Abstract
IMPORTANCE
The disease process leading to clinical type 1 diabetes often starts during the first years of life. Early exposure to complex dietary proteins may increase the risk of β-cell autoimmunity in children at genetic risk for type 1 diabetes. Extensively hydrolyzed formulas do not contain intact proteins.
OBJECTIVE
To test the hypothesis that weaning to an extensively hydrolyzed formula decreases the cumulative incidence of diabetes-associated autoantibodies in young children.
DESIGN, SETTING, AND PARTICIPANTS
A double-blind randomized clinical trial of 2159 infants with HLA-conferred disease susceptibility and a first-degree relative with type 1 diabetes recruited from May 2002 to January 2007 in 78 study centers in 15 countries; 1078 were randomized to be weaned to the extensively hydrolyzed casein formula and 1081 were randomized to be weaned to a conventional cows’ milk–based formula. The participants were observed to April 16, 2013.
INTERVENTIONS
The participants received either a casein hydrolysate or a conventional cows’ milk formula supplemented with 20% of the casein hydrolysate.
MAIN OUTCOMES AND MEASURES
Primary outcome was positivity for at least 2 diabetes-associated autoantibodies out of 4 analyzed. Autoantibodies to insulin, glutamic acid decarboxylase, and the insulinoma-associated–2 (IA-2) molecule were analyzed using radiobinding assays and islet cell antibodies with immunofluorescence during a median observation period of 7.0 years (mean, 6.3 years).
RESULTS
The absolute risk of positivity for 2 or more islet autoantibodies was 13.4% among those randomized to the casein hydrolysate formula (n = 139) vs 11.4% among those randomized to the conventional formula (n = 117). The unadjusted hazard ratio for positivity for 2 or more autoantibodies among those randomized to be weaned to the casein hydrolysate was 1.21 (95% CI, 0.94–1.54), compared with those randomized to the conventional formula, while the hazard ratio adjusted for HLA risk, duration of breastfeeding, vitamin D use, study formula duration and consumption, and region was 1.23 (95% CI, 0.96–1.58). There were no clinically significant differences in the rate of reported adverse events between the 2 groups.
CONCLUSIONS AND RELEVANCE
Among infants at risk for type 1 diabetes, the use of a hydrolyzed formula, when compared with a conventional formula, did not reduce the incidence of diabetes-associated autoantibodies after 7 years. These findings do not support a benefit from hydrolyzed formula.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00179777
Type 1 diabetes is characterized by selective loss of insulin-producing β cells in the pancreatic islets in genetically susceptible individuals. Overt clinical disable duration1 during which diabetes-associated autoantibodies appear in the peripheral circulation as markers of emerging β-cell autoimmunity. Several disease-related autoantibodies predict clinical type 1 diabetes including classical islet cell antibodies (ICA), insulin autoantibodies, autoantibodies to glutamic acid decarboxylase (GAD), and the tyrosine phosphatase-related insulinoma-associated 2 molecule (IA-2).2 In natural history studies from infancy, positivity for at least 2 autoantibodies signals a risk of approximately 60% for the development of clinical diabetes over 10 years, whereas the 10-year risk among those with a single autoantibody is about 15% and among those with no detectable autoantibodies less than 1%.3
Accumulating evidence suggests that β-cell autoimmunity emerges early in life.4,5 The incidence of type 1 diabetes is increasing among children in Europe and North America,6,7 although some studies suggest it may be stabilizing.8 This scenario implies that any measure aimed at primary prevention of type 1 diabetes, ie, prevention of the initiation of the diabetic disease process, has to be started in infancy. Early feeding may modify the risk of type 1 diabetes later in life. Some epidemiological and immunological studies suggest that exposure to complex foreign proteins in early infancy may increase the risk of β-cell autoimmunity and type 1 diabetes in genetically susceptible individuals,9–11 although others do not.12,13 A pilot study suggested that weaning to an extensively hydrolyzed casein formula (99.7% of the generated peptides having a molecular weight of less than 2000 Da) decreased the cumulative incidence of diabetes-associated autoantibodies in children with an affected first-degree relative and a risk-associated HLA genotype.14,15 This led to TRIGR (Trial to Reduce IDDM in the Genetically at Risk), with the study powered to assess the effect of the intervention on the development of type 1 diabetes by age 10 years. A prior prespecified end point, early humoral β-cell autoimmunity, is reported herein.
Methods
Study Design
We conducted a randomized, double-blind study in 78 study centers in 15 countries (eTable 1 in Supplement).16 Newborn infants who had a first-degree relative with type 1 diabetes were recruited between May 2002 and January 2007 and were observed to April 16, 2013, for this analysis. Randomization took place before birth or immediately after birth of the infants who met the inclusion criteria (Figure 1). The research assistant or investigator obtained the formula allocation code from the data management unit by completing the randomization form electronically. Randomization was balanced within each study center using a block size of 4. The randomization code will remain blinded to the participating families and all members of the study group, except for the data management and safety board and the principal investigator at the data management unit, until the study is completed in 2017. Written informed consent was obtained from the family before enrollment. The study was approved by the ethics committees of all participating centers. An agreement that the results would remain confidential until publication was in force between the members of the study group and Mead Johnson Nutrition, which provided the blinded color-coded study formulas.
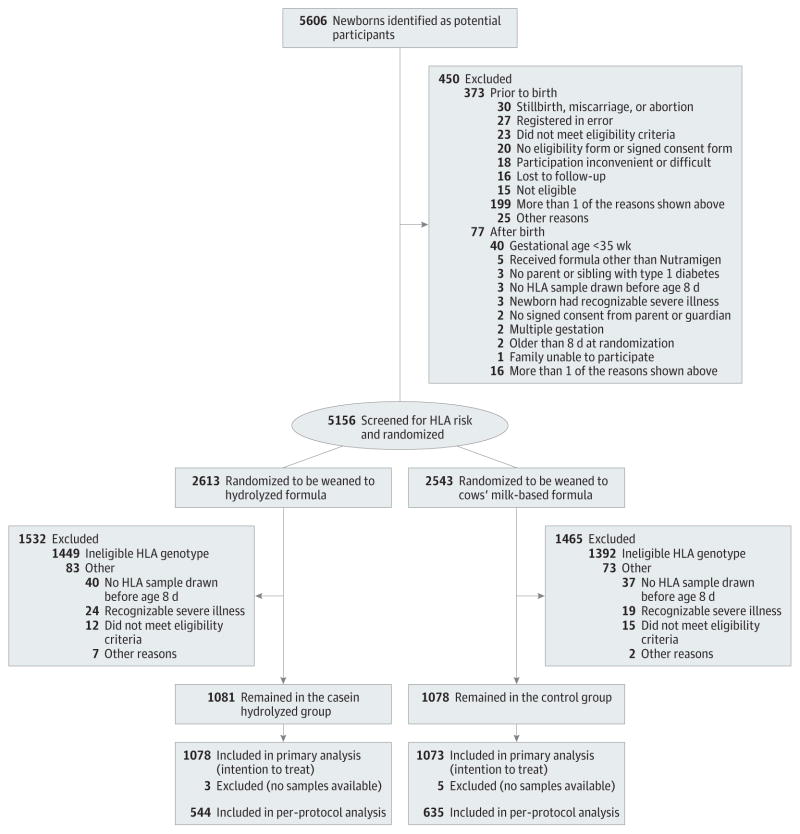
Figure 1.
Screening, Randomization, and Follow-up of TRIGR Study Infants
Presentation of clinical type 1 diabetes by age 10 years is studied as the primary outcome while positivity for 2 or more islet autoantibodies by age 6 years is a secondary outcome.
Blood Samples
Cord blood samples and follow-up blood samples were obtained from the trial participants at ages 3, 6, 9, 12, 18, and 24 months, and thereafter annually up to age 10 years. Serum samples were stored at −70°C until analyzed.
Dietary Intervention
Infants were randomly assigned weaning to either the experimental or control formulas that were produced specifically for this study. The experimental formula was an extensively hydrolyzed casein-based formula, while the control formula was composed of 80% intact cows’ milk protein and 20% hydrolyzed casein protein and formulated so that the taste and smell would be indistinguishable from the experimental formula. Study formulas were prepared and coded with the use of 4 colors by Mead Johnson. Newborn infants requiring supplemental feeding before randomization (eg, infants born at night or on weekends) received banked breast milk or Nutramigen.
Breastfeeding was practiced at the discretion of the participating mothers and maternal diets were unmodified. Breast-feeding was encouraged and exceeded national averages in both groups.17 The dietary intervention period lasted until the infant was at least 6 months of age, and if by that time the child had not received the study formula for at least 60 days, feeding of study formula was continued until 60 days of study formula exposure was reached, up to a maximum of 8 months of age. Parents were asked not to feed the infants any commercial or other baby foods containing bovine protein during the intervention period. Adherence to the protocol was monitored through regular family nutrition interviews (at age 0.5, 1, 2, 3, 4, 5, 6, 7, and 8 months) and by the analysis of cows’-milk antibodies in serum samples.
HLA Genotyping
Cord blood or a heel-stick blood sample collected on filter paper was immediately sent to the Turku, Finland (Europe and Australia) or Pittsburgh, Pennsylvania (North America) laboratories for HLA genotyping. HLA genotyping for the selected DQB1 and DQA1 alleles was performed using sequence-specific oligonucleotide hybridization, with quality control between the 2 laboratories carefully maintained. The following genotypes were regarded as eligible: (1) HLADQB1*02/DQB1*03:02 (high risk); (2) HLA-DQB1*03: 02/x (x not DQB1*02, DQB1*03:01, or DQB1*06:02) (moderate risk); (3) HLADQA1*05-DQB1*02/y (y not DQA1*02:01-DQB1*02, DQB1*03:01, DQB1*06:02, or DQB1*06:03) (mild risk); (4) HLADQA1*03-DQB1*02/y (y not DQA1*02:01-DQB1*02, DQB1*03:01, DQB1*06:02, or DQB1*06:03) (rare mild risk).
β-Cell Autoimmunity and Cows’ Milk Antibody Assays
ICAs were detected using indirect immunofluorescence. The other 3 autoantibodies were quantified with the use of specific radiobinding assays in the Scientific Laboratory, Children’s Hospital, University of Helsinki, Helsinki, Finland, with cutoff limits for positivity of 2.5 Juvenile Diabetes Foundation units for ICAs, 2.80 relative units for insulin autoantibodies, 5.36 relative units for GAD autoantibodies, and 0.77 relative units for IA-2 autoantibodies.18 The sensitivity and specificity for detecting existing type 1 diabetes of the assay for ICAs were 100% and 98%, respectively, in the fourth round of the international workshops on standardization of the ICA assay. The disease sensitivities and specificities of the assay for insulin autoantibodies were 44% and 98%, 82% and 97% for GAD autoantibodies, and 72% and 100% for IA-2 autoantibodies, respectively, in the 2005 Diabetes Antibody Standardization Program workshop. Maternal antibodies that were placentally transferred, as verified by their decreasing levels and disappearance from the infant’s circulation by age 18 months, were not included in the statistical analysis. Cows’ milk antibodies were measured from serum samples obtained from cord blood and at the age of 3, 6, and 9 months by enzyme-linked immunosorbent assay.
Statistical Analyses
The data management unit conducted the comparative analyses, as specified by protocol, when the unit had received the youngest child’s 6-year autoantibody results from the TRIGR core laboratory in Helsinki, Finland. The differences between the 2 groups with respect to the autoantibody titers and the duration of breastfeeding and study formula exposure were assessed using the Mann-Whitney U test. Statistical analyses were based on a longitudinal data set consisting of repeated measurements of several variables at standard time points. All statistical analyses were performed using SAS statistical software version 9.3.
Kaplan-Meier estimates and Cox proportional hazards regressions were used to analyze the association between the intervention and the risk of seroconversion to autoantibody positivity. The Cox regression analyses were adjusted for HLA risk, duration of breastfeeding, vitamin D use, study formula duration and consumption, and region (Finland, Canada, the United States, and other), as prespecified. The adjustment did not appreciably change the hazard ratio (HR), and therefore, the unadjusted log-rank test is the reported primary test of the end point.
The intention-to-treat principle was used for the analyses of seroconversion to autoantibody positivity. The analyses of seroconversion to autoantibody positivity were also performed according to treatment received (per-protocol analysis). A separate subanalysis of the Finnish participants (n = 424) was carried out as well. All tests were 2-tailed; P values of less than .05 were considered statistically significant. No imputations were performed due to missing values or loss to follow-up. Given a 95% CI and an estimated cumulative incidence of 2 or more autoantibodies of 9.9% by age 6 years in the control group, the study had a power of 80% to detect a 35% change in the end point, which represents a conservative estimate since a reduction in the cumulative incidence of multiple (≥2) antibodies in the range of 40% to 50% had been observed earlier in the TRIGR pilot.15 Similarly, the estimated cumulative incidence of at least 2 autoantibodies by age 6 years (9.9%) is a conservative estimate, since it represents the lower limit of the 95% CI for multiple autoantibody positivity among siblings in the Finnish DiMe study19 carrying the high-risk HLA genotype or one of the moderate-risk genotypes. The power of 80% results in 20% risk to miss a true difference between the groups.
Results
Altogether, 5606 potential participants were identified for the study during the 57 recruitment months; 92% consented, resulting in 5156 newborn infants available for randomization before or immediately after birth (Figure 1). A sample for HLA genotyping was obtained from cord blood or if not available, from capillary blood taken within 7 days of birth. Inclusion criteria were not met by 156 randomized participants (3.0%), mainly owing to prematurity (gestational age <36 weeks up to June 4, 2003, and subsequently <35 weeks) or families changing their minds about study participation.
Altogether, 2159 newborn infants (1142 boys [52.8%]) with an eligible HLA genotype (41.9% of the genotyped infants) continued in the intervention study. Five hundred sixteen infants (23.9%) carried the high-risk HLA genotype, 953 (44.1%) moderate-risk genotypes, 668 (31.0%) mild-risk genotypes, and 22 (1.0%) the rare mild-risk genotype. The first-degree relative with type 1 diabetes was the mother in 1055 infants (48.9%), the father in 723 (33.5%), a sibling in 308 (14.3%), and more than 1 first-degree family member in 73 participants (3.4%). The mean follow-up time for the detection of autoantibodies was 7.0 years (median, 6.3 years; range, 3 months-10.3 years).
Randomization resulted in balanced groups with 1078 infants in the experimental group and 1081 in the control group. There were no differences in the demographics or the distribution of HLA genotypes between the 2 groups (Table).
Table.
Demographic Characteristics of the Trial Participants
| Characteristics | No. (%) | |
|---|---|---|
| Casein Hydrolysate (n = 1081) | Control (n = 1078) | |
| Male sex | 576 (53.3) | 565 (52.4) |
| Gestational age, mean (SD), wk | 38.7 (1.6) | 38.8 (1.6) |
| Birth weight, mean (SD), g | 3585 (539) | 3625 (558) |
| Birth length, mean (SD), cm | 50.9 (2.8) | 51.1 (2.8) |
| Family history of type 1 diabetes | ||
| Mother | 531 (49.1) | 524 (48.6) |
| Father | 356 (32.9) | 367 (34.0) |
| Sibling | 151 (14.0) | 157 (14.6) |
| More than one family member | 43 (4.0) | 30 (2.8) |
| HLA genotype | ||
| HLADQB1*02/DQB1*0302 | 260 (24.1) | 256 (23.7) |
| HLA-DQB1*0302/x (x not DQB1*02, DQB1*0301, or DQB1*0602) | 478 (44.2) | 475 (44.1) |
| HLA-DQA1*05-DQB1*02/y (y not DQA1*0201-DQB1*02, DQB1*0301, DQB1*0602, or DQB1*0603) | 332 (30.7) | 336 (31.2) |
| HLA-DQA1*03-DQB1*02/y (y not DQA1*0201-DQB1*02, DQB1*0301, DQB1*0602, or DQB1*0603) | 11 (1.0) | 11 (1.0) |
Study Intervention
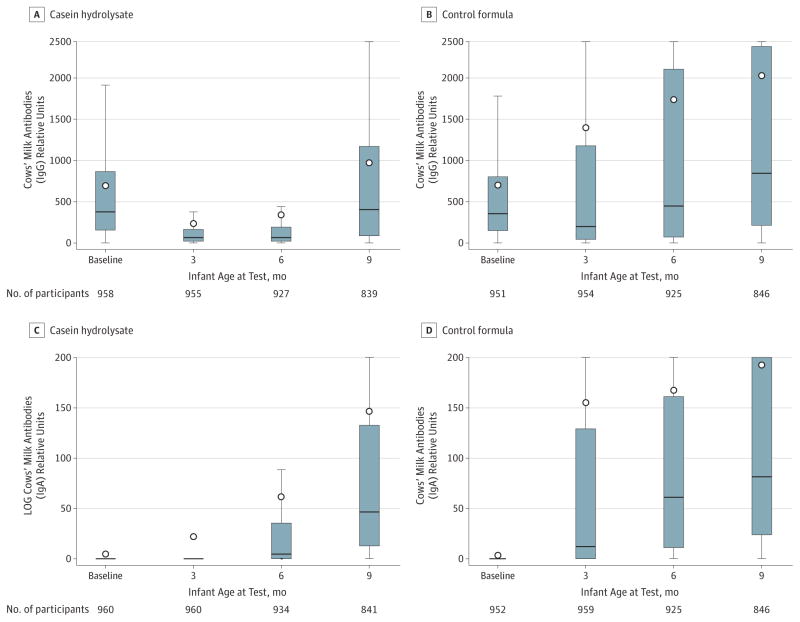
In the experimental group, 80.0% of the infants vs 80.9% in the control group were exposed to the study formula during the intervention period. The mean (SD) ages of the infants at the time of study formula introduction were 2.0 months (95% CI, 1.8–2.1) in the experimental group and 1.8 months (95% CI, 1.6–1.9) in the control group (P = .05). The mean duration of study-formula feeding was 10.2 weeks (95% CI, 9.7–10.8) in the experimental group vs 11.7 weeks (95% CI, 11.1–12.3) in the control group (P < .001). The mean per capita volumes of study formula consumed were 42.3 L (95% CI, 38.7–45.8) in the experimental group vs 48.7 L (95% CI, 45.1–52.4) in the control group (P = .01). The analysis of cows’ milk antibodies confirmed that the families adhered well with the dietary intervention resulting in clear differences in the antibody levels between the treatment groups (Figure 2).
Figure 2. Cows’ Milk Antibody Titers Over the First 9 Months of Life.
IgG (Panels A and B) and IgA (Panels C and D) class antibodies to cows’ milk (infant formula) in cord blood and at the ages of 3, 6, and 9 months in the casein hydrolysate group and control group. The bottom of the box plots indicate the 25th percentile and the top the 75th percentile. The circle symbols indicate the mean. The lower end of the whiskers represent the minimum observation and the upper end the maximum observation below the upper fence (1.5 interquartile range above the 75th percentile).
β-Cell Autoimmunity
During the follow-up period, 2070 children (95.9%; 1035 in each group) provided at least 1 blood sample for determination of diabetes-associated autoantibodies. The primary analysis of the autoantibody end point showed that 139 children in the experimental group (13.4%; 95% CI, 11.3%–15.5%) tested positive for 2 or more autoantibodies, as compared with 117 in the control group (11.3%; 95% CI, 9.4%–13.2%), At least 1 autoantibody developed in 431 of the children in the experimental group (41.6%; 95% CI, 38.6%–44.6%) and in 414 in the control group (40.0%; 95% CI, 37.0%–43.0%). Altogether, 136 of the 256 participants with multiple autoantibodies (53.1%) had only 1 autoantibody in their first positive sample. Among those children, insulin autoantibodies were the first to appear in 47 children (34.6%), GAD antibodies in 46 (33.8%), ICAs in 41 (30.1%), and IA-2 antibodies in 2 participants (1.5%). There was no difference between the 2 intervention groups regarding which autoantibody appeared first (eTable 3 in Supplement).
The earliest age at detection of autoantibodies was 3 months, and the latest seroconversion to date was at age 9 years. Among the children who tested positive for autoantibodies, there were no significant differences between the 2 treatment groups in the initial or maximal autoantibody titers (eTable 4 in Supplement). The cumulative incidences of at least 2 autoantibodies and of at least 1 autoantibody in the experimental and control groups by age at first detection are shown as parametric maximum-likelihood estimates in Figure 3. The cumulative incidences of each individual autoantibody in the 2 groups are presented in Figure 4. The corresponding cumulative incidences based on the per-protocol analyses are shown in eFigure 1 and eFigure 2 in the Supplement. The observed trends in the HRs were in the opposite direction when compared with the hypothesized benefit of the experimental formula. The unadjusted HR for positivity for 2 or more autoantibodies was 1.21 (95% CI, 0.94–1.54), while the adjusted HR for HLA risk, duration of breastfeeding, vitamin D use, study formula duration and consumption, and region was 1.23 (95% CI, 0.96–1.58). The unadjusted HR for positivity for 1 or more autoantibodies was 1.06 (95% CI, 0.93–1.22), whereas the adjusted HR was 1.09 (95% CI, 0.95–1.24). The subanalysis of the 424 Finnish participants showed an unadjusted HR of 1.20 (95% CI, 0.71–2.05; eFigure 3 in Supplement), whereas the adjusted HR for HLA risk, duration of breastfeeding, vitamin D use, and study formula duration and consumption was 1.33 (95% CI, 0.77–2.29).
Figure 3. Cumulative Survival Without at Least 2 Autoantibodies and at Least 1 Autoantibody.
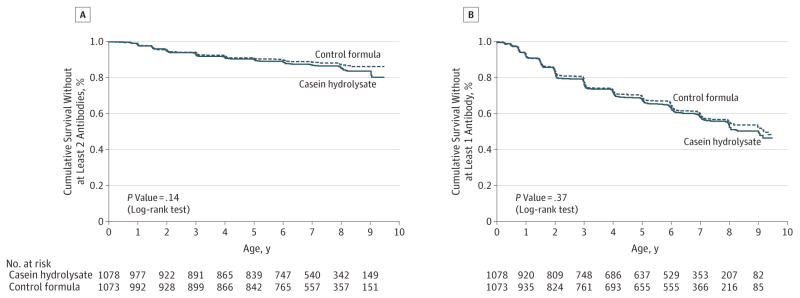
The cumulative survival without at least 2 autoantibodies (Panel A) and without at least 1 autoantibody (Panel B) is shown for the casein hydrolysate group and the control group according to the age of the children at the time the autoantibodies were detected.
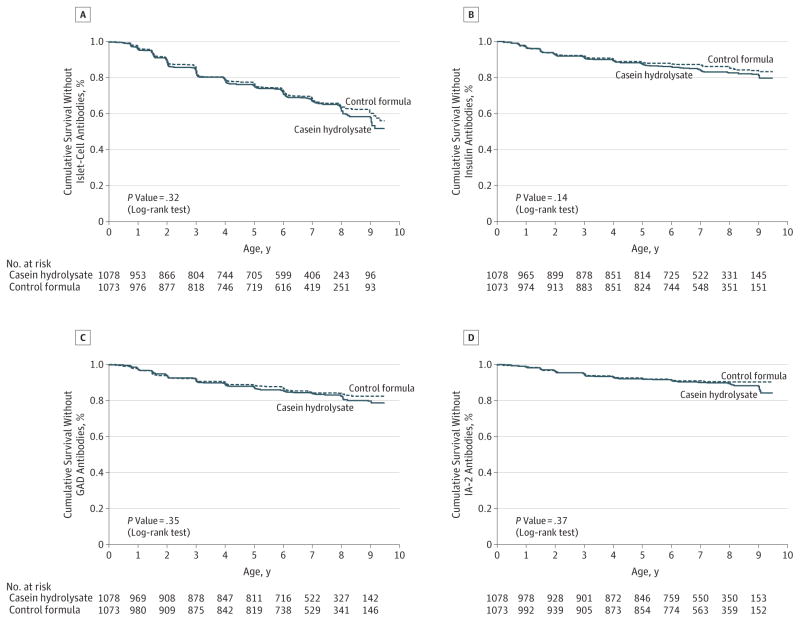
Figure 4. Cumulative Survival Without Islet Cell Antibodies, Insulin Autoantibodies, and Autoantibodies to Glutamic Acid Decarboxylase (GAD) and the Insulinoma-Associated–2 Molecule (IA-2).
The cumulative survival without islet cell antibodies (Panel A), insulin autoantibodies (Panel B), GAD antibodies (Panel C), and IA-2 autoantibodies (Panel D) is shown for the casein hydrolysate group and the control group according to the age of the children at the time the autoantibodies were detected.
Adverse Events
The children in the experimental group had a slightly lower rate of middle ear infections (eTable 5 in Supplement) when compared with children in the the control group (P = .04). Similar linear growth and weight gain were observed in both groups. There were no significant differences between the 2 groups in the rate of other adverse events during the total follow-up time.
Discussion
This study showed that in this large international trial in children with an HLA genotype conferring increased risk for type 1 diabetes and an affected first-degree relative, weaning to a highly hydrolyzed formula during infancy was not associated with any reduction in the signs of cumulative β-cell autoimmunity. This outcome is in contrast to data from the TRIGR pilot study,15 which reported that weaning to an extensively hydrolyzed formula in infancy was associated with an almost 50% reduction in the cumulative incidence of β-cell autoimmunity by the age of 10 years in similar children. The TRIGR pilot study was conducted in 230 Finnish children, while the current trial targets 2159 high-risk children from 15 different countries, the majority of the participants being from Canada, Finland, and United States.
The reasons for the discrepancy between the 2 studies are unclear. The large TRIGR study provides substantially stronger statistical power, although it includes a more heterogeneous study population than the pilot study. The proportion of children with a father with type 1 diabetes was about 10% higher in the pilot study compared with the large-scale trial (43% vs 34%). Although it is well established that the risk of type 1 diabetes is approximately 2 times higher among offspring of affected fathers than of affected mothers,20 this relatively small difference cannot explain the divergent results. The subanalysis of the 424 Finnish participants in the current trial showed that the results were very close to those seen in the total study cohort. Accordingly, it is unlikely that heterogeneity between the various populations involved in the TRIGR study would explain the contrasting outcome of the pilot study and this larger study. The pilot trial may have yielded a false-positive result.
The strengths of the current trial encompass a very high retention rate of the participants and documented dietary adherence. The fact that the study was performed in 15 countries on 3 continents also supports the generalizability of the results. The TRIGR study was planned to have 2 end points, ie, positivity for 2 autoantibodies by age 6 years and clinical diabetes by the age of 10 years. An important consideration is whether there is any justification to continue the follow-up of the trial participants given the current results. A recent combination of 3 natural history studies, ie, the Finnish DIPP study, the German BABYDIAB study, and the American DAISY study, has shown that positivity for 2 or more autoantibodies is associated with a risk of progression to clinical diabetes over the next 10 years of approximately 60% and approximately 80% over 15 years with variable rates of progression.3 Despite this, there is justification to continue the observation of the TRIGR children in order to analyze the study end point, which is clinical diabetes when the youngest child reaches 10 years in 3-years’ time. First, TRIGR is not a natural history study but an intervention trial, and the intervention may affect progression from islet autoimmunity to clinical disease. Such a scenario has been observed in nonobese diabetic mice, in which administration of an extensively hydrolyzed casein formula after weaning reduced the rate of autoimmune diabetes considerably with little effect on islet inflammation and autoimmunity.21 Second, the planned study was powered to detect the development of diabetes as the relevant end point. Third, the continued observation provides an opportunity to assess whether and how puberty may modify the progression to clinical disease.
This study has several limitations. The study participants were selected based on a positive family history for type 1 diabetes and an HLA genotype conferring risk for type 1 diabetes. Accordingly, the results are not directly generalizable to the background population. This study was not designed to test the effect of breastfeeding since random assignment of infants to breastfeeding or formula feeding would not be ethical. So far, we have not detected any effect of exclusive breastfeeding. Some prospective studies assessing the associations between infant feeding patterns and the development of β-cell autoimmunity in children who are at genetic risk for type 1 diabetes have not observed any associations between the duration of either exclusive or total breastfeeding and β-cell autoimmunity.10,11 However, a single-cohort study involving children in the general population showed that a shorter duration of breastfeeding was related to an increased risk of positivity for GAD autoantibodies, insulin autoantibodies, or both.22
The casein-based formula used as the intervention modality in this study is highly hydrolyzed and does not contain intact proteins. Less than 0.3% of the peptides have a molecular weight exceeding 2000 daltons. Accordingly, the formula is free of intact bovine insulin, which is present in cows’ milk.23 In a study by Vaarala et al,24 results showed that infants fed a conventional cows’ milk–based formula before the age of 3 months developed a strong immune response to bovine insulin, which differs from human insulin by 3 amino acids. Infants developing early signs of β-cell autoimmunity lacked the capacity to mount oral tolerance to bovine insulin. Sustained bovine insulin immunity might contribute to prediabetes progression, as weaning to an insulin-free formula reduced the cumulative incidence of autoantibodies by more than half in young children at genetic risk for type 1 diabetes.25
Our experience shows that a large-scale primary preventive dietary intervention aimed at decreasing the risk of type 1 diabetes is feasible. In contrast to the pilot study and supportive animal and uncontrolled human studies, weaning to a hydrolyzed formula in early infancy had no effect on the development of β-cell autoimmunity. It is, however, possible that the hydrolyzed formula affects the degree and rate of progression of autoimmunity to clinical diabetes in high-risk children, which will be ascertained in the TRIGR cohort by the 10-year follow-up. At the time of this publication, there is, however, no conclusive evidence to revise the current dietary recommendations for infants at high risk for type 1 diabetes.
Conclusions
Among infants at risk for type 1 diabetes, the use of a hydrolyzed formula compared with a conventional formula did not reduce the incidence of diabetes-associated autoantibodies after 7 years of follow-up. These findings do not support a benefit from hydrolyzed formula.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by the National Institute of Child Health and Development (NICHD), the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (grant numbers HD040364, HD042444, and HD051997), Canadian Institutes of Health Research, the Juvenile Diabetes Research Foundation International, and the Commission of the European Communities (specific RTD program, Quality of Life and Management of Living Resources, contract number QLK1-2002-00372 Diabetes Prevention). Other funding came from the European Foundation for the Study of Diabetes/Juvenile Diabetes Research Foundation/Novo Nordisk Focused Research Grant, Academy of Finland (Centre of Excellence in Molecular Systems Immunology and Physiology Research 2012–2017, decision no 250114), Dutch Diabetes Research Foundation, and Finnish Diabetes Research Foundation. Mead Johnson Nutrition provided the blinded color-coded study formulas.
Role of the Sponsors: Mead Johnson Nutrition, as well as all funding sources, had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Group Information: A complete list of the participants in the TRIGR Study Group is provided in eTable 1 in the Supplement.
Author Contributions: Dr Knip had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Knip, Akerblom, Becker, Dosch, Dupre, Fraser, Ilonen, Krischer, Kordonouri, Palmer, Savilahti, Vaarala, Virtanen.
Acquisition, analysis, or interpretation of data: Knip, Akerblom, Becker, Dupre, Fraser, Howard, Ilonen, Krischer, Lawson, Palmer, Vaarala, Virtanen.
Drafting of the manuscript: Knip, Becker, Dosch, Krischer, Kordonouri, Palmer.
Critical revision of the manuscript for important intellectual content: Knip, Akerblom, Becker, Dosch, Dupre, Fraser, Howard, Ilonen, Krischer, Kordonouri, Lawson, Palmer, Savilahti, Vaarala, Virtanen.
Statistical analysis: Becker, Krischer.
Obtained funding: Knip, Akerblom, Becker, Dosch, Dupre, Krischer, Lawson, Palmer.
Administrative, technical, or material support: Knip, Akerblom, Becker, Dupre, Fraser, Ilonen, Krischer, Palmer, Savilahti, Vaarala, Virtanen.
Study supervision: Akerblom, Becker, Dupre, Krischer, Palmer, Vaarala, Virtanen.
Additional Contributions: We thank Mead Johnson Nutrition for providing the study formulas free of charge and Jim W Hansen, MD, and Carol Berseth, MD, of that company for their collaboration and support. We also thank Gilman Grave, MD, project official for TRIGR (Trial to Reduce IDDM [insulin-dependent diabetes mellitus] in the Genetically at Risk) at the NICHD, for stimulating encouragement and collaboration over the years. Drs Hansen, Berseth, and Grave have not received any compensation in association with their contributions to this article. We acknowledge the dedicated and competent TRIGR staff at all clinical sites, data management unit, laboratories, research institutes, and administrative centers for their enthusiasm. We deeply thank all participating families for their commitment and look forward to their continued participation until the end of the study.
References
- 1.Knip M. Natural course of preclinical type 1 diabetes. Horm Res. 2002;57(suppl 1):6–11. doi: 10.1159/000053305. [DOI] [PubMed] [Google Scholar]
- 2.Knip M. Can we predict type 1 diabetes in the general population? Diabetes Care. 2002;25(3):623–625. doi: 10.2337/diacare.25.3.623. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler A-G, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziegler A-G, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48 (3):460–468. doi: 10.2337/diabetes.48.3.460. [DOI] [PubMed] [Google Scholar]
- 5.Kimpimäki T, Kupila A, Hämäläinen A-M, et al. The first signs of β-cell autoimmunity appear in infancy in genetically susceptible children from the general population: the Finnish Type 1 Diabetes Prediction and Prevention Study. J Clin Endocrinol Metab. 2001;86(10):4782–4788. doi: 10.1210/jcem.86.10.7907. [DOI] [PubMed] [Google Scholar]
- 6.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 7.Dabelea D, Mayer-Davis EJ, Saydah S, et al. SEARCH for Diabetes in Youth Study. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311 (17):1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harjutsalo V, Sund R, Knip M, Groop PH. Incidence of type 1 diabetes in Finland. JAMA. 2013;310(4):427–428. doi: 10.1001/jama.2013.8399. [DOI] [PubMed] [Google Scholar]
- 9.Virtanen SM, Räsänen L, Aro A, et al. Childhood Diabetes in Finland Study Group. Infant feeding in Finnish children less than 7 yr of age with newly diagnosed IDDM. Diabetes Care. 1991;14(5):415–417. doi: 10.2337/diacare.14.5.415. [DOI] [PubMed] [Google Scholar]
- 10.Norris JM, Barriga K, Klingensmith G, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA. 2003;290(13):1713–1720. doi: 10.1001/jama.290.13.1713. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler A-G, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes–associated autoantibodies. JAMA. 2003;290(13):1721–1728. doi: 10.1001/jama.290.13.1721. [DOI] [PubMed] [Google Scholar]
- 12.Virtanen SM, Kenward MG, Erkkola M, et al. Age at introduction of new foods and advanced beta cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes. Diabetologia. 2006;49(7):1512–1521. doi: 10.1007/s00125-006-0236-1. [DOI] [PubMed] [Google Scholar]
- 13.Knip M, Virtanen SM, Åkerblom HK. Infant feeding and the risk of type 1 diabetes. Am J Clin Nutr. 2010;91(5 suppl):1506S–1513S. doi: 10.3945/ajcn.2010.28701C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Åkerblom HK, Virtanen SM, Ilonen J, et al. National TRIGR Study Groups. Dietary manipulation of beta cell autoimmunity in infants at increased risk of type 1 diabetes: a pilot study. Diabetologia. 2005;48(5):829–837. doi: 10.1007/s00125-005-1733-3. [DOI] [PubMed] [Google Scholar]
- 15.Knip M, Virtanen SM, Seppä K, et al. Finnish TRIGR Study Group. Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med. 2010;363(20):1900–1908. doi: 10.1056/NEJMoa1004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Åkerblom HK, Krischer J, Virtanen SM, et al. TRIGR Study Group. The Trial to Reduce IDDM in the Genetically at Risk (TRIGR) study: recruitment, intervention and follow-up. Diabetologia. 2011;54 (3):627–633. doi: 10.1007/s00125-010-1964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorkio S, Cuthbertson D, Bärlund S, et al. TRIGR Study Group. Breastfeeding patterns of mothers with type 1 diabetes: results from an infant feeding trial. Diabetes Metab Res Rev. 2010;26(3):206–211. doi: 10.1002/dmrr.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parkkola A, Härkönen T, Ryhänen SJ, Ilonen J, Knip M Finnish Pediatric Diabetes Register. Extended family history of type 1 diabetes and phenotype and genotype of newly diagnosed children. Diabetes Care. 2013;36(2):348–354. doi: 10.2337/dc12-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumala P, Savola K, Petersen JS, et al. Prediction of insulin-dependent diabetes mellitus in siblings of children with diabetes—a population-based study. J Clin Invest. 1998;101(2):327–336. doi: 10.1172/JCI119879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warram JH, Krolewski AS, Gottlieb MS, Kahn CR. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med. 1984;311(3):149–152. doi: 10.1056/NEJM198407193110304. [DOI] [PubMed] [Google Scholar]
- 21.Karges W, Hammond-McKibben D, Cheung RK, et al. Immunological aspects of nutritional diabetes prevention in NOD mice: a pilot study for the cow’s milk-based IDDM prevention trial. Diabetes. 1997;46(4):557–564. doi: 10.2337/diab.46.4.557. [DOI] [PubMed] [Google Scholar]
- 22.Holmberg H, Wahlberg J, Vaarala O, Ludvigsson J ABIS Study Group. Short duration of breast-feeding as a risk-factor for beta-cell autoantibodies in 5-year-old children from the general population. Br J Nutr. 2007;97(1):111–116. doi: 10.1017/S0007114507210189. [DOI] [PubMed] [Google Scholar]
- 23.Aranda P, Sanchez L, Perez MD, Ena JM, Calvo M. Insulin in bovine colostrum and milk: evolution throughout lactation and binding to caseins. J Dairy Sci. 1991;74(12):4320–4325. doi: 10.3168/jds.S0022-0302(91)78627-X. [DOI] [PubMed] [Google Scholar]
- 24.Vaarala O, Knip M, Paronen J, et al. Cow’s milk formula feeding induces primary immunization to insulin in infants at genetic risk for type 1 diabetes. Diabetes. 1999;48(7):1389–1394. doi: 10.2337/diabetes.48.7.1389. [DOI] [PubMed] [Google Scholar]
- 25.Vaarala O, Ilonen J, Ruohtula T, et al. Removal of bovine insulin from cow’s milk formula and early initiation of beta-cell autoimmunity. Arch Pediatr Adolesc Med. 2012;166(7):608–614. doi: 10.1001/archpediatrics.2011.1559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.