Abstract
Objectives
To locate a plant with suitable phytochemicals for use as antimicrobial agents to control multidrug-resistant (MDR) bacteria as a complementary medicine, without host toxicity as monitored through cultured lymphocytes from human umbilical cord blood.
Methods
The methanol crude leaf extract of the plant Woodfordia fruticosa was subjected to antimicrobial assay in vitro with nine pathogenic MDR bacteria from clinical samples. This was followed by bioassay-guided fractionation with seven non-polar to polar solvents, gas chromatography–mass spectrometry analysis of the n-butanol fraction, and monitoring of the host toxicity of the leaf extract with in vitro grown lymphocytes from human umbilical cord blood.
Results
The leaf extract of W. fruticosa had a controlling capacity for MDR bacteria. The minimum inhibitory concentration and minimum bactericidal concentration of the n-butanol fraction were < 1.89 mg/mL extract and 9.63 mg/mL extract, respectively. The gas chromatography–mass spectrometry spectrum of the n-butanol fraction confirmed the presence of 13 peaks of different compounds with retention times of 9.11 minutes, 9.72 minutes, 10.13 minutes, 10.78 minutes, 12.37 minutes, 12.93 minutes, 18.16 minutes, 21.74 minutes, 21.84 minutes, 5.96 minutes, 12.93 minutes, 24.70 minutes, and 25.76 minutes. The six leading compounds were: diethyl phthalate: IUPAC name: diethyl benzene-1,2-dicarboxylate; 5-methyl-2-(1-methylethyl) phenol: IUPAC name: 5-methyl-2-propan-2-ylphenol; (E )-3,7-dimethylocta-2,6-diene-1-thiol: IUPAC name: (2Z)-3,7-dimethylocta-2,6-diene-1-thiol; 2,6,10-dodecatrien-1-ol, 3,7,11-trimethyl-, (E,E ): IUPAC name: 2,6,10-dodecatrien-1-ol; 3,7,11-trimethyl-, (E,E); 2-methoxy-4-(2-propenyl) phenol: IUPAC name: 2-methoxy-4-[(1E)-prop-1-en-1-yl]phenol; hexadecanoic acid: IUPAC name: hexadecanoic acid.
Conclusion
The presence of antimicrobial compounds that are therapeutically potent against MDR bacteria was confirmed in W. fruticosa. The crude leaf extract showed no host toxicity with human lymphocytes; the n-butanol fraction of the extract was the most suitable bioactive fraction. The terpenes isolated were: 5-methyl-2-(1-methylethyl) phenol, 2-methoxy-4-(2-propenyl) phenol, 2,6-octadien-1-ol, 3,7-dimethyl-(E)-2,6-octadienal, 3,7-dimethylcyclohexanol, and cyclohexanol, 2-methylene-5-(1-methylethenyl) which were reported to have specifically antimicrobial activity.
Keywords: gas chromatography–mass spectrometry analysis, human lymphocytes, multidrug-resistant bacteria, Woodfordia fruticosa
1. Introduction
Several plant species have been used by many generations of local ethnic tribes, especially those of the Kalahandi District, Odisha, India for holistic health care [1]. This practice has been validated by the Indian ayurvedic school, Indian traditional medicine, and Indian folklore medicine for several hundred plants [2]. In addition, a number of crude drugs known as aristha and asava are prepared, marketed, and consumed by much of the Indian population. Woodfordia fruticosa has many ethno-botanical roles as a traditional medicine, such as curing bowel disorders, dysentery, diarrhea, ulcers, and other infectious diseases, in addition to treating rheumatism [3–5]. This plant can cure peptic ulcers induced by Helicobacter pylori [6]. Therefore it was thought to be worthwhile to study its antibacterial activity against bacterial pathogens from clinical samples.
Infection and morbidity as a result of multidrug-resistant (MDR) bacteria in both community and hospital settings has been a problem for many decades – for example, methicillin-resistant Staphylococcus aureus (MRSA) is currently resistant to 23 antibiotic drugs [7]. Other pathogenic bacteria, such as various species of Acinetobacter, Pseudomonas, and Klebsiella have developed clonal nexuses so much that these, mainly Gram-negative, bacteria have been recorded as potent MDR bacteria in nosocomial surveys of patients in our hospital over the past 5 years [8–10]. The effect of MDR bacterial pathogens can be illustrated by the example of urinary tract infections (UTIs), which are common infections affecting > 50% of the population at some time in their life. UTIs are treated empirically with an antimicrobial stewardship program, but when the causative bacteria in repeated infections are found to be MDR, the failure of the empirical treatment can be devastating [9]. A patient with a UTI may initially have cystitis, which, if neglected or if the empirical treatment fails, leads to kidney infection (pyelonephritis). Ultimately, the infection may spread to other vulnerable zones such as the heart and lungs. This may cause a cough and an infection spread via the bloodstream from the kidneys may lead to endocarditis and terminal respiratory tract infections. To overcome this snowball effect of a UTI infection, in addition to mainstream treatment with antibiotic drugs, the use of a medicine from a complementary/supplementary source might be a prudent approach in view of the thousands of published research papers claiming that medicinal plants may have antimicrobial activities [1].
The resistant, or rather non-committal, attitude of mainstream medicinal practices has restrained the use of plethora of natural compounds from plant sources [11]. However, the most obvious method of treating a bacterial infection is to use antibiotics from microbes, i.e., from organisms with a similar heritage. If scaled up, crude plant extracts could be fractioned and the active antimicrobial fraction could be isolated and used as a complementary medicine together with the prescribed mainstream drug to control infectious diseases because no microbe, however well-equipped genetically by multidrug-resistance, can win over an array of phytocompounds.
This paper describes the antibacterial activities of crude leaf extracts of W. fruticosa and its fractions extracted using seven non-polar and polar solvents. The best solvent fraction was used to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values against MDR strains of nine pathogenic bacteria isolated from clinical samples. This work is better than other antimicrobial work with plants with standard bacterial reference strains from culture centers with undefined antibiotic sensitivity patterns of used bacteria, available in literature. The best solvent fraction was used for gas chromatography–mass spectroscopy (GC-MS) analysis to locate lead compounds that could be the coveted antimicrobial agents. The crude plant extract was tested for possible host toxicity by monitoring its activity against lymphocytes grown in vitro from human umbilical cord blood. The use of a bioactive fraction of a leaf extract of a plant without any host toxicity as a complementary antimicrobial agent for use alongside an antibiotic drug would be a novel approach against MDR bacteria.
2. Materials and methods
2.1. Collection of plant material and preparation of plant fractions
A methanolic extract was obtained from the dried leaf powders of W. fruticosa Kurz. via a 24-hour hot extraction method using a Soxhlet apparatus (Figure 1). The extract was filtered and the filtrate dried in vacuo. The crude methanol extract was subjected to a bioassay-guided fractionation by solubilizing in water followed by sequential partition with n-hexane, chloroform, ethyl acetate, dichloromethane, and n-butanol. The end product is referred to as the methanol fraction. Each collected fraction was concentrated under reduced pressure to form a dark residue.
Figure 1.

Woodfordia fruticosa Kurz.
2.2. Isolation and identification of pathogenic bacteria
Bacterial strains were isolated from clinical samples (urine, pus, swabs, or blood samples) from patients admitted to different wards of the hospital, including intensive care and neonatal intensive care units, wards, and cabins. The samples were cultured on suitable agar media and the bacterial isolates were identified using VITEK2 (Bimereux, New Delhi, India) and standard biochemical procedures following the Clinical and Laboratory Standards Institute (CLSI) guidelines [12]. Standard microbial type culture collection (MTCC), Chandigarh strains of bacteria were used as reference controls. Three Gram positive bacteria [MRSA, Streptococcus pyogenes, and vancomycin-resistant Enterococcus faecalis (VRE)] and six Gram-negative extended spectrum beta lactamase (ESBL) producing strains of bacteria (Acinetobacter baumannii, Klebsiella pneumoniae, Proteus vulgaris, Pseudomonas aeruginosa, Citrobacter freundii, and Proteus mirabilis) were isolated and used in this study.
2.3. Antibiotic susceptibility test
All of the nine bacterial strains used were subjected to an antibiotic sensitivity test using the Kirby–Bauer method or the disc diffusion method [13].
2.4. MRSA, vancomycin-resistant S. pyogenes, E. faecalis, and ESBL production
The isolated strains of S. aureus, S. pyogenes, and E. faecalis were subjected to the chromogenic agar media test and vancomycin screen agar plate test to confirm their MRSA and vancomycin-resistant S. pyogenes and Enterococcus (VRE) status, respectively [14]. Similarly, to determine the ESBL producers in the remaining six Gram negative bacteria, the double disc synergy test was used [8].
2.5. Antibacterial activity test by agar-well diffusion method
The antibacterial activities of the seven different solvent fractions (Merck, Mumbai, India; HiMedia, Mumbai, India; Sigma-Aldrich, Mumbai, India.) were determined using the agar-well diffusion method [14]. Linezolid (30 μg/mL) and imipenem (10 μg/mL) were used as reference controls for the Gram-positive and Gram-negative bacterial work, respectively. All the Gram-negative bacteria were ESBL producers; of the three Gram-positive bacterial species, S. aureus was MRSA and another two were resistant to vancomycin.
2.6. Determination of MIC and MBC
The MIC and MBC of the active n-butanol fraction were determined as described previously [13].
2.7. GC–MS analysis
The GC-MS analysis of the n-butanol fraction was carried out using an instrument equipped with a VF-5 ms fused-silica capillary column of 30 m length, 0.25 mm diameter, and 0.25 μm film thickness. An electron ionization system with an ionization energy of 70 eV was used as the detector. Helium gas (99.99%) was used as the carrier gas at the constant flow-rate of 1.51 mL/minute. The temperatures of the injector and mass transfer line were set at 200°C and 240°C, respectively. The oven temperature was programmed from 70°C to 220°C at 10°C/minute, held constant for 1 minute and finally increased to 300°C at 10°C/minute. Aliquots of 2 μL of the diluted samples were manually injected in the split-less mode with a split ratio of 1:40 and with a mass scan range of 50–600 AMU. The total running time of the GC–MS analysis was 60 minute.
2.8. Identification of compounds
The phytochemical components of the biologically active fraction (the n-butanol fraction) were identified by comparing their mass spectra fragmentations and retention indices with those stored in the following databases: NIST08.LIB (Stein SE National Institute of Standards and Technology, Mass Spectral Database and Software.Version 3.02, NIST, Gaithersburg, Md, USA, 1990) and WILEY8.LIB [15], and also with published data.
2.9. Toxicity testing of crude plant extract with lymphocytes grown in vitro from human cord blood monitored by the AO/EB staining method
2.9.1. Collection of lymphocytes
Umbilical cord blood (UCB) was collected in a sterile 15- or 50-mL sized Falcon tube (Tarson, Kolkata, India) with an aliquot of 100 μL or 250 μL of 1000 IU heparin (HiMedia, Mumbai, India), immediately after the delivery of an infant. The blood sample (<15–50 mL) was stored at 4°C until use. Lymphocytes were isolated immediately or within 24 hours of collection. To isolate the lymphocytes, the collected UCB sample was diluted with an equal volume of phosphate-buffered saline (PBS) solution. The mixture was then loaded carefully into a centrifuge tube for over-layering with lymphocyte separating medium (HiMedia), which was one-third the total volume of the mixture. The mixture was centrifuged at 1800 × g for 25 minutes at 22–24°C. Four heavy to light layers (red blood cells, lymphocyte separating medium, buffy coat, and plasma) were seen. The buffy coat layer with mononuclear cells was carefully removed from the tube. After the addition of another aliquot of PBS to the separated cells of the buffy coat layer at a ratio of 1:1, the sample was centrifuged again at 2000 × g for 5 minutes. The lymphocyte pellet was cultured and the cells were counted using a haemocytometer [15].
2.9.2. Growth of lymphocytes
After separation, the lymphocytes derived from UCB were diluted to a density of 1 × 106 cells/mL with the required volume of Dulbecco's Modified Eagle's Medium (DMEM, low glucose; HiMedia) and were loaded into a six-well culture plate (Tarson) containing 15% fetal bovine serum (Sigma, Sigma-Aldrich, Mumbai, India), 1% penicillin–streptomycin, and 1% sodium pyruvate, along with graded concentrations of plant extract for the in vitro growth of lymphocytes. The stock solution was prepared by dissolving 100 mg of plant extract in an aliquot of 100 mL of triple-distilled water to give a concentration of 1000 mg/L and the stock solution was stored at 4°C for further use. A total volume of 2 mL was maintained in each well of the culture plate using the plant extract solution. The cells were incubated with different concentrations of plant extract (0 mg/L, 20 mg/L, 40 mg/L, 60 mg/L, 80 mg/L, 100 mg/L, 120 mg/L, 140 mg/L, 160 mg/L, 180 mg/L, 200 mg/L, 220 mg/L, 240 mg/L, 260 mg/L, 280 mg/L, and 300 mg/L) and grown in an incubator at 37°C in an atmosphere of 5% CO2 for 24 hours [15].
2.9.3. Monitoring toxicity with lymphocytes
The viability of lymphocytes grown in the presence of graded concentrations of plant extract was determined by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The MTT solution was prepared at a concentration of 5 mg/mL in PBS. After 24 hours of plant extract treatment in six-well culture plate, 80 μL of MTT solution were added to each well to study the toxicity effect. The plate was stored in an incubator (37°C, 5% CO2) for 4 hours. It was found that the media containing the cells and a toxin changed to a blue color after incubation with MTT. The samples were then gently centrifuged at 1000 rpm = 157 × g for 10 minute at 22°C. The supernatant was removed and the pellet was dissolved in 1 mL of 100% dimethyl sulfoxide (DMSO) and stored in the incubator for 1 hour. A purple color was seen. The optical density was measured with a spectrophotometer at 570 nm [16]:
| (1) |
MTT in DMSO solution was taken as the blank. Probits of the observed lethality percentage were used for the analysis of toxicity.
2.9.4. Comet assay
Lymphocytes were cultured with different concentrations of the plant extract and the DNA damage in the harvested cells were determined by the neutral comet assay. Slides were coated with 1% agarose and allowed to dry in air. The lymphocyte pellets obtained by centrifugation of the cultured cells were washed with PBS and the pellet was mixed with three times the cell volume of the pellet with low melting point agarose 1% in a sol state. The mixture of cells and low melting point agarose sol was placed over the agarose-coated slide, which was then kept at 4°C for 10 minutes until dry. The dried slides were submerged into a pre-cooled lysing solution of 2.5 M NaCl, 100 mM EDTA, 10 mM pH 7.4 Tris, 1% Triton-X 100, and 10 mM dithiothreitol. The mixture was stored at 4°C in the dark for about 2 hours. The slides were subsequently removed and placed in an electrophoresis buffer with 500 mM NaCl, 100 mM Tris, 1 mM EDTA, and 0.2% DMSO, for 20 minutes. The slides were transferred to a horizontal gel electrophoresis chamber with fresh electrophoretic buffer. Electrophoresis was carried out at 10 V and 250 mA for 60 minutes. After electrophoresis the slides were washed in PBS for 5 minutes and placed in a neutralizing solution with 50% ethanol and 20 mM Tris for 5 minutes, then washed again in PBS. After 5 minutes the slides were stained with ethidium bromide solution. The slides were observed under a fluorescence microscope at 400 × magnification and the comets were scored. Probits of observed lethality percentage values calculated from the percentage values of observed comets due to the treatment of plant extract were used for the analysis of toxicity [15].
3. Results
3.1. Antibiotic susceptibility of isolated bacteria
Antibiotic susceptibility tests for three Gram-positive and six Gram-negative bacterial isolates were carried out; 18 antibiotics from seven groups were used against Gram-positive bacteria; 16 of 18 antibiotics (except oxacillin and vancomycin) were used against the Gram-negative bacteria. The MRSA strain was found to be sensitive to two antibiotics (ciprofloxacin and chloramphenicol), but was resistant to the other 16 antibiotics. Similarly, A. baumannii was resistant to 14 antibiotics and was sensitive to two antibiotics (gentamicin and chloramphenicol). Antibiograms of other three Gram-positive bacteria and five Gram-negative bacteria were recorded (Table 1). All the isolated Gram-negative bacteria were also ESBL producers.
Table 1.
Antibiogram of clinically isolated pathogenic Gram-positive and Gram-negative bacteria.
| Bacteria | Susceptibility to prescribed antibioticsa |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminoglycosides |
β-lactams |
Cephalosporins |
Fluoroquinolones |
Glycopeptides |
Sulfonamides |
Stand-alone drugs |
||||||||||||
| Ac | Ge | Am | Ak | Ox | Pt | Ce | Cf | Ci | Gf | Na | No | Of | Va | Cot | Ch | Nf | Te | |
| MRSAb | R | R | R | R | R | R | R | R | S | R | R | R | R | R | R | S | R | I |
| Streptococcus pyogenesb | R | R | R | R | R | R | R | R | S | R | R | R | R | R | S | S | R | R |
| VREb | R | R | R | R | R | R | R | R | S | R | R | R | R | R | S | S | R | R |
| Klebsiella pneumoniae | R | R | R | R | Nd | R | R | R | R | R | R | R | R | Nd | R | R | R | R |
| Acinetobacter baumannii | R | S | R | R | Nd | R | R | R | R | R | R | R | R | Nd | R | R | R | R |
| Citrobacter freundii | S | S | R | R | Nd | S | R | R | S | R | S | S | S | Nd | R | S | I | S |
| Proteus mirabilis | R | R | R | R | Nd | R | R | R | I | R | R | R | R | Nd | R | R | R | R |
| Proteus vulgaris | R | R | R | R | Nd | R | R | R | I | R | R | R | R | Nd | R | R | I | R |
| Pseudomonas aeruginosa | R | I | R | R | Nd | R | R | R | S | R | R | R | R | Nd | R | R | R | R |
I = moderately sensitive; MRSA = methicillin-resistant Staphylococcus aureus; R = resistant; S = sensitive; VRE = vancomycin-resistant Enterococcus.
Antibiotics (μg/disc): Ac = amikacin 30; Ak = amoxyclav 30; Am = ampicillin 10; Ce = ceftriaxone 30; Cf = cefpodoxime 10; Ch = chloramphenicol 30; Ci = ciprofloxacin 5; Co-t = co-trimoxazole 25; Ge = gentamicin 10; Gf = gatifloxacin 5; Na = nalidixic acid 30; Nf = nitrofurantoin 300; No = norfloxacin 10; Of = ofloxacin 5; Ox = oxacillin 30; Pt = piperacillin/tazobactam 100/10; Te = tetracycline 30; Va = vancomycin 30; Nd = not done.
Gram-positive bacteria.
3.2. Antibacterial activities of the seven solvent fractions
The antibacterial activities of the seven solvent fractions were monitored by the agar-well diffusion method on separate lawn cultures of nine bacterial isolates (3 Gram-positive and 6 Gram-negative bacteria). The n-butanol leaf fraction of W. fruticosa registered the maximum diameter of the size zone of inhibition against MRSA (37 mm), followed by C. freundii (33 mm). The methanolic fraction registered the maximum size of zone inhibition against MRSA (30 mm). The n-hexane and dichloromethane fractions registered very low antibacterial activity compared with the other five solvent fractions. The antibacterial activities of all the other fractions were recorded (Table 2).
Table 2.
Antibacterial assay by agar-well diffusion method of hot solvent leaf extract fractions of Woodfordia fruticosa against MDR strains of bacteria.a
| Bacteria | n-Hexane | Chloroform | Ethyl acetate | Dichloromethane | Acetone | Butanol | Methanol | Linezolid/imipenem (30/10 μg/mL) |
|---|---|---|---|---|---|---|---|---|
| Methicillin-resistant Staphylococcus aureus | 10 | 21 | 23 | 29 | 23 | 37 | 30 | 29 |
| Streptococcus pyogenes | 15 | 22 | 19 | 19 | 18 | 26 | 26 | 29 |
| VRE | 17 | 15 | 19 | 25 | 26 | 29 | 29 | 33 |
| Acinetobacter baumannii | 8 | 12 | 22 | 19 | 13 | 28 | 26 | 31 |
| Citrobacter freundii | 11 | 18 | 24 | 23 | 19 | 33 | 30 | 26 |
| Proteus mirabilis | 10 | 19 | 23 | 29 | 19 | 23 | 22 | 29 |
| Proteus vulgaris | 18 | 18 | 25 | 19 | 15 | 32 | 26 | 26 |
| Pseudomonas aeruginosa | 9 | 23 | 22 | 25 | 19 | 34 | 27 | 29 |
Results given as diameter of the zone of inhibition (mm).
3.3. MIC and MBC values
The MIC and MBC values of the n-butanol fraction were determined as it registered the maximum level of antibacterial activity. An MIC value of 0.37 mg/mL of the n-butanol fraction was recorded against MRSA, S. pyogenes, and C. freundii; a value of 0.141 mg/mL was recorded against A. baumannii. An MIC value of 1.89 mg/mL was recorded against P. mirabilis and P. aeruginosa, while 0.83 mg/mL was the MIC value against VRE and P. vulgaris (Table 3). An MBC value of 1.89 mg/mL of the n-butanol fraction was registered against MRSA, S. pyogenes, A. baumannii, and C. freundii; a value of 4.27 mg/mL was registered against VRE, whereas P. mirabilis, P. vulgaris, and P. aeruginosa had an MBC value of 9.63 mg/mL (Table 3).
Table 3.
Minimum inhibitory concentration and minimum bactericidal concentration of the best bioactive n-butanol fraction of Woodfordia fruticosa against multidrug-resistant bacterial strains (mg/mL).
| Strain |
n-Butanol |
|
|---|---|---|
| MIC | MBC | |
| Methicillin-resistant Staphylococcus aureus | 0.37 | 1.89 |
| Streptococcus pyogenes | 0.37 | 1.89 |
| VRE | 0.83 | 4.27 |
| Acinetobacter baumannii | 0.14 | 1.89 |
| Citrobacter freundii | 0.37 | 1.89 |
| Proteus mirabilis | 1.89 | 9.63 |
| Proteus vulgaris | 0.83 | 9.63 |
| Pseudomonas aeruginosa | 1.89 | 9.63 |
MBC = minimum bactericidal concentration; MIC = Minimum inhibitory concentration.
3.4. Phytochemical analysis and GC-MS study
The phytochemical screening of the n-butanol leaf fraction of W. fruticosa revealed the presence of alkaloids, glycosides, terpenoids, steroids, saponins, and tannins. The results of the GC-MS analysis led to the identification of different compounds from the n-butanol fraction (Figure 2). The structures of the compounds were based on the analysis of the fragmentation pattern of the mass spectra, a direct comparison of their spectral data with the chemical profiles in the National Institute of Standards and Technology (NIST) library, Gaithersburg, Md,D, USA, and comparisons with published mass spectra.
Figure 2.
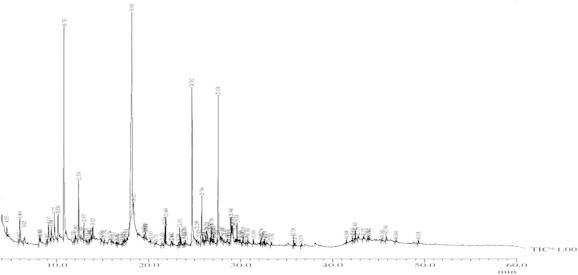
Gas chromatography–mass spectrometry chromatogram of the n-butanol fraction of W. fruticosa.
The chemical profiles of the identified compounds, together with their retention time, percentage peak area, molecular formula, molecular weight, structure, nature of compound, and reported activity are given in Table 4. The studies of the biologically active compounds in W. fruticosa by GC–MS analysis clearly showed the presence of 13 compounds, namely: (1) diethyl phthalate (26.77%); (2) 5-methyl-2-(1-methylethyl) phenol (13.37%); (3) (E)-3,7-dimethylocta-2,6-diene-1-thiol (10.71%); (4) 2,6,10-dodecatrien-1-ol-3,7,11-trimethyl-(E,E) (9.15%); (5) 2-methoxy-4-(2-propenyl) phenol (3.42%); (6) hexadecanoic acid (2.88%); (7) 1,6-octadien-3-ol-3,7-dimethyl (1.64%), (8) cyclohexanol, 2-methylene-5-(1-methylethe (1.98%); (9) 2,6-octadien-1-ol, 3,7-dimethyl-, (E)- (1.97%); (10) 2,6-octadienal, 3,7-dimethyl- (1.84%); (11) 2,6-octadien-1-ol, 3,7-dimethyl-, acetate, (E)-(1.18%); (12) tetradecanoic acid (1.00%), benzyl benzoate (1.21%); and (13) 10,12-hexadecadien-1-ol (1.14%).
Table 4.
Phyto-components identified in butanol fraction of leaves of Woodfordia fruticosa.
| Peak | Retention time (min) | Area | Area (%) | Molecular weight | Molecular formula | Name | Chemical nature | Chemical structure |
|---|---|---|---|---|---|---|---|---|
| 1 | 9.113 | 4,131,489 | 1.98 | 152 | C10H16O | Cyclohexanol, 2-methylene-5-(1-methylethenyl (Isocarveol) | Monocyclic terpene alcoholic derivative | 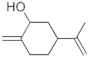 |
| 2 | 9.727 | 4,102,469 | 1.97 | 154 | C10H18O | 2,6-Octadien-1-ol, 3,7-dimethyl-, (E)-(Geraniol) | Alicyclic monoterpene alcohol | |
| 3 | 10.136 | 3,839,900 | 1.84 | 152 | C10H16O | 2,6-Octadienal, 3,7-dimethyl (Citral) | Alicyclic monoterpene aldehyde | 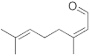 |
| 4 | 10.783 | 27,829,208 | 13.37 | 150 | C10H14O | 5-Methyl-2-(1-methylethyl) phenol (Thymol) | Monocyclic terpene phenol | 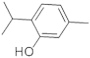 |
| 5 | 12.374 | 7,115,681 | 3.42 | 164 | C10H12O2 | 2-Methoxy-4-(2-propenyl) phenol (Eugenol) | Monocyclic terpene phenolic ether |  |
| 6 | 12.937 | 2,452,534 | 1.18 | 196 | C12H20O2 | 2,6-Octadien-1-ol, 3,7-dimethyl-, acetate, (E)-(Geranyl acetate) | Alicyclic monoterpene ester | |
| 7 | 18.163 | 55,723,750 | 26.77 | 222 | C12H14O4 | Diethyl phthalate | Diester of phthalic acid |  |
| 8 | 21.744 | 2,090,511 | 1.00 | 228 | C14H28O2 | Tetradecanoic acid (Myristic acid) | Saturated fatty acid | |
| 9 | 21.840 | 2,509,298 | 1.21 | 212 | C14H12O2 | Benzyl benzoate | Aromatic ester | |
| 10 | 5.969 | 3,421,471 | 1.64 | 154 | C10H18O | 1,6-Octadien-3-ol, 3,7-dimethyl (Linalool) | Alicyclic monoterpene alcoholic | |
| 11 | 12.937 | 2,452,534 | 1.18 | 196 | C12H20O2 | 2,6-Octadien-1-ol, 3,7-dimethyl-, acetate, (E)- (Geranyl acetate) | Alicyclic monoterpene ester | |
| 12 | 24.702 | 22,293,627 | 10.71 | 170 | C10H18S | (E)-3,7-dimethylocta-2,6-diene-1-thiol (Thiogeraniol) | Alicyclic monoterpene thiol |  |
| 13 | 25.764 | 5,994,750 | 2.88 | 256 | C6H32O2 | Hexadecanoic acid (palmitic acid) | Saturated fatty acid |
The GC–MS spectrum confirmed the presence of 13 peaks of different compounds with retention times of 9.11 minute, 9.72 minute, 10.13 minute, 10.78 minute, 12.37 minute, 12.93 minute, 18.16 minute, 21.74 minute, 21.84 minute, 5.96 minute, 12.93 minute, 24.70 minute, and 25.76 minute, respectively (Figure 3A−M). The mass spectrometer characterized the compounds at different times to identify the chemical nature and structure of the eluted compounds. The large compounds fragment into small compounds giving rise to peaks at different m/z ratios.
Figure 3.
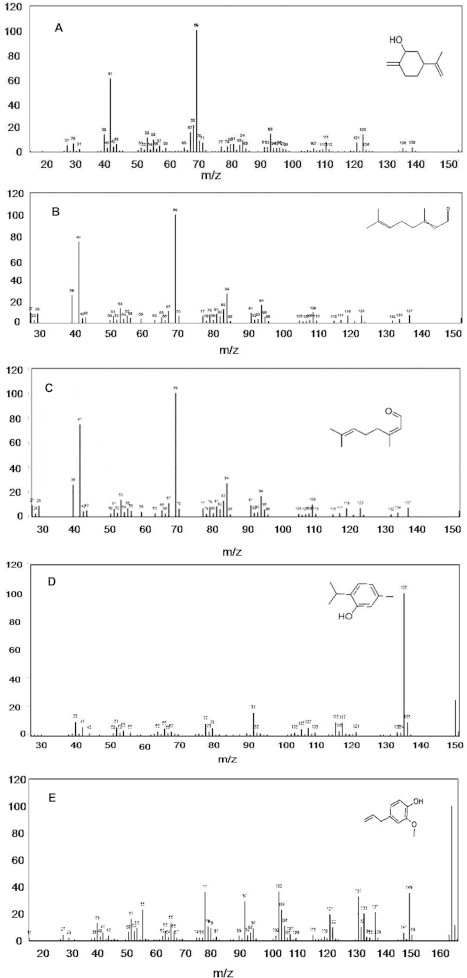
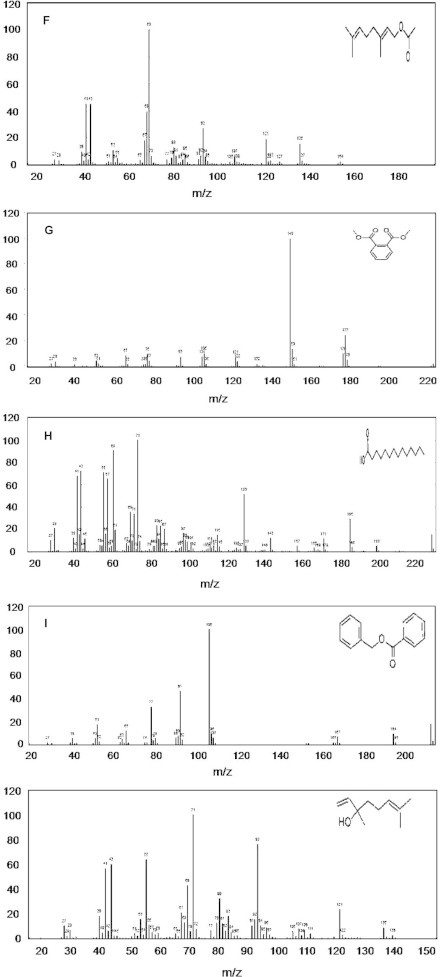
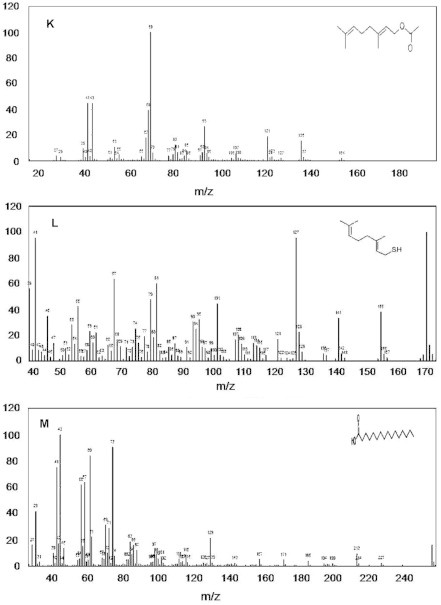
Mass spectra of 13 compounds, with structures as an insert in individual figures. (A) cyclohexanol, 2-methylene-5-(1-methylethenyl); (B) 2,6-octadien-1-ol, 3,7-dimethyl-, (E)-; (C) 2,6-octadienal, 3,7-dimethyl-; (D) phenol, 5-methyl-2-(1-methylethyl)-; (E) phenol, 2-methoxy-4-(2-propenyl)-; (F) 2,6-octadien-1-ol, 3,7-dimethyl-, acetate, (E)-; (G) diethyl phthalate; (H) tetradecanoic acid; (I) benzyl benzoate; (J) 1,6-octadien-3-ol, 3,7-dimethyl; (K) 2,6-octadien-1-ol, 3,7-dimethyl-, acetate, (E)-; (L) (E)-3,7-dimethylocta-2,6-diene-1-; and (M) hexadecanoic acid.
3.5. Assessment of plant toxicity
The percentage lethality values recorded from data sets of the MTT assay and its probit were used to construct a plot which was then used for extrapolation to compute the individual lethal concentration (LC) values (LC25, LC50, and LC75) for each method (Figure 4). The individual MIC, highest permissive concentration, and LC100 values were taken directly from the experiments.
Figure 4.
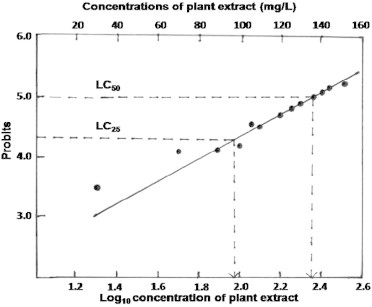
Probits of the percentage lethality values were plotted against the log10 concentration of plant extracts in the toxicity study of human lymphocytes.
3.5.1. MTT assay
The cells without a toxin had a higher OD570 value than the cells treated with different concentrations of plant extract. The cell density gradually decreased from 20 mg/L to 300 mg/L. Experimentally, the MIC value for lymphocytes was 20 mg/L. Probit values and log10 values of the concentrations of plant extracts in the plot yielded log10values for LC25 and LC50 of 1.99 and 2.38, respectively. These log10 concentration values generated LC values of 97.72 (LC25) and 239.88 mg/L (LC50) (Table 5).
Table 5.
Probit transformations of percentage lethality values during crude plant extract toxicity to human lymphocytes growing in Dulbecco's Modified Eagle's Medium, assessed by 3-[4, 5-dimethylthiazol-2-yl] 2,5-diphenyl tetrazolium bromide assay.
| Concentration of plant extract (mg/L) | Log10 concentrations of plant extract | OD | PL of cells by MTT Assay | Probits of MTT Assay |
|---|---|---|---|---|
| 0 | – | 1.984 | 0 | – |
| 20 | 1.30 | 1.861 | 7.8 | 3.58 |
| 40 | 1.60 | 1.843 | 8.7 | 3.64 |
| 60 | 1.77 | 1.619 | 20.0 | 4.15 |
| 80 | 1.90 | 1.619 | 20.0 | 4.15 |
| 100 | 2.0 | 1.541 | 23.9 | 4.29 |
| 120 | 2.07 | 1.432 | 29.4 | 4.45 |
| 140 | 2.14 | 1.376 | 32.2 | 4.53 |
| 160 | 2.20 | 1.210 | 40.6 | 4.76 |
| 180 | 2.25 | 1.143 | 44.0 | 4.84 |
| 200 | 2.30 | 1.101 | 46.1 | 4.90 |
| 220 | 2.34 | 1.087 | 46.8 | 4.91 |
| 240 | 2.38 | 1.004 | 51.0 | 5.02 |
| 260 | 2.41 | 0.987 | 51.8 | 5.04 |
| 280 | 2.44 | 0.922 | 55.1 | 5.12 |
| 300 | 2.47 | 0.845 | 59.0 | 5.22 |
MTT = 3-[4, 5-dimethylthiazol-2-yl] 2,5-diphenyl tetrazolium bromide; OD = optical density; PL = percentage lethality; – = not applicable.
3.5.2. Comet assay
Single-cell gel electrophoresis was carried out to study the DNA damage in cells treated with different concentrations of plant extract (Figure 5A and B). No comet was found in the cells treated with different concentrations of plant extract.
Figure 5.
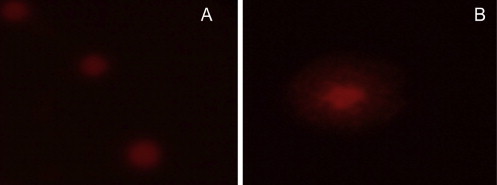
Comet assay with lymphocytes. (A) Control cells; and (B) cells after treatment with 300 mg/L plant extract.
4. Discussion
The antibiogram patterns of three Gram-positive and five Gram-negative bacterial strains clearly indicated resistance to most of the currently used antibiotics. In clinical management, this means that a doctor would not be able to choose an antibiotic to treat a patient empirically and this scenario of multiple resistance may cause the bacteria spread in both hospital and community settings. This work clearly indicated that the n-butanol fraction was the leading or active fraction of the leaf extract and that it could control eight MDR bacteria. The GC–MS analysis of the n-butanol fraction of W. fruticosa indicated the presence of 13 compounds, of which the predominant compounds were determined.
MRSA strains (40.1% of total isolates) reported from Nepal were resistant to antibiotics (amikacin, cephalexin, ciprofloxacin, norfloxacin, and trimethoprim/sulfamethoxazole), apart from penicillin derivatives, but all the strains were vancomycin-sensitive [17]. Daptomycin is now often seen as to be effective against MRSA [18,19]. In Brazil, urinary and respiratory tract infections, boils, and surgical wound infections yielded MRSA at a prevalence of 40–60% in nosocomial settings and the presence of the mecA gene in those strains was demonstrated [20]. It was reported from Malaysia that, among 287 bacterial isolates, 45% were Gram-positive bacteria with S. aureus (40%), group B Streptococci (25%), and Enterococcus sp. (9%); of the rest, 52% were Gram-negative bacterial with Proteus sp. (25%), P. aeruginosa (25%), K. pneumoniae (15%), and E. coli (9%). Susceptibility of the Gram-negative bacteria to imipenem and amikacin and the Gram-positive bacteria to vancomycin was recorded [21]. MRSA is invasive through the eye [22] and by the intravenous mode [23].
Antibacterial studies of the effect of plant extracts on non-resistant bacteria have been reported previously [11]. Long-term hospitalization may lead to extraneous infection of a patient from MDR bacterial isolates, particularly MRSA, P. aeruginosa, Klebsiella sp., and A. baumannii. It was also reported that 51.5% of patients infected with MRSA had already been infected at the time of admission to hospital, suggesting the introduction of a new MRSA strain onto the hospital from the community [24]. Moreover, in England and Wales, < 2% of S. aureus strains were methicillin resistant in 1990, but in 2002 about 42% of S. aureus were MRSA. It has been estimated that 300,000 infections with MRSA led to 5000 deaths [25]. Nevertheless, vancomycin has always been the drug of choice against MRSA infections. Our results recorded the isolation of vancomycin-resistant S. aureus, which has been an additional clinical problem in this hospital [26]. Complementary medicines are developed on the principle of “comparative effectiveness research” and isolated phyto-compounds could be promoted, when the source plant has no host toxicity, as seen with W. fruticosa. There is increasing interest in correlating phytochemical compounds with their biological activity [27].
In a study from Mysore, India, W. fruticosa was reported to have antibacterial activity against the standard MTCC strains of the Gram-positive pathogens S. aureus and Streptococcus faecalis, with a zone of inhibition > 21 mm, which was larger than the zone of inhibition of the positive control, the antibiotic gentamicin. In the same study, the same plant showed a great deal of antibacterial activity against other standard MTCC Gram-negative pathogens, particularly against Salmonella paratyphi B, Shigella boydii and Shigella dysenteriae [28]. In this study, both standard MTCC strains and clinical isolates from various sources with different resistant patterns of the three Gram-positive strains were used. It was seen that W. fruticosa could control in vitro the Gram-positive bacteria used.
As an example, crude phyto-extract of a lesser-known plant, Combretum albidum, has been recorded having a synergistic effect on the action of the antibiotic ceftriaxone against P. aeruginosa that was resistant to both ceftriaxone and several other antibiotics. The LC25 value of its leaf extract was 97.72 mg/mL with human lymphocytes and the level of LC50 was 239.88 mg/mL [16]. The LC25 value of the crude methanol leaf extract at 97.72 mg/L with human lymphocytes was much greater than the MBC value of the n-butanol fraction. This study clearly corroborated the work with the methanol leaf extract of W. fruticosa; the crude leaf extract of W. fruticosa was non-toxic to human lymphocytes cultured in vitro. Thus this plant could possibly help control of MDR pathogens potent enough cause public health problems. Phyto-compounds such as alkaloids, glycosides, terpenoids, steroids, saponins, and tannins were present in the leaf extract and these compounds have contributed to the recorded control of MDR bacteria [29]. This plant could be used as a part of an integrative treatment of the pathogen as antimicrobial agents of non-microbial origin along with mainstream antimicrobial drugs.
The GC–MS analysis of the n-butanol fraction of W. fruticosa revealed the presence of a number of secondary metabolites that have therapeutic properties, such as antibacterial, antifungal, antiseptic, anthelmintic, anti-inflammatory, antihemolytic, anticancer, antioxidant, antiparasitic, antidiabetic, and wound-healing activities [30]. The compounds with higher percentages in peak areas, namely, diethyl phthalate (26.77), 5-methyl-2-(1-methylethyl) phenol (13.37), dimethylocta-2,6-diene-1-thiol (10.71), 2-methoxy-4-(2-propenyl) phenol (3.42), and hexadecanoic acid (2.88) are present in the n-butanol fraction. These six compounds have previously been reported to have medicinal properties. Diethyl phthalate has antimicrobial, acetylcholinesterase, and neurotoxic activity [31]. The saturated fatty acid, hexadecanoic acid, has a wide range of activity, such as anticancer, antimicrobial, antioxidant, and antihemolytic activity [32,33]. The monocyclic phenolic compound, 5-methyl-2-(1-methylethyl) phenol has antibacterial, antifungal, antiseptic, and antihelmintic activities [34]. The compound 2-methoxy-4-(2-propenyl) phenol, has been reported to have antibacterial, antimicrobial, antiseptic, anesthetic, and anticancer properties [35]. The terpenes isolated in this study consisted of 5-methyl-2-(1-methylethyl) phenol, 2-methoxy-4-(2-propenyl) phenol, 2,6-octadien-1-ol, 3,7-dimethyl-(E)-, 2,6-octadienal, 3,7-dimethyl-, cyclohexanol, 2-methylene-5-(1-methylethenyl). Terpenes exhibit antimicrobial activity; the monoterpenes and sesquiterpenes are active against bacteria and fungi [36]. The terpenes isolated were 5-methyl-2-(1-methylethyl) phenol, 2-methoxy-4-(2-propenyl) phenol, 2,6-octadien-1-ol, 3,7-dimethyl-(E)-, 2,6-octadienal, 3,7-dimethyl-, cyclohexanol, 2-methylene-5-(1-methylethenyl), which could have specific antimicrobial activity.
This study confirms the presence of therapeutically potent antimicrobial compounds in the n-butanol fraction of the leaf extract of W. fruticosa for the control of MDR pathogenic bacteria. The crude leaf extract has no host toxicity with human lymphocytes and the n-butanol fraction of the extract is the most suitable bioactive fraction. The antibacterial activity of the n-butanol fraction could be due to the presence of monoterpenes, diesters of phthalic acid, saturated fatty acids, and monocyclic phenolic compounds.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
We are grateful to Dr P.K. Naik, Department of Biotechnology Guru Ghasidas Vishwavidyalaya, Central University, Bilaspur, MP, India, for the help in the GC–MS work. D.D. is an INSPIRE Fellow (IF 10503) from Department of Science and Technology, New Delhi, India. We are grateful to Professor Dr. SC Si, Dean, School of Pharmaceutical Sciences, Siksha ‘O’ Anusandhan University, Bhubaneswar, India for encouragement. This work is a part of PhD thesis of DD, submitted to Utkal University, Bhubaneswar, 2014.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Dubey D., Rath S., Sahu M.C. Antimicrobials of plant origin against multidrug resistant bacteria including the TB bacterium and economics of plant-drugs – introspection. Indian J Trad Knowl. 2012 Apr;11(2):225–233. [Google Scholar]
- 2.Dubey D., Sahu M.C., Rath S. Antibacterial activity of medicinal plants used by aborigines of Kalahandi, Orissa India against multidrug resistant bacteria. Asian Pac J Trop Biomed. 2012 Feb;2(2):S546–S554. [Google Scholar]
- 3.Chopra R.N., Nayar S.L., Chopra I.C. CSIR; Delhi: 1956. Glossary of Indian medicinal plants. [Google Scholar]
- 4.Shome U., Mehrotra S., Sharma H.P. Pharmacognostic studies on the flower of Woodfordia fruticosa Kurz. Proc Ind Acad Sci (Plant Sci) 1981 Aug;4:335–351. [Google Scholar]
- 5.Khare C.P. Springer; Berlin: 2004. Encyclopaedia of Indian medicinal plants: rational western therapy, ayurvedic and other traditional usage, botany; pp. 483–484. [Google Scholar]
- 6.Chung J.G. Inhibitory actions of ellagic acid on growth and arylamine N-acetyltransferase activity in strains of Helicobacter pylori from peptic ulcer patients. Microbios. 1998;93(375):115–127. [PubMed] [Google Scholar]
- 7.Dubey D., Rath S., Sahu M.C. A report on infection dynamics of inducible clindamycin resistance of Staphylococcus aureus isolated from a teaching hospital in India. Asian Pac J Trop Biomed. 2013 Feb;3(2):148–153. doi: 10.1016/S2221-1691(13)60040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahu M.C., Dubey D., Rath S. Multidrug resistance of Pseudomonas aeruginosa as known from surveillance of nosocomial and community infections in an Indian teaching hospital. J Pub Health. 2012 Aug;20(4):413–423. [Google Scholar]
- 9.Mishra M.P., Debata N.K., Padhy R.N. Surveillance of multidrug resistant uropathogenic bacteria in hospitalized patients – an Indian study. Asian Pac J Trop Biomed. 2013 Apr;3(4):15–24. doi: 10.1016/S2221-1691(13)60071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rath S., Dubey D., Sahu M.C. Surveillance of ESBL producing multidrug resistant Escherichia coli in a teaching hospital in India. Asian Pac J Trop Dis. 2014 Apr;4(2):40–49. [Google Scholar]
- 11.Dubey D., Rath S., Sahu M.C. Status of multidrug resistance in tubercle bacillus and phytochemicals for the control. J Pub Health. 2013 Feb;21(1):115–119. [Google Scholar]
- 12.Clinical and Laboratory Standards Institute . Clinical and Laboratory Standards Institute; 2011. Performance standard for antimicrobial susceptibility testing: twenty-first informational supplement. Document M200-S21, Wayne, PA, USA. [Google Scholar]
- 13.Dubey D., Padhy R.N. Antibacterial activity of Lantana camara L. against multidrug resistant pathogens from ICU patients of a teaching hospital. J Herbal Med. 2013 Jun;3(2):5–75. [Google Scholar]
- 14.Dubey D., Padhy R.N. Surveillance of multidrug resistance of two Gram-positive pathogenic bacteria in a teaching hospital and in vitro efficacy of 30 ethnomedicinal plants used by an aborigine of India. Asian Pac J Trop Dis. 2012 Aug;29(4):73–81. [Google Scholar]
- 15.McLafferly FW. 5th edition. John Wiley & Sons; New York, USA: 1989. Registry of mass spectral data. [Google Scholar]
- 16.Sahu M.C., Patnaik R., Padhy R.N. In vitro combination-efficacy of ceftriaxone and leaf extract of Combretum albidum G. Don against multidrug resistant Pseudomonas aeruginosa and host-toxicity testing with human lymphocytes. J Acut Med. 2014 Mar;4(1):26–37. [Google Scholar]
- 17.Tiwari H.K., Das A.K., Sapkota D. Methicillin resistant Staphylococcus aureus: prevalence and antibiogram in a tertiary care hospital in western Nepal. J Infect Devel Countries. 2009 Oct;3(9):681–684. doi: 10.3855/jidc.86. [DOI] [PubMed] [Google Scholar]
- 18.Sorlozano A., Gutiérrez J., Roman J. Activity of daptomycin against multiresistant clinical isolates of Staphylococcus aureus and Streptococcus agalactiae. Microb Drug Resist. 2009 Jun;15(2):125–127. doi: 10.1089/mdr.2009.0899. [DOI] [PubMed] [Google Scholar]
- 19.Holloway K. Antimicrobial resistance: the facts. Essential drug monitor. WHO. 2000;29:7–8. [Google Scholar]
- 20.Perez L., Dias C., Azevedol P.A. Agar dilution and agar screen with cefoxitin and oxacillin: what is known and what is unknown in detection of methicillin-resistant Staphylococcus aureus. J Med Microbiol. 2008 Aug;57(8):954–956. doi: 10.1099/jmm.0.46992-0. [DOI] [PubMed] [Google Scholar]
- 21.Raja N.S. Microbiology of diabetic foot infections in a teaching hospital in Malaysia: a retrospective study of 194 cases. J Microbiol Immunol Infect. 2007 Feb;40(1):39–44. [PubMed] [Google Scholar]
- 22.Kato T., Hayasaka S. Methicillin-resistant Staphylococcus aureus and methicillin-resistant coagulase-negative Staphylococci from conjunctivas of preoperative patients. Jpn J Ophthalmol. 1998 Dec;42(6):461–465. doi: 10.1016/s0021-5155(98)00047-1. [DOI] [PubMed] [Google Scholar]
- 23.Berman D.S., Schaefler S., Simberkoff S. Staphylococcus aureus colonization in intravenous drug abusers, dialysis patients and diabetics. J Infect Dis. 1987 Apr;155(4):829–831. doi: 10.1093/infdis/155.4.829-a. [DOI] [PubMed] [Google Scholar]
- 24.Slonczewski J.L., Foster J.W. WW Norton; New York: 2009. Microbiology, an evolving science; pp. 1–1039. [Google Scholar]
- 25.Carnicer-Pont D., Bailey K.A., Mason B.W. Risk factors for hospital-acquired methicillin-resistant Staphylococcus aureus bacteraemia: a case control study. Epidemiol Infect. 2006 Dec;134(6):1167–1173. doi: 10.1017/S0950268806006327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubey D., Rath S., Sahu M.C. Surveillance of infection status of drug resistant Staphylococcus aureus in an Indian teaching hospital. Asian Pac J Trop Dis. 2013 Apr;3(2):133–142. doi: 10.1016/S2221-1691(13)60040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvamangai C., Bhaskar A. GC MS analysis of phytocomponents in the methanolic extract of Eupatorim triplinerve. Int J Drug Dev Res. 2012 Dec;4(4):148–153. [Google Scholar]
- 28.Kumaraswamy M.V., Kavitha H.U., Satish S. Antibacterial potential of extracts of Woodfordia fruticosa Kurz. on human pathogens. World J Med Sci. 2008;3(2):93–96. [Google Scholar]
- 29.Nascimento G.F., Juliana L., Paulo C.F. Antibacterial activity of plant extracts and phytochemcicals on antibiotic resistant bacteria. Brazilan J Microbiol. 2000 Dec;31(4):247–256. [Google Scholar]
- 30.Nitha A., Ansil P.N., Prabha S.P. Preventive and curative effect of Woodfordia fruticosa Kurz. flowers on thioacetamide induced oxidative stress in rats. Asian Pac J Trop Biomed. 2012 Feb;2(2):S757–S764. [Google Scholar]
- 31.Velanganni J., Kadamban D., Ramamoorthy D. Phytochemical screening and antimicrobial activity of the stem of Mallotus philippensis (Lam.) Muell. Arg. Var. Philippensis. Int J Pharmacol Pharmaceut Sci. 2011 Mar;3(2):160–163. [Google Scholar]
- 32.Agoramoorthy G., Chandrasekaran M., Venkatesalu V. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Brazilian J Microbiol. 2007 Sep;38(4):739–742. [Google Scholar]
- 33.Wei L.S., Wee W., Siong J.Y.F. Characterization of anticancer, antimicrobial, antioxidant properties and chemical compositions of Peperomia pellucida leaf extract. Acta Med Iran. 2011;49(10):670–674. [PubMed] [Google Scholar]
- 34.Khadem S., Marles R.J. Monocyclic phenolic acids; hydroxy- and polyhydroxybenzoic acids: occurrence and recent bioactivity studies. Molecule. 2010 Nov;15(11):7985–8005. doi: 10.3390/molecules15117985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao A., Zhang Y., Muend S. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the tor pathway. Antimicrob Agent Chemother. 2010 Dec;54(12):5062–5069. doi: 10.1128/AAC.01050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Habtemariam S., Gray A.L., Waterman P.G. A new antibacterial sesquiterpene from Premna oligitricha. J Nat Prod. 1993 Jan;56(1):140–143. doi: 10.1021/np50091a022. [DOI] [PubMed] [Google Scholar]


