive for two autoantibodies, one for three and one for all four. Five of the 15 children became antibody negative during the observation period. The data on gender, T1D in the family and HLA genotypes in the participants is presented in Table 1. Written informed consent was obtained from the mother before enrollment. The study was approved by the Joint Ethics Committees of the participating hospitals in accordance with the Declaration of Helsinki.
Table 1.
Characteristics of children diagnosed with T1D, AAB+, and control children who were randomized to be weaned to a CM-based formula in the trial to reduce insulin-dependent diabetes mellitus in the genetically at risk pilot study
| Characteristics | T1D (n = 8), n (%) | AAB+ (n = 15), n (%) | Controls (n = 71), n (%) |
|---|---|---|---|
| Males | 4 (50) | 8 (53) | 39 (55) |
| T1D in the mother | 1 (12) | 6 (40) | 30 (42) |
| HLA-DQB1 | |||
| *02/*0302 | 3 (37.5) | 3 (20) | 17 (24) |
| *0302/xa | 4 (50) | 5 (33) | 27 (38) |
| *02/yb | 1 (12.5) | 7 (47) | 27 (38) |
AAB+, children who developed at least one disease-associated autoantibody; HLA, human leukocyte antigen; T1D, type 1 diabetes. Values are the number of children, with proportions given in the parentheses. No differences were seen between the groups.
x not DQB1*02, *0301, *0602, *0603.
y not DQB1*0301, *0302, *0602, *0603.
Methods
Disease-associated autoantibodies
Autoantibodies to glutamic acid decarboxylase (GAD65), IA-2, and insulin were measured by specific radiobinding assays, and islet cell antibodies by conventional immunofluorescence, from cord blood samples and subsequent samples taken at 3, 6, 9, 12, 24, 36, 60, and 84 months of age as described (18).
IgG and IgA class antibodies to CM proteins
Blood samples taken from the infants at birth and at the age of 3, 6, 9, 12, 18, 24, and 36 months were analyzed for IgG and IgA class antibodies to CM formula, BLG, BSA, and CAS by using ELISA techniques as previously described (9, 10). The individual levels of antibodies were compared with the standard serum sample with very high levels of CM and BLG antibodies and the levels of antibodies were expressed as arbitrary unit (AU).
ELISA for antibodies to BI
BI-binding IgG antibodies were determined using an ELISA method (19). Polystyrene plates were coated with insulin (1 μg/well) by incubating the plates with BI (Sigma, St Louis, MO, USA) in phosphate-buffered saline (PBS) at 4°C overnight. The plates were washed with Tween 20–PBS (0.05%) and residual coated with 1% human serum albumin (HSA). Samples were diluted 1:20 in PBS containing 0.2% HSA, 0.05% Tween and incubated on the plates at room temperature for 2 h. After washes, alkaline phosphatase-conjugated rabbit antihuman IgG antibodies (Jackson Immunoresearch, West Grove, PA, USA) were used as the secondary antibody and p-nitrophenyl phosphatase tablets (Sigma) as a substrate, and absorbance was read on a spectrophotometer. Results were expressed as optical density (OD) units.
TT antibody assay
Antibodies to TT were measured by ELISA. Maxisorb plates (Nunc, Roskilde, Denmark) were coated overnight at 4°C with TT at a concentration of 1 μg/mL. After washing with 0.05% Tween–PBS, residual coating was performed with 1% HSA–PBS. Plasma samples were diluted at 1:800 in 0.2% HSA 0.05% Tween–PBS and incubated for 2 h at room temperature. Alkaline phosphatase-conjugated rabbit antihuman IgG (Fc) (Jackson Immunoresearch) was diluted 1:3000 in 0.2% HSA 0.05% Tween–PBS and incubated for 90 min at room temperature. After washing with 0.05% Tween–PBS, p-nitrophenyl phosphate (Sigma) was added and after 30 min incubation, the absorbance was read at 405 nm. Results were expressed as OD units.
HLA genotyping
The method for HLA genotyping has been described previously (20, 21). Genotyping was performed on cord blood EDTA samples. HLA-DQB1 and HLA-DQA1 alleles were analyzed by a method developed for screening T1D susceptibility on the basis of the presence of alleles associated with risk or protection against T1D. This two-step screening technique is based on the hybridization of PCR products with lanthanide-labeled probes detected by time-resolved fluorometry.
Statistical analysis
The Kruskal–Wallis test was used as pretest for comparison of three unrelated groups, and the Mann–Whitney U test was applied for comparison of two unrelated groups. The antibody levels are expressed as medians (interquartile range). The Chi-squared test was used to compare different groups with regard to gender, HLA genotypes, and diabetes in the family. The area under the curve (AUC) method was used to compare the CM protein antibody levels between the groups (22). The statistical analyses were performed with SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA). A p value of <0.05 was considered statistically significant.
Results
Characteristics of early infant feeding
No difference was observed in the duration of exclusive breast-feeding (p = 0.97) or total breast-feeding (p = 0.23) between children diagnosed with T1D, subjects who developed at least one disease-associated autoantibody, and autoantibody-negative subjects. In addition, no difference was seen in the age at introduction of CM-based infant formula between the three groups (p = 0.99) (Table 2). A ninety of 94 infants received the formula before the age of 6 months. Three children in the control group were exposed to the CM formula for the first time at the ages of 7, 7.5, and 8 months, and one among the subjects who developed at least one disease-associated autoantibody was exposed to the CM formula for the first time at the age of 6.2 months.
Table 2.
The duration of exclusive and total breast-feeding and the age of introduction of the study formula (medians and ranges in months) among children diagnosed with T1D, AAB+, and control children who were randomized to be weaned to a cow’s milk-based formula in the trial to reduce insulin-dependent diabetes mellitus in the genetically at risk pilot study. No differences were seen between the groups
| T1D (n = 8) | AAB+ (n = 15) | Controls (n = 71) | |
|---|---|---|---|
| Duration of exclusive breast-feeding (months) | 1.4 (0.2–4) | 1 (0.2–6) | 1 (0–5.5) |
| Duration of total breast-feeding (months) | 4.9 (0.2–15) | 4.0 (0.5–14) | 8.0 (0.2–23) |
| Age of introduction of study formula (months) | 1.4 (0.2–5) | 1 (0.2–6.2) | 1.1 (0–8) |
AAB+, children who developed at least one disease-associated autoantibody; HLA, human leukocyte antigen; T1D, type 1 diabetes.
IgG and IgA antibody levels to CM proteins
The IgA antibody levels to CM formula differed at the age of 9 months between the three study groups [3.14 (1.59–9.64), 3.38 (0.18–15.62), and 0.97 (0.27–2.13), p = 0.024]. The IgA antibody levels to CM formula were increased in children diagnosed with T1D and tended to be higher in children who developed at least one disease-associated autoantibody compared with the controls (p = 0.022 and p = 0.068, respectively). IgA class antibody levels to total CM formula at the age of 9 months are shown in Fig. 1.
Fig. 1.
Immunoglobulin A antibody levels to cow’s milk formula at the age of 9 months in children who later progressed to type 1 diabetes (T1D), children who developed at least one disease-associated autoantibody (AAB+) during 7 years follow-up, and autoantibody-negative subjects, that is, the control group. The horizontal lines represent median values and p values derived from the Mann–Whitney U test are shown in the figure. CM, cow’s milk; NS, not significant.
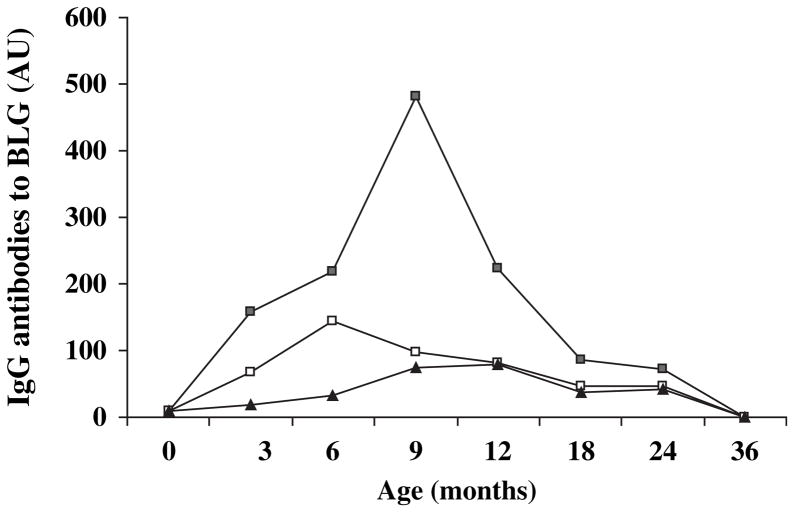
The IgG antibody levels to BLG were higher in children diagnosed with T1D compared with children who developed at least one disease-associated antibody and with the controls at 6 months [218.5 (36–444), 143 (19.5–812), and 32.0 (4–137.5), p = 0.035, respectively], but not at other time points. IgG antibody levels to BLG were higher in children with subsequent T1D compared with controls (p = 0.049). At the age of 3 months, the medians were 158.5, 68.3, and 19.2, respectively (p = 0.22), at 9 months 481.8, 98.8, and 75.0 (p = 0.08), at 12 months 223, 81.5, and 78.4 (p = 0.34), at 18 months 86.9, 47, and 38 (p = 0.47), and at 24 months 71.8, 45.5, and 41.1 (p = 0.62). In addition, based on the AUC analysis, the IgG antibody levels to BLG were increased during the time period from 3 to 18 months of age among the children who later progressed to T1D compared with the controls (p = 0.028) (Fig. 2).
Fig. 2.
Median levels of immunoglobulin G antibodies to beta-lactoglobulin (BLG) at the age of 0, 3, 6, 9, 12, 18, 24, and 36 months in children diagnosed with type 1 diabetes (■), children who developed at least one disease-associated antibody (□), and antibody-negative subjects, that is, the control group (▲). The IgG antibody levels to BLG were increased during the time period from 3 to 18 months of age among the children who later developed to T1D compared with the controls (comparison of the area under the curve; p = 0.028).
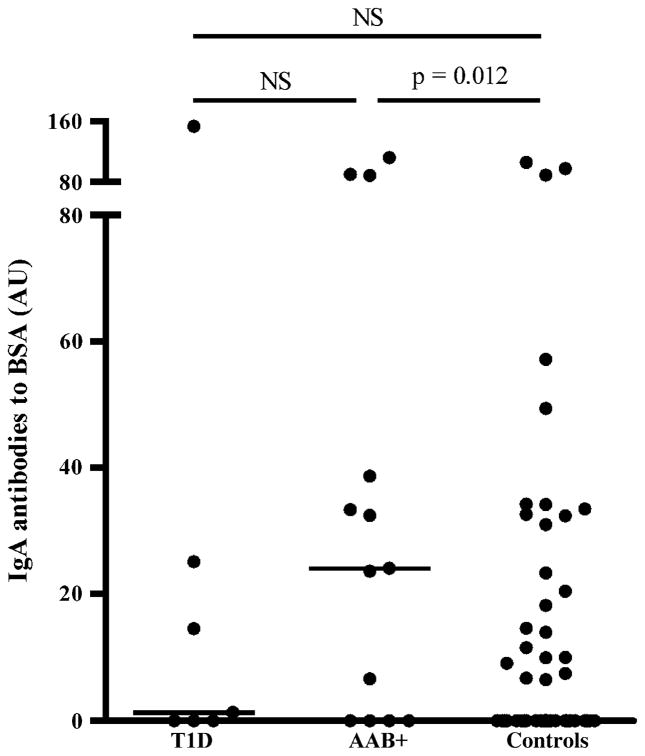
The IgA antibody levels to BSA differed at the age of 9 months between the groups [1.3 (0–25.1), 24.1 (0–63.6), and 0 (0–14), respectively, p = 0.037]. The IgA antibody levels to BSA were increased in children who developed at least one disease-associated antibody compared with the controls (p = 0.012; Fig. 3). The IgG and IgA levels to CAS, IgG levels to CM and BSA, and IgA levels to BLG did not differ between the groups during the follow-up period up to the age of 36 months.
Fig. 3.
Immunoglobulin A antibody levels to bovine serum albumin (BSA) at the age of 9 months in children who later progressed to type 1 diabetes (T1D), children who developed at least one disease-associated autoantibody (AAB+) during 7 years follow-up, and autoantibody-negative subjects, that is, the control group. The horizontal lines represent median values and p values derived from the Mann–Whitney U test are shown in the figure. NS, not significant.
Antibody production to BI
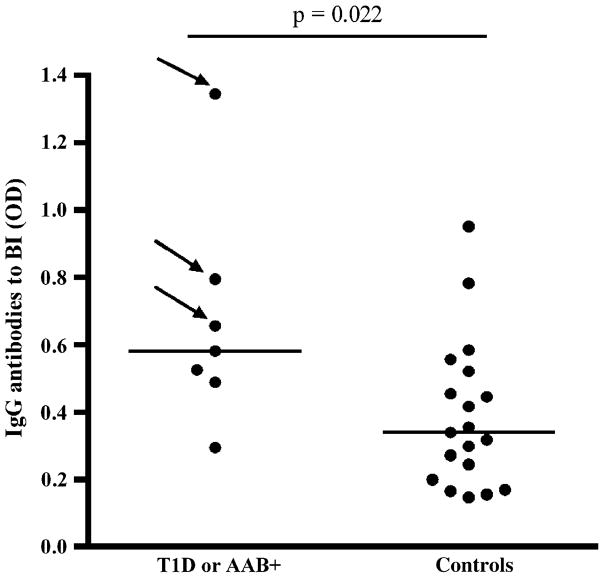
As offspring of mothers with T1D have transplacental transfer of insulin antibodies, children with a diabetic mother were excluded from the analysis of IgG antibodies to BI, and we analyzed only the children with a diabetic father or sibling. The IgG antibody levels binding to BI were higher at 18 months of age in children who developed at least one disease-associated autoantibody compared with autoantibody-negative subjects [0.58 (0.49–0.79) vs. 0.34 (0.20–0.52), p = 0.022, Fig. 4]. All the infants were exposed to CM formula before the age of 3 months.
Fig. 4.
Immunoglobulin G antibody levels to bovine insulin (BI) at the age of 18 months in subjects with a diabetic father or sibling among children who developed at least one disease-associated autoantibody [type 1 diabetes (T1D) or AAB+] during 7 years follow-up and among autoantibody-negative children, that is, the control group. The horizontal lines represent median values and p values derived from the Mann–Whitney U test are shown in the figure. The children who later progressed to T1D are marked with an arrow.
Antibody levels to TT
The antibody levels to TT differed at the age of 12 months between the groups [1.46 (0.54–1.63), 0.53 (0.35–0.69), and 0.79 (0.48–1.11), respectively, p = 0.024]. The levels were decreased in children who developed at least one disease-associated antibody compared with children with T1D or controls (p = 0.031 and p = 0.038, respectively).
Discussion
In this study, we demonstrated an enhanced reactivity to dietary CM proteins already in infancy in children who later progressed to clinical T1D. Accordingly, children later affected by T1D respond to at least some early dietary antigens differently from children with similar HLA-conferred susceptibility to T1D who remain non-diabetic. We suggest that the stronger immune reactivity to oral antigens during infancy is because of increased gut permeability and/or delayed maturation of the gut immune system in children who later develop T1D.
In our study, the IgG levels to BLG decreased by the age of 2 years in all children who received the CM-based formula before the age of 6–8 months. This is in agreement with earlier reports showing that feeding with CM formula leads in healthy children to stimulation of the humoral immune response to CM proteins that is most conspicuous when CM proteins are given in early infancy (23). Also the T-cell responses to CM proteins later declined, implying the development of oral tolerance, which is established along with increasing age in healthy children (14, 23). The introduction of dietary CM proteins in infancy is a strong immunogenic stimulus and seems to be associated with inflammatory changes too. Exposure to CM is associated with transient increase in the permeability of the gut (24) and unspecific general stimulation of the immune system reflected by, for example, increased levels of soluble intercellular adhesion molecule (ICAM)-1 in infants fed with CM-based formulas when compared with infants fed with a highly hydrolyzed formula (25).
The increased levels of antibodies to BLG and other CM proteins in early life in children with later T1D may be associated with increased gut permeability. Increased intestinal permeability has indeed been shown in patients with T1D (26, 27) and prediabetes (28). Furthermore, signs of enhanced immune activation, such as increased expression of major histocompatibility complex class II antigens, ICAM-1, and cytokines have been observed in the intestine of children with T1D (29). Interestingly, increased permeability occurs transiently in BB rats, an animal model of human T1D, early in life and precedes the development of autoimmune diabetes (30). Altogether, we implicate that the small intestine is involved in the T1D disease process, and aberrancies in handling the dietary antigens are seen already before the clinical disease.
The results of the TRIGR pilot study in which hydrolyzed formula was used during the first 6–8 months of life showed that elimination of CM protein during infancy decreased the development of beta-cell autoimmunity (17). This suggests that the elimination of CM proteins during early infancy, that is, postponing exposure to CM, might result in a reduced risk of T1D. It is possible that CM contains a diabetogenic antigen, for example, BI, or early introduction of CM induces unspecific stimulation of the immune system that contributes to the risk of T1D in the infants with slow intestinal maturation and genetic risk.
We have earlier reported that dietary BI induces antibodies in infants and the levels of antibodies binding to BI detected by ELISA were increased during the first 2 years of life in children who developed at least two diabetes-associated autoantibodies. In contrast, the levels tended to decrease during the follow-up in autoantibody-negative children (16). This is in accordance with our finding here showing that the IgG-binding antibodies to BI were increased at the age of 18 months in children with disease-associated autoantibodies compared with autoantibody-negative subjects. Antibody levels to BI were analyzed in children with an affected father or an affected sibling, because insulin antibodies are transferred to the fetal circulation in offspring of T1D mothers with such antibodies to exogenous insulin (31), and these antibodies have been shown earlier to modulate the response to dietary insulin in infants (19).
To rule out the possibility that increased response to CM proteins is a consequence of a general hyperimmune state related to the autoimmune process and T1D, we studied the level of IgG antibodies to TT. The children participating in the TRIGR pilot study in Finland were vaccinated according to the national vaccination program: the pertussis–diphtheria–tetanus vaccine was given at the age of 3, 4, and 5 months, the Hemophilus influenzae vaccine at 4 and 6 months of age, and poliovirus at the age of 6 and 12 months. In our study, decreased levels of antibodies to TT were seen at the age of 12 months in subjects who were auto-antibody positive when compared with those later diagnosed with T1D or controls. The significance of this finding remains open. In contrast to TT antibodies, antibodies to different dietary antigens were increased systemically in children at risk for T1D, which strengthens the observation of aberrant oral immune responses in such children.
In this study, there was no difference in the antibody levels to CM proteins according to HLA risk alleles for T1D (data not shown). Previously it has been reported that children with T1D carrying either HLA DR3 or DR4 had similar levels of BLG and CM antibodies (7). Enterovirus infections or other infections may have interactions with dietary risk factors because they might change the local cytokine environment in the gut or the permeability of the gut (32). In our study, there was no difference between the groups in the levels of C-reactive protein (CRP) concentrations that were determined with an ultrasensitive CRP assay (data not shown). Thus, infection as explanation for the increased antibody levels to dietary proteins was not supported by the CRP levels.
In conclusion, these findings suggest that early introduction of CM proteins results in aberrant humoral immune responses to various CM proteins in children who later progress to T1D when compared with HLA-matched unaffected controls. This implies that a dysregulated immune response to dietary antigens is an early event in the pathogenesis of T1D, and may explain why early exposure to CM proteins is a risk factor for T1D only in a minority of children, that is, among those with a dysfunctional gut immune system.
Acknowledgments
This study was supported by grants from Academy of Finland, European Commission (BMH4-CT96-0233), the Juvenile Diabetes Foundation International (File # 195003), Helsinki University Central Hospital, University of Helsinki, Finnish Diabetes Research Foundation, the Novo Nordisk Foundation, Medical Research Foundation of Tampere University Hospital, The Dorothea Olivia, Karl Walter and Jarl Walter Perklén Foundation, and the Liv och Hölsa Fund.
We are grateful to Marja Salonen, Tarja Tenkula, Anne Björk, and Kristiina Merentie, for excellent work in the project office and with the study subjects. We thank Sirpa Anttila, Susanna Heikkilä, Sirpa Pohjola, Riitta Päkkilä and Päivi Salmijärvi for their skilful technical assistance in the autoantibody assays and Terttu Louhio for skillful technical assistance in measuring levels of CM antibodies. In addition, we thank Matti Koski, for assistance in database work. We also thank all the local study nurses and dietary advisors and participating families for good collaboration.
References
- 1.Virtanen SM, Räsänen L, Ylönen K, et al. Early introduction of dairy products associated with increased risk of IDDM in Finnish children. Diabetes. 1993;42:1786–1790. doi: 10.2337/diab.42.12.1786. [DOI] [PubMed] [Google Scholar]
- 2.Åkerblom HK, Knip M. Putative environmental factors in type 1 diabetes. Diabetes Metab Rev. 1998;14:31–67. doi: 10.1002/(sici)1099-0895(199803)14:1<31::aid-dmr201>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Kimpimäki T, Erkkola M, Korhonen S, et al. Short-term exclusive breastfeeding predisposes young children with increased genetic risk of type 1 diabetes to progressive beta-cell immunity. Diabetologia. 2001;44:63–69. doi: 10.1007/s001250051581. [DOI] [PubMed] [Google Scholar]
- 4.Virtanen SM, Knip M. Nutritional risk predictors of beta-cell autoimmunity and type 1 diabetes at a young age. Am J Clin Nutr. 2003;78:1053–1067. doi: 10.1093/ajcn/78.6.1053. [DOI] [PubMed] [Google Scholar]
- 5.Elliott RB, Martin JM. Dietary protein: a trigger of insulin-dependent diabetes in the BB rat? Diabetologia. 1984;26:297–299. doi: 10.1007/BF00283653. [DOI] [PubMed] [Google Scholar]
- 6.Elliott RB, Reddy SN, Bibby NJ, Kida K. Dietary prevention of diabetes in the non-obese diabetic mouse. Diabetologia. 1988;31:62–64. doi: 10.1007/BF00279136. [DOI] [PubMed] [Google Scholar]
- 7.Savilahti E, Åkerblom HK, Tainio VM, Koskimies S. Children with newly diagnosed insulin dependent diabetes mellitus have increased levels of cow’s milk antibodies. Diabetes Res. 1988;7:137–140. [PubMed] [Google Scholar]
- 8.Dahlquist G, Savilahti E, Landin-Olsson M. An increased level of antibodies to beta-lactoglobulin is a risk determinant for early-onset type 1 (insulin-dependent) diabetes mellitus independent of islet cell antibodies and early introduction of cow’s milk. Diabetologia. 1992;35:980–984. doi: 10.1007/BF00401429. [DOI] [PubMed] [Google Scholar]
- 9.Savilahti E, Saukkonen TT, Virtala ET, Tuomilehto J, Åkerblom HK The Childhood Diabetes in Finland (DiMe) Study Group. Increased levels of cow’s milk and beta-lactoglobulin antibodies in young children with newly diagnosed IDDM. Diabetes Care. 1993;16:984–989. doi: 10.2337/diacare.16.7.984. [DOI] [PubMed] [Google Scholar]
- 10.Saukkonen T, Savilahti E, Vaarala O, et al. Children with newly diagnosed insulin-dependent diabetes mellitus have increased levels of antibodies to bovine serum albumin but not to ovalbumin. Diabetes Care. 1994;17:970–976. doi: 10.2337/diacare.17.9.970. [DOI] [PubMed] [Google Scholar]
- 11.Vaarala O, Klemetti P, Savilahti E, Reijonen H, Ilonen J, Åkerblom HK. Cellular immune response to cow’s milk beta-lactoglobulin in patients with newly diagnosed IDDM. Diabetes. 1996;45:178–182. doi: 10.2337/diab.45.2.178. [DOI] [PubMed] [Google Scholar]
- 12.Klemetti P, Savilahti E, Ilonen J, Åkerblom HK, Vaarala O. T-cell reactivity to wheat gluten in patients with insulin-dependent diabetes mellitus. Scand J Immunol. 1998;47:48–53. doi: 10.1046/j.1365-3083.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- 13.Vaarala O. Gut and induction of immune tolerance in type 1 diabetes. Diabetes Metab Res Rev. 1999;15:353–361. doi: 10.1002/(sici)1520-7560(199909/10)15:5<353::aid-dmrr59>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Vaarala O, Saukkonen T, Savilahti E, Klemola T, Åkerblom HK. Development of immune response to cow’s milk proteins in infants receiving cow’s milk or hydrolyzed formula. J Allergy Clin Immunol. 1995;96:917–923. doi: 10.1016/s0091-6749(95)70229-6. [DOI] [PubMed] [Google Scholar]
- 15.Vaarala O, Paronen J, Otonkoski T, Åkerblom HK. Cow milk feeding induces antibodies to insulin in children—a link between cow milk and insulin-dependent diabetes mellitus? Scand J Immunol. 1998;47:131–135. doi: 10.1046/j.1365-3083.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- 16.Vaarala O, Knip M, Paronen J, et al. Cow’s milk formula feeding induces primary immunization to insulin in infants at genetic risk for type 1 diabetes. Diabetes. 1999;48:1389–1394. doi: 10.2337/diabetes.48.7.1389. [DOI] [PubMed] [Google Scholar]
- 17.Åkerblom HK, Virtanen SM, Ilonen J, et al. Dietary manipulation of beta cell autoimmunity in infants at increased risk of type 1 diabetes: a pilot study. Diabetologia. 2005;48:829–837. doi: 10.1007/s00125-005-1733-3. [DOI] [PubMed] [Google Scholar]
- 18.Kukko M, Virtanen SM, Toivonen A, et al. Geographic variation in risk HLA-DQB1 genotypes for type 1 diabetes and signs of beta-cell autoimmunity within a high incidence country. Diabetes Care. 2004;27:676–681. doi: 10.2337/diacare.27.3.676. [DOI] [PubMed] [Google Scholar]
- 19.Paronen J, Knip M, Savilahti E, et al. Effect of cow’s milk exposure and maternal type 1 diabetes on cellular and humoral immunization to dietary insulin in infants at genetic risk for type 1 diabetes. Diabetes. 2000;49:1657–1665. doi: 10.2337/diabetes.49.10.1657. [DOI] [PubMed] [Google Scholar]
- 20.Ilonen J, Reijonen H, Herva E, et al. Rapid HLA-DQB1 genotyping for four alleles in the assessment of risk for IDDM in the Finnish population. Diabetes Care. 1996;19:795–800. doi: 10.2337/diacare.19.8.795. [DOI] [PubMed] [Google Scholar]
- 21.Sjöroos M, Itiä A, Ilonen J, Reijonen H, Lövgren T. Triple-label hybridization assay for type-1 diabetes-related HLA alleles. BioTechniques. 1995;18:870–877. [PubMed] [Google Scholar]
- 22.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tainio VM, Savilahti E, Arjomaa P, Salmenperä L, Perheentupa J, Siimes MA. Plasma antibodies to cow’s milk are increased by weaning and consumption of unmodified milk, but production of plasma IgA and IgM cow’s milk antibodies is stimulated even during exclusive breast-feeding. Acta Paediatr Scand. 1988;77:807–811. doi: 10.1111/j.1651-2227.1988.tb10760.x. [DOI] [PubMed] [Google Scholar]
- 24.Catassi C, Bonucci A, Coppa GV, Carlucci A, Giorgi PL. Intestinal permeability duting the first month: effect of natural versus artificial feeding. J Pediatr Gastroenterol Nutr. 1995;21:383–386. doi: 10.1097/00005176-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Paronen J, Vaarala O, Savilahti E, Saukkonen T, Åkerblom HK. Soluble adhesion molecules and oral antigen feeding in infants. Pediatr Res. 1996;40:276–279. doi: 10.1203/00006450-199608000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Mooradian AD, Morley JE, Levine AS, Prigge WF, Gebhard RL. Abnormal intestinal permeability to sugars in diabetes mellitus. Diabetologia. 1986;29:221–224. doi: 10.1007/BF00454879. [DOI] [PubMed] [Google Scholar]
- 27.Carratu R, Secundulfo M, De Magistris L, et al. Altered intestinal permeability to mannitol in diabetes mellitus type 1. J Pediatr Gastroenterol Nutr. 1999;28:264–269. doi: 10.1097/00005176-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Bosi E, Molteni L, Radaelli MG, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49:2824–2827. doi: 10.1007/s00125-006-0465-3. [DOI] [PubMed] [Google Scholar]
- 29.Westerholm-Ormio M, Vaarala O, Pihkala P, Ilonen J, Savilahti E. Immunologic activity in the small intestine mucosa of pediatric patients with type 1 diabetes. Diabetes. 2003;52:2287–2295. doi: 10.2337/diabetes.52.9.2287. [DOI] [PubMed] [Google Scholar]
- 30.Neu J, Reverte CM, Mackey AD, et al. Changes in intestinal morphology and permeability in the biobreeding rat before the onset of type 1 diabetes. J Pediatr Gastroenterol Nutr. 2005;40:589–595. doi: 10.1097/01.mpg.0000159636.19346.c1. [DOI] [PubMed] [Google Scholar]
- 31.Hämäläinen AM, Savola K, Kulmala PK, et al. Disease-associated autoantibodies during pregnancy and at birth in families affected by type 1 diabetes. Clin Exp Immunol. 2001;126:230–235. doi: 10.1046/j.1365-2249.2001.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyöty H, Hiltunen M, Knip M, et al. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Diabetes. 1995;44:652–657. doi: 10.2337/diab.44.6.652. [DOI] [PubMed] [Google Scholar]