Abstract
The incidence of type 1 diabetes has been increasing rapidly among children in most European countries over the last decades. Despite of the known strong genetic component in the disease only environmental factors can explain such a rapid change. The increase in incidence has been most conspicuous in the youngest age group, which emphasizes the importance of infancy and early environmental exposures. Nutritional and infectious factors affecting the young child or even the mother during pregnancy have been implicated to be important in the pathogenesis. The identification of single factors has been extremely difficult as reflected by many controversial reports on their importance. This difficulty may also be due to the heterogeneity of the disease mechanisms. Multiple mechanisms in different pathways may ultimately be responsible for beta-cell destruction. In most cases the disease is probably caused by a complex interplay between multiple factors including distinct genetic polymorphisms and environmental effects. Exploration of these pathways is needed for the development of effective preventive measures. The implementation of primary prevention trials will ultimately prove the value of various concepts generated for the disease pathogenesis.
Keywords: type 1 diabetes, primary prevention trial, infant nutrition
Introduction
Type 1 diabetes (T1D) is one of the most common chronic diseases starting in childhood. Although insulin treatment has become more and more sophisticated, T1D is still associated with increased morbidity due to disease-related complications affecting the cardiovascular, renal and nervous systems, which also shorten the life expectancy of affected subjects. The disease causes a considerable burden to the patients and their families and increases healthcare costs considerably in the society. The rapid increase in disease incidence during the last decades in the Western world reflects the importance of a changing environment (1). For example in Finland the incidence of T1D in children is now more than five-fold higher than that reported 55 years ago (2, 3). The mechanisms and aetiological factors causing the disease are still unresolved and poorly known. One might expect that by understanding these mechanisms also preventive measures could be developed to reduce the numbers of affected individuals – on the other hand successful prevention trials will eventually prove the value of the hypotheses created.
Pathogenesis of type 1 diabetes
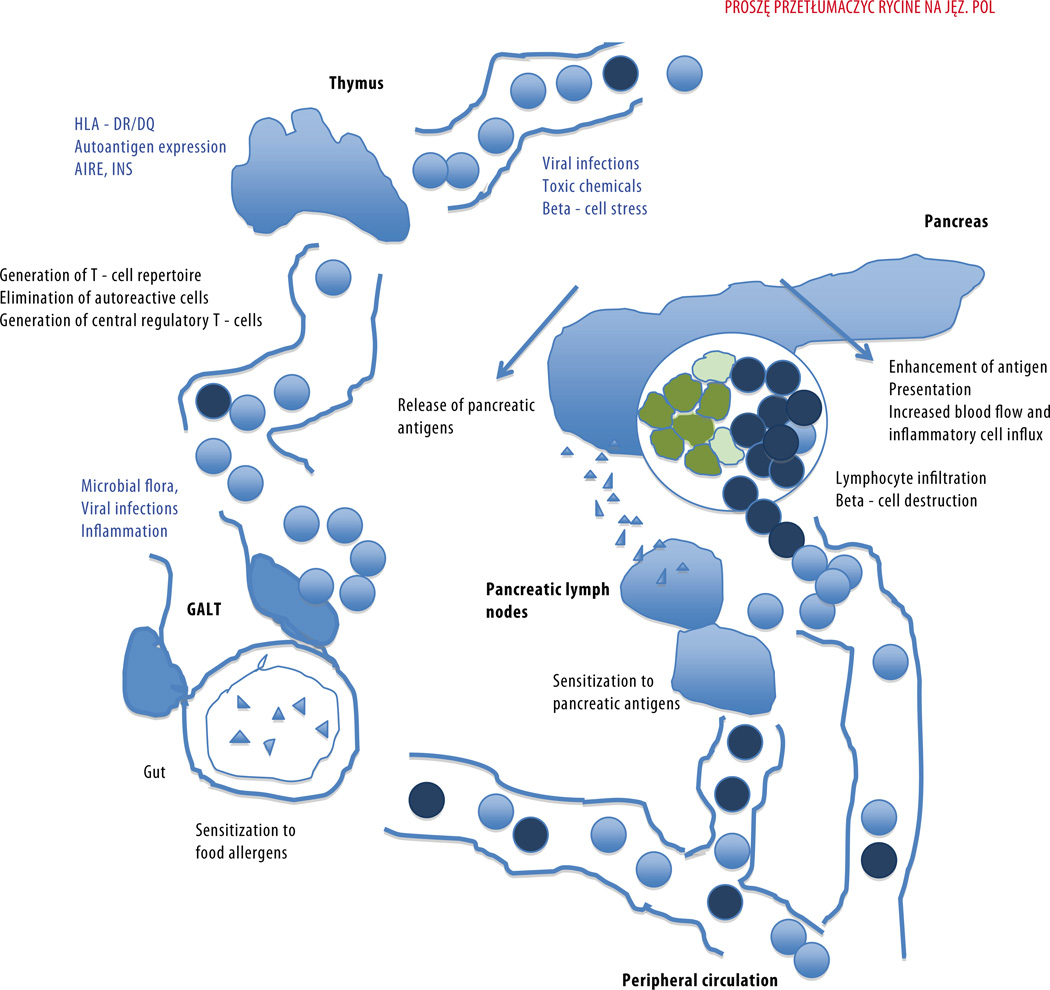
Metabolic derangement in T1D is caused by failing insulin secretion due to the destruction of the beta cells in the pancreatic islets. In a majority of the cases this destruction is caused by an immune-mediated process reflected histologically by the lymphocytic infiltration into the islets, i.e. the insulitis (4). The majority of the infiltrating lymphocytes are CD8 positive, apparently cytotoxic T cells. They are accordingly regarded as central effectors but their generation requires the presentation of islet-specific antigens to CD4 positive helper T-cells by dendritic cells. Apparently this process takes place in lymph nodes draining the pancreatic tissue. Various environmental determinants may exert their influence either on the initiation of the immune-mediated process by destroying beta cells and releasing their intracellular molecules to be recognized by immune cells or by enhancing the initiated autoimmune response later on. This enhancement may be associated with inflammation-linked effects. Infection-associated inflammation caused by virus induced mediators such as interferon also increase the expression of cell surface components like HLA and adhesion molecules needed in immune recognition, increase vascular permeability and attract inflammatory cells.
Although autoimmune destruction is the major mechanism in beta-cell damage, rare cases of T1D without autoimmune markers have been described especially in Far East. Very rapid beta-cell destruction without autoimmune markers in so called fulminant T1D is suggested to be caused by a virus infection (5).
Environmental factors operative in early life
The increase in incidence of T1D has been steepest among children under the age of 5 years. It has also been observed that autoantibodies appearing during the first years of life have the highest predictive value (6). Accordingly a lot of attention has been focused on infancy when the maturation of the immune system is taking shape in interaction with the microbial world. The fundamental role of gut microbiota has been recently reviewed in this process (7). The so called hygiene hypothesis was established to explain the increased incidence of allergy and atopy in the Western world but has more recently been expanded to also explain the increase seen in autoimmune diseases, such as type 1 diabetes (8). According to this hypothesis the lack of microbial contacts in early life needed to stimulate the developing immune system results in susceptibility to allergy and autoimmunity. The exact nature of useful microbial stimuli is still poorly characterized but children raised in a rural environment in contact with farm animals seem to be relatively protected from allergy (9). The concept of useful microbes has been experimentally tested in allergy using a variety of probiotic bacteria with some preventive effect reported (10).
The introduction of food and the dietary composition in infancy has been intensively studied and discussed. The initial stimulus for these studies was a report in 1984 describing an inverse association between the duration of breastfeeding and the incidence of T1D (11). A short breastfeeding time is naturally strongly associated with early introduction of infant formula, which is mainly produced from cow’s milk in developed countries. Controversial results have since been obtained when the effect of early introduction of cow’s milk and/or the duration of breast-feeding has been studied in relation to the emergence of signs of beta-cell autoimmunity. A meta-analysis concluded that the effect of early introduction of cow’s milk was primary, and that the relatively weak predisposing effect detected is a true one (12), while a subsequent meta-analysis questioned such an effect (13). The role of cow’s milk in the development of T1D has also been supported by findings of increased antibody levels to various constituents of cow’s milk in subjects with preclinical and clinical T1D (14–16). However, increased immune responses have as well been described to other food components such as wheat proteins possibly indicating a deviation in gut-specific immune responses covering a large spectrum of food antigens (17–19).
The possible mechanisms explaining the association between cow’s milk and diabetes may be multiple. Various components such as bovine serum albumin and bovine insulin have been implicated to be capable of inducing immune responses cross-reacting with autologous molecules (20, 21). The introduction of cow’s milk-based formula into infant nutrition has been shown to induce antibodies recognizing both bovine and human insulin by an EIA assay (22). Antigenic stimulation by food proteins may be deleterious also as such in an immature gut. Cow’s milk-based formula has been shown to increase the levels of circulating soluble adhesion molecules compared to hydrolyzed formula (23). The replacement of normal formula with a hydrolysate after weaning has been shown to effectively reduce diabetes in the NOD mouse model (24).
Other factors may also affect the type of immunisation. Enteral virus infections at an early age can enhance immunisation to nutritional molecules as demonstrated by increased levels of antibodies against food antigens in children with rotavirus infection at the time of the introduction of these foods (Ahmed 1998). Early rotavirus infections also increase the humoral autoimmune response to insulin induced by bovine insulin in cow’s milk-based-formula (25).
Heterogeneity in pathogenesis
It is likely that beta-cell destruction can be caused by various mechanisms, also within the entity of autoimmune or type 1A diabetes. It is remarkable that various autoantibodies are prone to appear in defined age groups and to show distinct genetic correlations (26–28). Insulin autoantibodies have been detected at a higher frequency in young children with T1D, and in follow-up studies of high-risk children they most often are the first autoantibody specificity to appear (29). In contrast, GAD antibodies are the most common autoantibody reactivity observed in older children and adult patients with T1D. IA-2 antibodies usually appear later than the other autoantibodies, and their presence is associated with a very high disease risk. The phenomenon of antigen spreading, which may be of essential importance in the pathogenesis of autoimmune disease, is confusing the distinct patterns of the appearance of specific autoantibodies. Antigenic spreading implies, that the specificity of the autoimmune response is widening. Initially only one autoantigen is recognized by the immune system, but later on, autoantibodies or specific T cells to several autoantigens in the same tissue are seen. The first autoantibody might still reflect the specific environmental effect causing e.g. the autoimmune response due to molecular mimicry between e.g. food or microbial antigens and an autoantigen. Follow-up studies in cohorts of children at increased genetic risk for T1D like DAISY, DIPP and TEDDY will undoubtedly provide more exact data on these phenomena.
Genetic factors affecting various pathways
It is well known, that genetic susceptibility is of utmost importance in T1D (30). Genes within the HLA gene complex are important determinants of disease risk like in many other autoimmune diseases. The HLA association is conspicuously strong but it is also strikingly complex. HLA-DR-DQ haplotypes are the major determinants, haplotypes associated with various degree of both susceptibility and protection can be identified. Among the high-risk Finnish population the disease risk in childhood defined by these haplotypes varies between 10% and 0.03% in groups including 2% and 49%, respectively, of the total population. Even within the HLA gene region alleles within the class I locus seem also to modify the disease risk as well as several poorly defined loci. In addition to HLA currently at least 40 non-HLA loci are associated with T1D (31). Insulin, PTPN22, CTLA-4 and IL-2A are among the best known and strongest risk genes. Although each non-HLA gene is associated with a relatively weak effect, their combined effect on disease susceptibility is approximately of the same magnitude as the estimated HLA effect.
HLA molecules are important in antigen presentation and define which antigen derived peptides are presented to T cells in various phases of the immune response. CD4 positive helper T cells recognize their antigenic peptide in the context of class II molecules and cytotoxic effector CD8 cells in the context of class I molecules. This emphasizes the importance of various HLA alleles in molecular mimicry as specific cross reactions between autologous and microbial or other environmental antigens are allele specific (32). HLA molecules play also a central role in the generation of the T-cell repertoire in the thymus where 90% of the precursor cells are destroyed because of too low or too high affinity to complexes of HLA and autologous peptides. Interestingly the expression of HLA-DQ molecules associated most strongly with T1D susceptibility is high in thymus. The same selection affects the development of central regulatory T cells. Ectopic expression of several peripheral molecules in the thymus is important for tolerance formation as seen in the rare APS-1 syndrome combining Addison disease, hypoparathyroidism and mucocutaneous candidiasis and quite often also including T1D and hypothyroidism. The syndrome is caused by mutations in the AIRE gene necessary for thymic expression of autologous molecules (33). The disease-associated alleles of the insulin gene have also been connected to lower level of thymic expression of insulin and thereby to a weaker capacity for tolerance induction (34).
Specific HLA genotypes are known to be associated with specific immunological features, the HLA-DR4-DQ8 haplotype with the presence of insulin and IA-2 autoantibodies at disease manifestation whereas the HLA-DR3-DQ2 haplotype is associated with GAD autoantibodies (27, 35). HLA-DR4, the insulin gene and PTPN22 risk alleles also appear to be associated with the autoimmunity characterized by insulin autoantibodies as the first autoantibody to appear in young children (36, 37). We have observed that the PTPN22 risk allele was increased only among autoantibody-positive children who had received cow’s milk-based formula before the age of 6 months implicating a gene-environmental interaction (38). There are some results suggesting that HLA-associated risk is required for the effect of cow’s milk induced formula on T1D risk to emerge but on the other hand studies based on HLA-risk selected follow-up cohorts have not been able to confirm any effect of early introduced cow’s milk formula on subsequent risk for progression to T1D (39–41).
Possibilities of primary prevention
Prevention of T1D can target various phases of the long period preceding clinical disease. Primary prevention refers to efforts started very early, before the appearance of diabetes-associated autoantibodies as a marker of an ongoing autoimmune process. It may even be directed to the mother during pregnancy if events during foetal life can be shown to affect the risk of diabetes later on. Studies exploring this possibility have suggested e.g. that the consumption of cod liver oil by the mother during pregnancy might be protective or identified viral infections during pregnancy as risk factors for subsequent diabetes (42–44). The intervention in the NIP trial testing the beneficial effect of supplemention with omega-3 fatty acids is also initiated during pregnancy, and probiotics used in allergy prevention studies have often been initiated during pregnancy as the colonization of the newborn infant is largely defined by maternal microbiota in the birth channel and gut (45).
The potential importance of early diet in infancy is the background of several dietary interventions. Interestingly animal models have not produced clear answers to questions related to the potential diabetogenicity of single food constituents but reduction of spontaneous autoimmune diabetes in NOD mice has been obtained by hydrolyzed formulas in which antigenic proteins have been digested into smaller peptides. Weaning to such a diet will prevent the early immunisations to constituents in cow’s milk that otherwise might lead to cross-reactions through molecular mimicry, and it will also prevent non-specific inflammatory reactions in the gut associated with the early introduction of cow’s milk-based formula or actually with any type of food containing foreign molecules.
There are several dietary intervention studies ongoing or planned (tab. I). The largest ongoing study is the Trial to Reduce IDDM in the Genetically at Risk (TRIGR), which compares weaning to highly hydrolyzed formula to a conventional formula (46, 47). The duration of the TRIGR intervention is until the age of at least 6 months with the goal of a minimum daily formula exposure time of 2 months. The full-scale study started in 2002 and 2160 newborn infants with an affected first-degree relative and a risk-associated HLA genotype were recruited. This multicenter study is run in 12 European countries, Australia, Canada and USA. The recruitment phase of the study was completed by February 2007, and the first endpoint, i.e. positivity for at least two diabetes-associated autoantibodies and/or clinical diabetes by the age of 6 years will be reached in February 2013 when all study participants have been observed for at least 6 years. The final outcome assessment will be based on the development of clinical diabetes by the age of 10 years. The study has been designed by the expectation that about 10% of the children will develop multiple autoantibodies by the age of 6 years, and the study was planned to have 80% power to detect a reduction of 40% in the appearance of these autoantibodies. A drop-out rate of 20% and a frequency of 10% of exclusive breast feeding up to the age of 6 months are expected. In addition to the measurement of diabetes-associated autoantibodies (ICA, IAA, GADA and IA-2A) antibodies to cow’s milk proteins will be analyzed and used as a marker of compliance in secondary analyses which will be performed after the primary analysis based on the intention to treat principle. The primary comparison between treatment arms will be performed using the Kaplan-Meier method and long-rank statistics with respect to time until beta-cell autoimmunity or T1D develops. Secondary analyses will take into account milk exposure as a potential confounding factor. A number of subgroup analyses are planned to help identify individuals more likely to benefit from the treatment. Such subgroups include gender, gender of relative with T1D, genetic risk group etc.
Table I.
Examples of ongoing primary prevention studies
| Study Badanie |
Intervention Interwencja | Present stage / Stan obecny | Study group / Grupa badana | References Literatura |
|---|---|---|---|---|
| TRIGR | cow’s milk-based hyrdrolyzed formula mieszanka hydrolizowana na bazie mleka krowiego | full scale study proper ongoing prowadzone badania właściwe w pełnym zakresie | 2160 HLA – screened infants with a first-degree relative with T1D | 46, 47 |
| 2160 niemowląt z krewnymi I stopnia z cukrzycą typu 1 poddane skriningowi | ||||
| BABYDIET | postponement of gluten introduction opóżnione wprowadzenie glutenu | pilot study completed zakończone badania pilotażowe | 50 HLA – screened infants with a first-degree relative with T1D | 49 |
| 50 niemowląt podddanych skriningowi HLA z krewnymi z cukrzycą typu 1 | ||||
| PRODIA | probiotics during infancy probiotyki w okresie niemowlęcym | pilot study completed zakończone badania pilotażowe | 264 HLA – screened infants from the general population | 52 |
| 264 niemowlęta, skrining HLA z populacji ogólnej | ||||
| NIP | docosahexaenoic acid (DHA) to pregnant women and infants podawanie kwasu dokosaheskanowego (DHA) kobietom w ciąży i niemowlętom | pilot study ongoing prowadzone badania pilotażowe | 97 HLA – screened infants with a first-degree relative with T1D | 45 |
| 98 niemowląt, skrining HLA z krewnymi z cukrzycą typu 1 | ||||
| PREVEFIN | D-vitamin supplementation and cow’s milk-based hydrolyzed formula suplementacja witaminą D i mieszanką hydrolizowaną na bazie mleka krowiego | study proper ongoing prowadzone badania właściwe | 120 HLA – screened high risk infants from the general population | 51 |
| 120 niemowląt, skrining HLA z populacji ogólnej | ||||
| FINDIA | insulin-free cow’s milk-based formula and hydrolyzed formula mieszanka na bazie mleka krowiego bez insuliny i mieszanka hydrolizowana | pilot study ongoing prowadzone badania pilotażowe | 1106 HLA – screened infants from the general population | 20 |
| 1106 niemowląt, skrining HLA z ogólnej populacji | ||||
The results of the second pilot study of TRIGR published in 2005 were encouraging. In that study comprising 242 newborn infants with an affected first-degree relative and a risk associated HLA-DQB1 genotype the experimental formula reduced the appearance of ICA or any autoantibody by around 50% during the mean follow-up time of 4.7 years (48).
Another ongoing pilot study tests the hypothesis that the culprit in cow’s milk-based formula actually is bovine insulin (20). The FINDIA trial tests a cow’s milk-based formula from which bovine insulin has been removed.
Pilot interventions aimed at primary prevention and concentrating on other specific food constituents are also ongoing or completed. The BABYDIET study analyses the effect of postponement of gluten introduction based on the hypothesis of its deleterious effect (49). A pilot trial (NIP) testing the effect of supplementation with an omega-3 fatty acid, docosahexaenoic acid (DHA), is also in progress including 97 children with a risk-associated HLA genotype and an affected family member (45). These children get DHA supplementation up to the age of 36 months starting during the last trimester of pregnancy or before the age of 5 months. This approach is supported by a recent study where low intake of omega-3 fatty acids was associated with diabetes-associated autoimmunity. The result was also supported by the observation that the content of these fatty acids in erythrocyte membranes of autoantibody positive children was lower than in matched controls (50). Supplementation with vitamin D is as well used in pilot prevention studies. In an Italian study vitamin D supplementation is combined with a hydrolyzed formula (51). Probiotic bacteria have been used with the aim of preventing T1D-associated autoimmunity but the results of the completed pilot trial do not allow any conclusion on their effect but demonstrate the safety and feasibility of the approach (52).
The time-table of ongoing studies does not unfortunately allow any swift conclusions on their effects, especially as most of them are still pilot studies and full-scale studies are still in the planning phase. The outcome of full-scale intervention trials are, however, needed before issuing any strong recommendations concerning infant nutrition in relation to subsequent risk of T1D. Prevention studies targeting infectious diseases are still totally lacking. The historical experience with several microbial vaccines introduced during last decades have, however, not clearly decreased the incidence of T1D.
Fig. 1.
Several alternative mechanisms and their combinations may be important in immune mediated beta-cell destruction
Footnotes
Konflikt interesów: nie zgłoszono
References
- 1.Patterson CC, Dahlquist GG, Gyurus E, et al. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373:2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 2.Tuomilehto J, Karvonen M, Pitkaniemi J, et al. Record-high incidence of type I (insulin-dependent) diabetes mellitus in Finnish children. The Finnish Childhood Type I Diabetes Registry Group. Diabetologia. 1999;42:655–660. doi: 10.1007/s001250051212. [DOI] [PubMed] [Google Scholar]
- 3.Harjutsalo V, Sjoberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777–1782. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- 4.Daneman D. Type 1 diabetes. Lancet. 2006;367:847–858. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- 5.Imagawa A, Hanafusa T, Uchigata Y, et al. Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care. 2003;26:2345–2352. doi: 10.2337/diacare.26.8.2345. [DOI] [PubMed] [Google Scholar]
- 6.Hummel M, Bonifacio E, Schmid S, et al. Brief communication: early appearance of islet autoantibodies predicts childhood type 1 diabetes in offspring of diabetic parents. Ann. Intern. Med. 2004;140:882–886. doi: 10.7326/0003-4819-140-11-200406010-00009. [DOI] [PubMed] [Google Scholar]
- 7.Vaarala O, Atkinson MA, Neu J. The “perfect storm” for type 1 diabetes. The complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555–2562. doi: 10.2337/db08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kvity S, Agmon-Levin N, Blank M, et al. Infections and autoimmunity – friends or foes. Trends Immunol. 2009 doi: 10.1016/j.it.2009.05.005. in press. [DOI] [PubMed] [Google Scholar]
- 9.Riedler J, Braun-Fahrlander C, Eder W, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 10.Savilahti E, Kuitunen M, Vaarala O. Pre and probiotics in the prevention and treatment of food allergy. Curr. Opin. Allergy Clin. Immunol. 2008;8:243–248. doi: 10.1097/ACI.0b013e3282ffb134. [DOI] [PubMed] [Google Scholar]
- 11.Borch-Johnsen K, Joner G, Mandrup-Poulsen T, et al. Relation between breast-feeding and incidence rates of insulin-dependent diabetes mellitus. A hypothesis. Lancet. 1984;2:1083–1086. doi: 10.1016/s0140-6736(84)91517-4. [DOI] [PubMed] [Google Scholar]
- 12.Gerstein HC. Cow’s milk exposure and type I diabetes mellitus. A critical overview of the clinical literature. Diabetes Care. 1994;17:13–19. doi: 10.2337/diacare.17.1.13. [DOI] [PubMed] [Google Scholar]
- 13.Norris JM, Scott FW. A meta-analysis of infant diet and insulin-dependent diabetes mellitus: do biases play a role? Epidemiology. 1996;7:87–92. doi: 10.1097/00001648-199601000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Sarugeri E, Dozio N, Meschi F, et al. Cellular and humoral immunity against cow’s milk proteins in type 1 diabetes. J. Autoimmunol. 1999;13:365–373. doi: 10.1006/jaut.1999.0327. [DOI] [PubMed] [Google Scholar]
- 15.Savilahti E, Saukkonen TT, Virtala ET, et al. Increased levels of cow’s milk and beta-lacto-globulin antibodies in young children with newly diagnosed IDDM. The Childhood Diabetes in Finland Study Group. Diabetes Care. 1993;16:984–989. doi: 10.2337/diacare.16.7.984. [DOI] [PubMed] [Google Scholar]
- 16.Luopajarvi K, Savilahti E, Virtanen SM, et al. Enhanced levels of cow’s milk antibodies in infancy in children who develop type 1 diabetes later in childhood. Pediatr. Diabetes. 2008;9:434–441. doi: 10.1111/j.1399-5448.2008.00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mojibian M, Chakir H, Lefebvre DE, et al. Diabetes-specific HLA-DR-restricted proinflammatory T-cell response to wheat polypeptides in tissue transglutaminase antibody-negative patients with type 1 diabetes. Diabetes. 2009;58:1789–1796. doi: 10.2337/db08-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacFarlane AJ, Burghardt KM, Kelly J, et al. A type 1 diabetes-related protein from wheat (Triticum aestivum). cDNA clone of a wheat storage globulin, Glb1, linked to islet damage. J. Biol. Chem. 2003;278:54–63. doi: 10.1074/jbc.M210636200. [DOI] [PubMed] [Google Scholar]
- 19.Klemetti P, Savilahti E, Ilonen J, et al. T-cell reactivity to wheat gluten in patients with insulin-dependent diabetes mellitus. Scand. J. Immunol. 1998;47:48–53. doi: 10.1046/j.1365-3083.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- 20.Vaarala O, Paronen J, Otonkoski T, et al. Cow milk feeding induces antibodies to insulin in children – a link between cow milk and insulin-dependent diabetes mellitus? Scand. J. Immunol. 1998;47:131–135. doi: 10.1046/j.1365-3083.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- 21.Martin JM, Trink B, Daneman D, et al. Milk proteins in the etiology of insulin-dependent diabetes mellitus (IDDM) Ann. Med. 1991;23:447–452. doi: 10.3109/07853899109148088. [DOI] [PubMed] [Google Scholar]
- 22.Vaarala O, Knip M, Paronen J, et al. Cow’s milk formula feeding induces primary immunization to insulin in infants at genetic risk for type 1 diabetes. Diabetes. 1999;48:1389–1394. doi: 10.2337/diabetes.48.7.1389. [DOI] [PubMed] [Google Scholar]
- 23.Paronen J, Vaarala O, Savilahti E, et al. Soluble adhesion molecules and oral antigen feeding in infants. Pediatr. Res. 1996;40:276–279. doi: 10.1203/00006450-199608000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Karges W, Hammond-McKibben D, Cheung RK, et al. Immunological aspects of nutritional diabetes prevention in NOD mice: a pilot study for the cow’s milk-based IDDM prevention trial. Diabetes. 1997;46:557–564. doi: 10.2337/diab.46.4.557. [DOI] [PubMed] [Google Scholar]
- 25.Mäkelä M, Vaarala O, Hermann R, et al. Enteral virus infections in early childhood and an enhanced type 1 diabetes-associated antibody response to dietary insulin. J. Autoimmun. 2006;27:54–61. doi: 10.1016/j.jaut.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Sabbah E, Savola K, Ebeling T, et al. Genetic, autoimmune, and clinical characteristics of childhood- and adult-onset type 1 diabetes. Diabetes Care. 2000;23:1326–1332. doi: 10.2337/diacare.23.9.1326. [DOI] [PubMed] [Google Scholar]
- 27.Vandewalle CL, Falorni A, Lernmark A, et al. Associations of GAD65- and IA-2-autoantibodies with genetic risk markers in new-onset IDDM patients and their siblings. The Belgian Diabetes Registry. Diabetes Care. 1997;20:1547–1552. doi: 10.2337/diacare.20.10.1547. [DOI] [PubMed] [Google Scholar]
- 28.Graham J, Hagopian WA, Kockum I, et al. Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes. 2002;51:1346–1355. doi: 10.2337/diabetes.51.5.1346. [DOI] [PubMed] [Google Scholar]
- 29.Kupila A, Keskinen P, Simell T, et al. Genetic risk determines the emergence of diabetes-associated autoantibodies in young children. Diabetes. 2002;51:646–651. doi: 10.2337/diabetes.51.3.646. [DOI] [PubMed] [Google Scholar]
- 30.Alizadeh BZ, Koeleman BP. Genetic polymorphisms in susceptibility to type 1 diabetes. Clin. Chim. Acta. 2008;387:9–17. doi: 10.1016/j.cca.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and metaanalysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 2009 doi: 10.1038/ng.381. [ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wucherpfennig KW. T cell receptor crossreactivity as a general property of T cell recognition. Mol. Immunol. 2004;40:1009–1017. doi: 10.1016/j.molimm.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Mathis D, Benoist C. Aire. Ann. Rev. Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 34.Pugliese A. Central and peripheral autoantigen presentation in immune tolerance. Immunology. 2004;111:138–146. doi: 10.1111/j.0019-2805.2003.01804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabbah E, Savola K, Kulmala P, et al. Disease-associated autoantibodies and HLA-DQB1 genotypes in children with newly diagnosed insulin-dependent diabetes mellitus (IDDM). The Childhood Diabetes in Finland Study Group. Clin. Exp. Immunol. 1999;116:78–83. doi: 10.1046/j.1365-2249.1999.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walter M, Albert E, Conrad M, et al. IDDM2/insulin VNTR modifies risk conferred by IDDM1/HLA for development of type 1 diabetes and associated autoimmunity. Diabetologia. 2003;46:712–720. doi: 10.1007/s00125-003-1082-z. [DOI] [PubMed] [Google Scholar]
- 37.Hermann R, Laine AP, Veijola R, et al. The effect of HLA class II, insulin and CTLA4 gene regions on the development of humoral beta cell autoimmunity. Diabetologia. 2005;48:1766–1775. doi: 10.1007/s00125-005-1844-x. [DOI] [PubMed] [Google Scholar]
- 38.Lempainen J, Vaarala O, Mäkelä M, et al. Interplay between PTPN22 C1858T polymorphism and cow’s milk formula exposure in type 1 diabetes. J. Autoimmunol. 2009;33:155–164. doi: 10.1016/j.jaut.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Pérez-Bravo F, Carrasco E, Gutierrez-Lopez MD, et al. Genetic predisposition and environmental factors leading to the development of insulin-dependent diabetes mellitus in Chilean children. J. Mol. Med. 1996;74:105–109. doi: 10.1007/BF00196786. [DOI] [PubMed] [Google Scholar]
- 40.Kostraba JN, Cruickshanks KJ, Lawlerheavner J, et al. Early exposure to cow’s milk and solid foods in infancy, genetic predisposition, and risk of IDDM. Diabetes. 1993;42:288–295. [PubMed] [Google Scholar]
- 41.Virtanen SM, Kenward MG, Erkkola M, et al. Age at introduction of new foods and advanced beta cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes. Diabetologia. 2006;49:1512–1521. doi: 10.1007/s00125-006-0236-1. [DOI] [PubMed] [Google Scholar]
- 42.Stene LC, Joner G. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Am. J. Clin. Nutr. 2003;78:1128–1134. doi: 10.1093/ajcn/78.6.1128. [DOI] [PubMed] [Google Scholar]
- 43.Dahlquist GG, Forsberg J, Hagenfeldt L, et al. Increased prevalence of enteroviral RNA in blood spots from newborn children who later developed type 1 diabetes: a population-based case-control study. Diabetes Care. 2004;27:285–286. doi: 10.2337/diacare.27.1.285. [DOI] [PubMed] [Google Scholar]
- 44.Svensson J, Lindberg B, Jonsson B, et al. Intrauterine exposure to maternal enterovirus infection as a risk factor for development of autoimmune thyroiditis during childhood and adolescence. Thyroid. 2004;14:367–370. doi: 10.1089/105072504774193203. [DOI] [PubMed] [Google Scholar]
- 45.Chase HP, Lescheck E, Rafkin-Mervis L, et al. Nutritional intervention to prevent type 1 diabetes a pilot trial. ICAN: Infant, Child, & Adolescent Nutrition. 2009;1:98–107. [Google Scholar]
- 46.TRIGR Study Group. Study design of the Trial to Reduce IDDM in the Genetically at Risk (TRIGR) Pediatr. Diabetes. 2007;8:117–137. doi: 10.1111/j.1399-5448.2007.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Åkerblom HK, Knip M, Becker D, et al. The TRIGR trial: testing the potential link between weaning diet and type 1 diabetes. Immun. Endoc. & Metab. Agents in Med. Chem. 2007;7:251–263. [Google Scholar]
- 48.Åkerblom HK, Virtanen SM, Ilonen J, et al. Dietary manipulation of beta cell autoimmunity in infants at increased risk of type 1 diabetes: a pilot study. Diabetologia. 2005;48:829–837. doi: 10.1007/s00125-005-1733-3. [DOI] [PubMed] [Google Scholar]
- 49.Schmid S, Buuck D, Knopff A, et al. BABYDIET, a feasibility study to prevent the appearance of islet autoantibodies in relatives of patients with type 1 diabetes by delaying exposure to gluten. Diabetologia. 2004;47:1130–1131. doi: 10.1007/s00125-004-1420-9. [DOI] [PubMed] [Google Scholar]
- 50.Norris JM, Yin X, Lamb MM, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007;298:1420–1428. doi: 10.1001/jama.298.12.1420. [DOI] [PubMed] [Google Scholar]
- 51.Lorini R, Minicucci L, Napoli F, et al. Screening for type 1 diabetes genetic risk in newborns of continental Italy. Primary prevention (Prevefin Italy) – preliminary data. Acta Biomed. 2005;76(suppl. 3):31–35. [PubMed] [Google Scholar]
- 52.Ljungberg M, Korpela R, Ilonen J, et al. Probiotics for the prevention of beta cell autoimmunity in children at genetic risk of type 1 diabetes – the PRODIA study. Ann. N. Y. Acad. Sci. 2006;1079:360–364. doi: 10.1196/annals.1375.055. [DOI] [PubMed] [Google Scholar]