Abstract
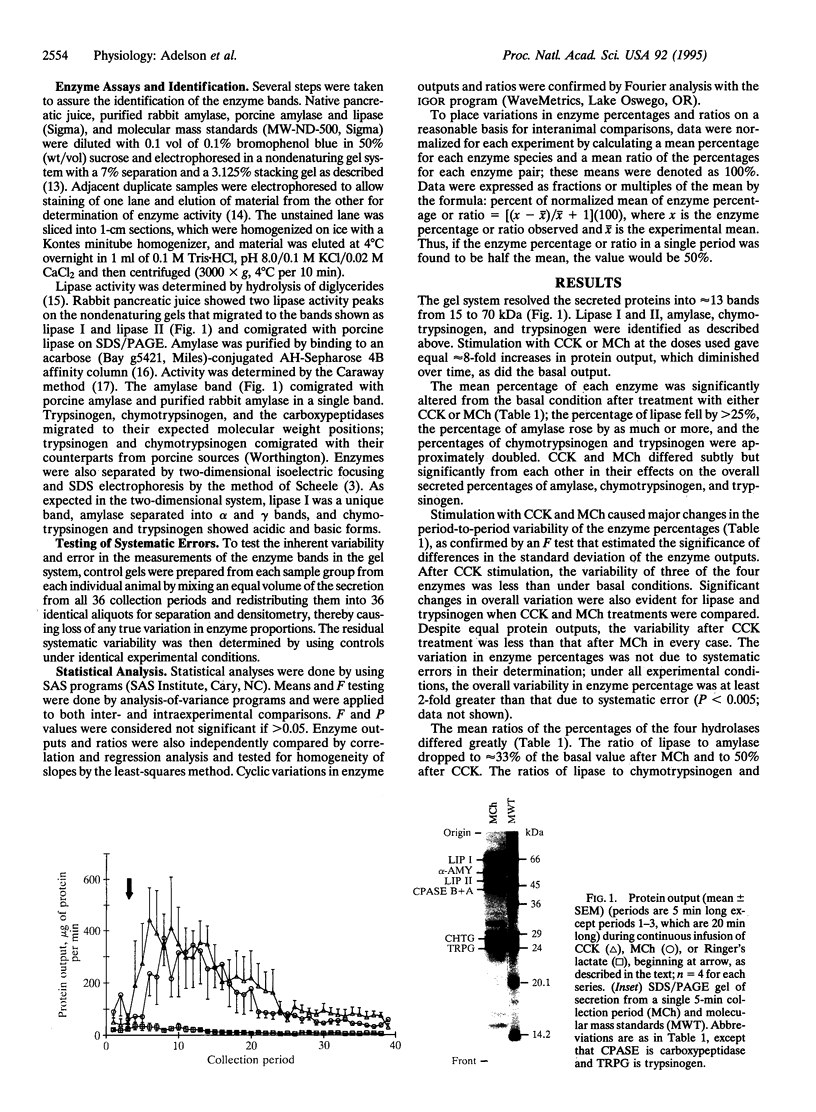
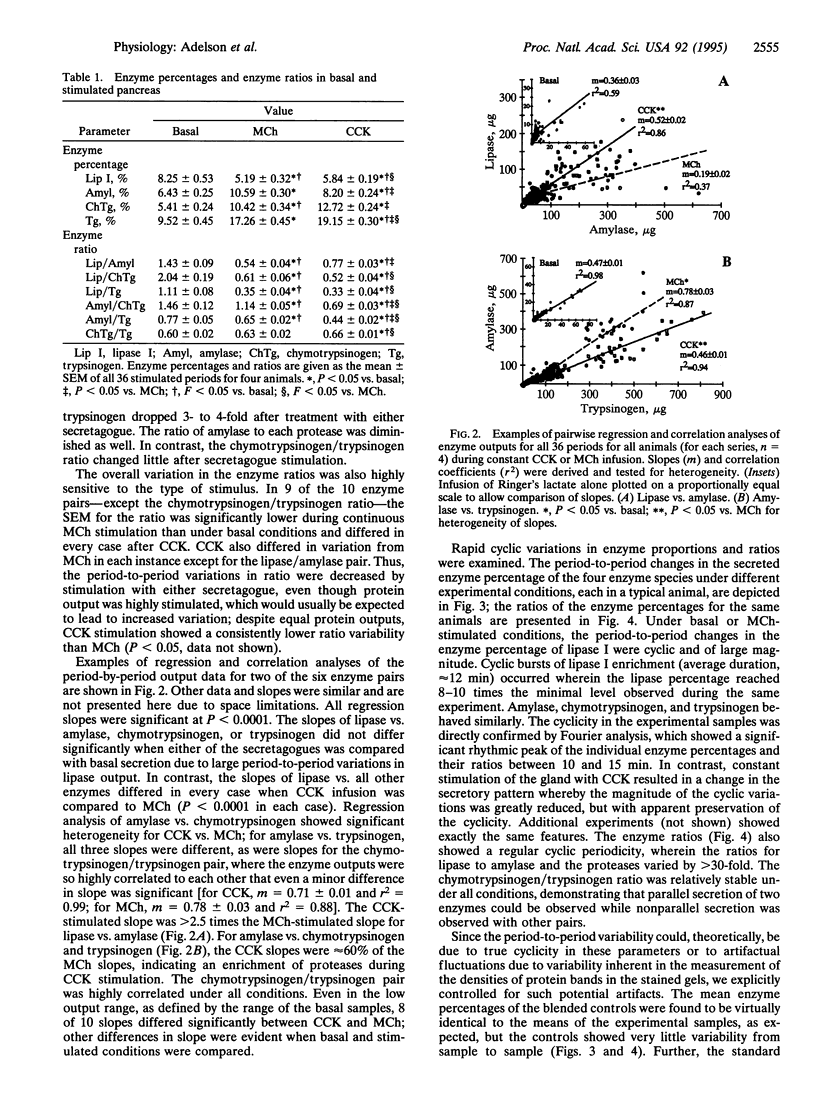
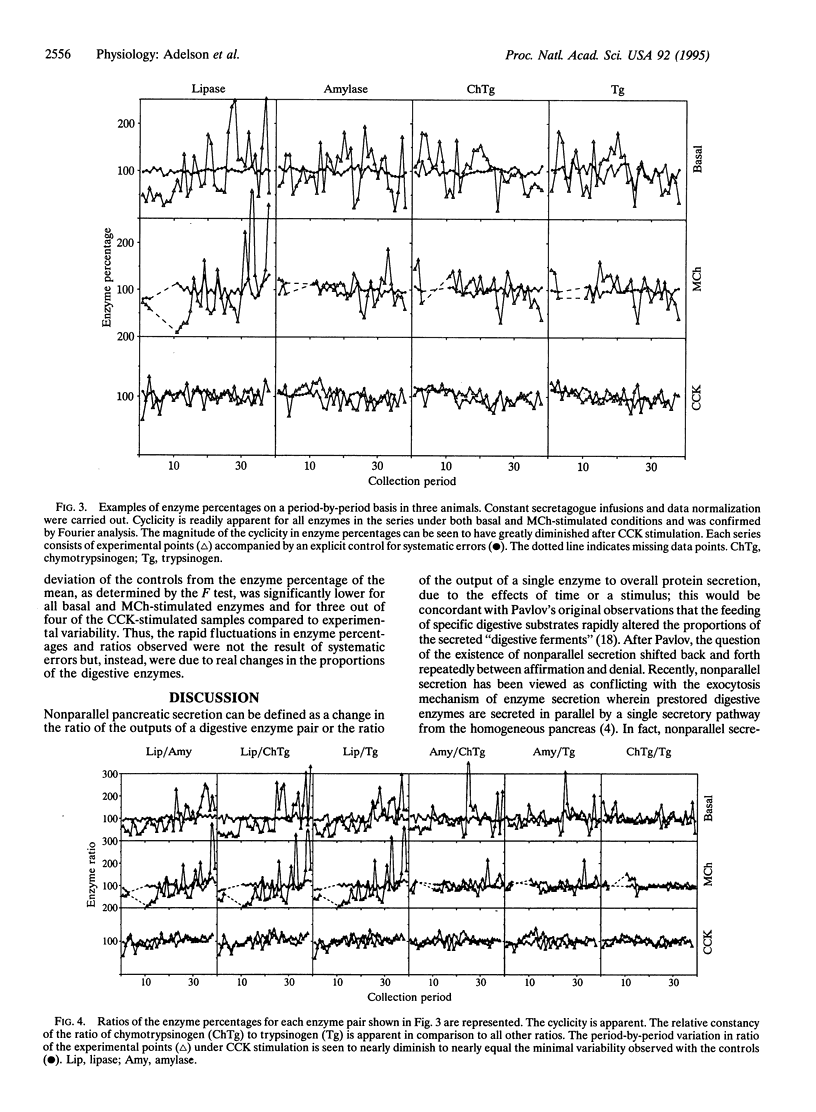
The role and mechanism of nonparallel pancreatic secretion of digestive enzymes, in which enzyme proportions change in rapidly regulated fashion, remain controversial. Secretion was collected from male 2.2-kg New Zealand rabbits in 5-min intervals for 3 h under basal conditions or constant stimulation with cholecystokinin (CCK; 0.1 microgram per kg per h i.v.) or methacholine chloride (MCh; 40 micrograms per kg per h i.v.). Both CCK and MCh produced an 8-fold stimulation of protein output. Enzymes were separated by SDS/PAGE and quantitated by densitometry of Coomassie blue-stained gels. Under both basal conditions and constant MCh infusion, rapid neurosecretory-like 12-min cyclic changes occurred in the proportions of amylase, lipase I, chymotrypsinogen, and trypsinogen. During constant infusion their percentages changed as much as 10-fold, and their ratios cycled by as much as 30-fold. The mean percentage for the entire infusion period for lipase I declined > 25% with CCK or MCh, for amylase it rose approximately 30%, and for chymotrypsinogen and trypsinogen it doubled (for all, P < 0.05). CCK and MCh elicited subtly but significantly different mean enzyme percentages and enzyme ratios (P < 0.05) for amylase, chymotrypsinogen, and trypsinogen; these differences were also confirmed by regression and correlation analyses. The changes in enzyme percentages and ratios were explicitly consistent with secretagogue-caused shifts in the intrapancreatic enzyme secretory sources. Nonparallel secretion of digestive enzymes occurs routinely, even during constant stimulation, and is due to cyclic neurosecretory-like secretion from heterogeneous intrapancreatic sources.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelson J. W., Miller P. E. Heterogeneity of the exocrine pancreas. Am J Physiol. 1989 May;256(5 Pt 1):G817–G825. doi: 10.1152/ajpgi.1989.256.5.G817. [DOI] [PubMed] [Google Scholar]
- Adelson J. W., Miller P. E. Pancreatic secretion by nonparallel exocytosis: potential resolution of a long controversy. Science. 1985 May 24;228(4702):993–996. doi: 10.1126/science.2408334. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burrill P. H., Brannon P. M., Kretchmer N. A single-step purification of rat pancreatic and salivary amylase by affinity chromatography. Anal Biochem. 1981 Nov 1;117(2):402–405. doi: 10.1016/0003-2697(81)90798-3. [DOI] [PubMed] [Google Scholar]
- CARAWAY W. T. A stable starch substrate for the determination of amylase in serum and other body fluids. Am J Clin Pathol. 1959 Jul;32(1):97–99. doi: 10.1093/ajcp/32.1_ts.97. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Erlanson-Albertsson C., Larsson A., Duan R. Secretion of pancreatic lipase and colipase from rat pancreas. Pancreas. 1987;2(5):531–535. doi: 10.1097/00006676-198709000-00007. [DOI] [PubMed] [Google Scholar]
- Gilliland E. L., Glazer G. Parallel secretion of enzymes by the rabbit pancreas. J Physiol. 1980 Jun;303:33–41. doi: 10.1113/jphysiol.1980.sp013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer G., Steer M. L. Requirements for activation of trypsinogen and chymotrypsinogen in rabbit pancreatic juice. Anal Biochem. 1977 Jan;77(1):130–140. doi: 10.1016/0003-2697(77)90297-4. [DOI] [PubMed] [Google Scholar]
- Keim V., Rohr G. Influence of secretagogues on asynchronous secretion of newly synthesized pancreatic proteins in the conscious rat. Pancreas. 1987;2(5):562–567. doi: 10.1097/00006676-198709000-00012. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liebow C. Nonparallel pancreatic secretion: its meaning and implications. Pancreas. 1988;3(3):343–351. doi: 10.1097/00006676-198805000-00017. [DOI] [PubMed] [Google Scholar]
- Maouyo D., Guan D., Rivard N., Adelson J. W., Morisset J. Stability of circadian and minor cycles of exocrine pancreatic secretion in atropine- and MK-329-infused rats. Am J Physiol. 1995 Feb;268(2 Pt 1):G251–G259. doi: 10.1152/ajpgi.1995.268.2.G251. [DOI] [PubMed] [Google Scholar]
- Maouyo D., Sarfati P., Guan D., Morisset J., Adelson J. W. Circadian rhythm of exocrine pancreatic secretion in rats: major and minor cycles. Am J Physiol. 1993 Apr;264(4 Pt 1):G792–G800. doi: 10.1152/ajpgi.1993.264.4.G792. [DOI] [PubMed] [Google Scholar]
- Miller P. E., Adelson J. W. Proteins are secreted from heterogeneous prestored sources in the exocrine pancreas. Am J Physiol. 1987 Jun;252(6 Pt 1):G768–G775. doi: 10.1152/ajpgi.1987.252.6.G768. [DOI] [PubMed] [Google Scholar]
- Mroz E. A., Lechene C. Pancreatic zymogen granules differ markedly in protein composition. Science. 1986 May 16;232(4752):871–873. doi: 10.1126/science.2422756. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Petersen C. C., Kasai H. Calcium and hormone action. Annu Rev Physiol. 1994;56:297–319. doi: 10.1146/annurev.ph.56.030194.001501. [DOI] [PubMed] [Google Scholar]
- Rothman S. S. The digestive enzymes of the pancreas: a mixture of inconstant proportions. Annu Rev Physiol. 1977;39:373–389. doi: 10.1146/annurev.ph.39.030177.002105. [DOI] [PubMed] [Google Scholar]
- Rothman S., Liebow C., Grendell J. Nonparallel transport and mechanisms of secretion. Biochim Biophys Acta. 1991 Jul 22;1071(2):159–173. doi: 10.1016/0304-4157(91)90023-p. [DOI] [PubMed] [Google Scholar]
- Scheele G. A., Palade G. E. Studies on the guinea pig pancreas. Parallel discharge of exocrine enzyme activities. J Biol Chem. 1975 Apr 10;250(7):2660–2670. [PubMed] [Google Scholar]
- Scheele G. A. Two-dimensional gel analysis of soluble proteins. Charaterization of guinea pig exocrine pancreatic proteins. J Biol Chem. 1975 Jul 25;250(14):5375–5385. [PubMed] [Google Scholar]
- Tartakoff A. M., Jamieson J. D., Scheele G. A., Palade G. E. Studies on the pancreas of the guinea pig. Parallel processing and discharge of exocrine proteins. J Biol Chem. 1975 Apr 10;250(7):2671–2677. [PubMed] [Google Scholar]
- Thorn P., Lawrie A. M., Smith P. M., Gallacher D. V., Petersen O. H. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 1993 Aug 27;74(4):661–668. doi: 10.1016/0092-8674(93)90513-p. [DOI] [PubMed] [Google Scholar]



