Abstract
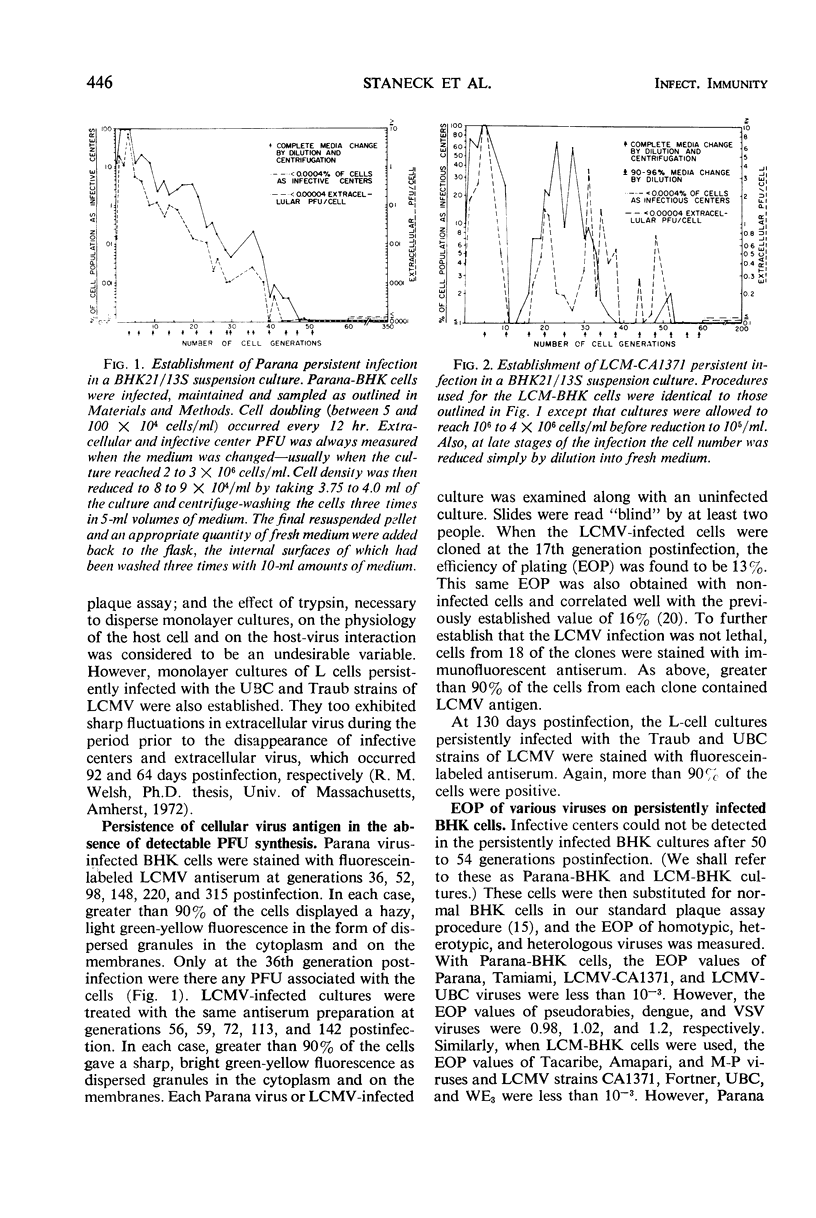
Persistent infections were established in suspension cultures of BHK21/13S cells with both Parana and lymphocytic choriomeningitis viruses. Four generations after infection with either virus, more than 90% of the cells scored as infective centers, with concomitant peaks in extracellular virus yields. In both cultures the synthesis of detectable plaque-forming units (PFU) ceased about the 50th generation postinfection, and this condition was maintained until the 350th cell generation when the cultures were discontinued. The generation time of each culture was identical to that of uninfected parent controls, and at no time were cytopathic effects evident. In spite of the absence of infectivity, over 90% of the cells sampled at various times contained viral antigen demonstrable by immunofluorescence. When either of these persistently infected cell lines was substituted for normal cells in the standard plaque assay, very low efficiencies of plating were observed for homotypic and heterotypic viruses. Plaque formation by several heterologous viruses was virtually unaffected. The mechanism of homotypic plaque exclusion in both cell lines was shown to occur beyond the virion adsorption stage. The original infecting virus genome persisted in both cell lines after standard virus was no longer detectable. This was shown with the lymphocytic choriomeningitis virus-infected cells after storage in liquid nitrogen. After thawing, such cells were found to synthesize standard virus for a brief period. Although the Parana virus-infected cells did not behave this way, the growth medium from these cells would initiate PFU synthesis in normal cells within 36 hr after infection.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEN-PORAT T., KAPLAN A. S. The chemical composition of herpes simplex and pseudorabies viruses. Virology. 1962 Mar;16:261–266. doi: 10.1016/0042-6822(62)90246-5. [DOI] [PubMed] [Google Scholar]
- Camyre K. P., Pfau C. J. Biophysical and biochemical characterization of lymphocytic choriomeningitis virus. IV. Strain differences. J Virol. 1968 Mar;2(3):161–166. doi: 10.1128/jvi.2.3.161-166.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANOFF A. Studies on mixed infection with Newcastle disease virus. I. Isolation of Newcastle disease virus mutants and tests for genetic recombination between them. Virology. 1959 Dec;9:636–648. doi: 10.1016/0042-6822(59)90154-0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Jennings W. L., Lewis A. L., Sather G. E., Pierce L. V., Bond J. O. Tamiami virus in the Tampa Bay area. Am J Trop Med Hyg. 1970 May;19(3):527–536. doi: 10.4269/ajtmh.1970.19.527. [DOI] [PubMed] [Google Scholar]
- Justines G., Johnson K. M. Immune tolerance in Calomys callosus infected with Machupo virus. Nature. 1969 Jun 14;222(5198):1090–1091. doi: 10.1038/2221090a0. [DOI] [PubMed] [Google Scholar]
- Lehmann-Grube F. A carrier state of lymphocytic choriomeningitis virus in L cell cultures. Nature. 1967 Feb 25;213(5078):770–773. doi: 10.1038/213770a0. [DOI] [PubMed] [Google Scholar]
- Lehmann-Grube F., Slenczka W., Tees R. A persistent and inapparent infection of L cells with the virus of lymphocytic choriomeningitis. J Gen Virol. 1969 Jul;5(1):63–81. doi: 10.1099/0022-1317-5-1-63. [DOI] [PubMed] [Google Scholar]
- Liebhaber H., Gross P. A. The structural proteins of rubella virus. Virology. 1972 Mar;47(3):684–693. doi: 10.1016/0042-6822(72)90558-2. [DOI] [PubMed] [Google Scholar]
- Mims C. A. Immunofluorescence study of the carrier state and mechanism of vertical transmission in lymphocytic choriomeningitis virus infection in mice. J Pathol Bacteriol. 1966 Apr;91(2):395–402. doi: 10.1002/path.1700910214. [DOI] [PubMed] [Google Scholar]
- Molomut N., Padnos M. Inhibition of transplantable and spontaneous murine tumours by the M-P virus. Nature. 1965 Dec 4;208(5014):948–950. doi: 10.1038/208948a0. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., HUNTER H. S. PURIFICATION AND PARTIAL CHEMICAL ANALYSIS OF SINDBIS VIRUS. Virology. 1963 Jul;20:433–445. doi: 10.1016/0042-6822(63)90092-8. [DOI] [PubMed] [Google Scholar]
- Pfau C. J., Trowbridge R. S., Welsh R. M., Staneck L. D., O'Connell C. M. Arenaviruses: inhibition by amantadine hydrochloride. J Gen Virol. 1972 Feb;14(2):209–211. doi: 10.1099/0022-1317-14-2-209. [DOI] [PubMed] [Google Scholar]
- Pulkkinen A. J., Pfau C. J. Plaque size heterogeneity: a genetic trait of lymphocytic choriomeningitis virus. Appl Microbiol. 1970 Jul;20(1):123–128. doi: 10.1128/am.20.1.123-128.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBIN H., BALUDA M., HOTCHIN J. E. The maturation of Western equine encephalomyelitis virus and its release from chick embryo cells in suspension. J Exp Med. 1955 Feb 1;101(2):205–212. doi: 10.1084/jem.101.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Webb P. A., Peters C. J. Serological relationship of the Tacaribe complex of viruses to lymphocytic choriomeningitis virus. J Virol. 1970 Mar;5(3):289–292. doi: 10.1128/jvi.5.3.289-292.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustigian R. Persistent infection of cells in culture by measles virus. I. Development and characteristics of HeLa sublines persistently infected with complete virus. J Bacteriol. 1966 Dec;92(6):1792–1804. doi: 10.1128/jb.92.6.1792-1804.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLESINGER W., FRANKEL J. W. Adaptation of the New Guinea B strain of dengue virus to suckling and to adult swiss mice; a study in viral variation. Am J Trop Med Hyg. 1952 Jan;1(1):66–77. doi: 10.4269/ajtmh.1952.1.66. [DOI] [PubMed] [Google Scholar]
- STOKER M., MACPHERSON I. SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Nature. 1964 Sep 26;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- TRAUB E. Multiplication of LCM virus in lymph node and embryo cells from non-tolerant and tolerant mice. Arch Gesamte Virusforsch. 1962;11:473–486. doi: 10.1007/BF01241301. [DOI] [PubMed] [Google Scholar]
- Trapido H., Sanmartín C. Pichindé virus, a new virus of the Tacaribe group from Colombia. Am J Trop Med Hyg. 1971 Jul;20(4):631–641. [PubMed] [Google Scholar]
- WILSNACK R. E., ROWE W. P. IMMUNOFLUORESCENT STUDIES OF THE HISTOPATHOGENESIS OF LYMPHOCYTIC CHORIOMENINGITIS VIRUS INFECTION. J Exp Med. 1964 Nov 1;120:829–840. doi: 10.1084/jem.120.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb P. A., Johnson K. M., Hibbs J. B., Kuns M. L. Parana, a new Tacaribe complex virus from Paraguay. Arch Gesamte Virusforsch. 1970;32(4):379–388. doi: 10.1007/BF01250066. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Pfau C. J. Determinants of lymphocytic choriomeningitis interference. J Gen Virol. 1972 Feb;14(2):177–187. doi: 10.1099/0022-1317-14-2-177. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Trowbridge R. S., Kowalski J. B., O'Connell C. M., Peau C. J. Amantadine hydrochloride inhibition of early and late stages of lymphocytic choriomenigitis virus-cell interactions. Virology. 1971 Sep;45(3):679–686. [PubMed] [Google Scholar]


