Abstract
Objective
To evaluate the effects and costs of three doses of behavioral weight-loss treatment delivered via Cooperative Extension Offices in rural communities.
Design and Methods
Obese adults (N=612) were randomly assigned to low, moderate or high doses of behavioral treatment (i.e., 16, 32 or 48 sessions over two years) or to a control condition that received nutrition education without instruction in behavior modification strategies.
Results
Two-year mean reductions in initial body weight were 2.9% (95% Credible Interval=1.7–4.3), 3.5% (2.0–4.8), 6.7% (5.3–7.9), and 6.8% (5.5–8.1) for the control, low, moderate, and high-dose conditions, respectively. The moderate-dose treatment produced weight losses similar to the high-dose condition and significantly larger than the low-dose and control conditions (posterior probability > .996). The percentages of participants who achieved weight reductions ≥ 5% at two years were significantly higher in the moderate-dose (58%) and high-dose (58%) conditions compared with low-dose (43%) and control (40%) conditions (posterior probability > .996). Cost-effectiveness analyses favored the moderate-dose treatment over all other conditions.
Conclusion
A moderate dose of behavioral treatment produced two-year weight reductions comparable to high-dose treatment but at a lower cost. These findings have important policy implications for the dissemination of weight-loss interventions into communities with limited resources.
Trial Registration
ClinicalTrials.gov number, NCT00912652.
Keywords: Behavior Therapy, Cost effectiveness, Weight Management Programs, Treatment Outcomes, Dissemination
INTRODUCTION
Rural communities in the U.S. have higher rates of obesity and obesity-related chronic diseases than urban areas (1–3) yet little research attention has been given to the treatment of obesity in rural areas (4). Research demonstrating that lifestyle interventions can produce weight losses of sufficient magnitude to improve health has yet to be translated for implementation and dissemination into rural communities (4–6). Indeed, most weight-loss trials have been efficacy studies, conducted with middle-class, urban and suburban participants and delivered by teams of experts working in academic medical centers (7). Very few trials have been conducted in medically underserved community settings with treatment delivered by local staff. Moreover, the costs associated with high-dose treatment regimens, such as those employed in efficacy trials, represent a significant barrier to dissemination into rural community settings, which have very limited resources for the provision of weight-loss interventions (4,8).
The existing infrastructure of the United States Department of Agriculture (USDA) Cooperative Extension Service, which has more than 2900 offices nationwide and whose mission includes nutrition and health, may serve as a valuable resource for researching and disseminating lifestyle interventions into rural communities (9–11). For example, findings from the TOURS (Treatment of Obesity in Underserved Rural Settings) Trial (12) showed promising findings for a long-term, lifestyle intervention delivered through Extension offices in rural counties. However, the high dose of treatment in the TOURS Trial (i.e., 50 sessions over 18 months) poses a significant obstacle to implementation in rural communities. Thus, it is important to determine whether lower doses of behavioral treatment would produce clinically meaningful, long-term weight reductions, when administered in underserved community settings.
We conducted a single-blind, multi-site, randomized controlled trial (RCT) in obese adults to evaluate the effects of three doses of behavioral lifestyle treatment (low = 16, moderate = 24, and high = 48 sessions) on two-year changes in body weight, compared to a nutrition education control condition. The low (LOW) intensity intervention reflected a dose of treatment commonly delivered in primary care, community and worksite settings (13–15), whereas the high (HIGH) dose corresponded to a level typically provided in efficacy trials such as the Look AHEAD Study (16). The moderate (MOD) intensity condition comprised an intermediate dose of treatment that we expected (a) would demonstrate larger long-term weight losses than the low dose and control conditions and (b) would produce a similar percentage of participants achieving clinically significant, long-term losses as the high-dose treatment but at a lower cost.
METHODS AND PROCEDURES
Participants
The study was approved by the University of Florida Institutional Review Board. Participants included 612 adults, 21–75 years of age, with a body-mass index (BMI, kg/m2) ≥ 30 and ≤ 45. Eligible participants were free of uncontrolled hypertension and diabetes and had no active (within 12 months) manifestations of cardiovascular, cerebrovascular, renal, or hepatic disease. The use of medications known to affect body weight, a weight change ≥ 4.5 kg in the preceding six months, and musculoskeletal conditions that precluded walking for 30 min were also exclusionary criteria. Psychosocial contraindications included substance abuse and clinically significant depression.
Recruitment and Screening
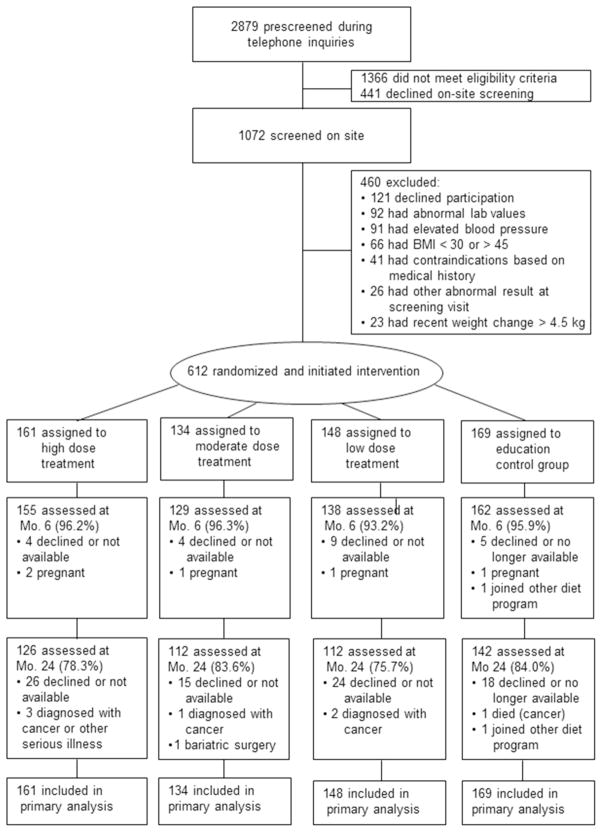
Study announcements were mailed to households in ten rural counties in northern Florida. All ten counties held designations in whole or in part as “Health Professional Shortage Areas” (17). In response to the mailings, 1072 adults who made telephone inquiries about the study and met basic eligibility criteria were invited to an orientation/screening session wherein the study was described and informed consent was obtained. Height, weight, and blood pressure were measured by a study nurse who also took a medical history, drew a fasting sample of blood, and obtained a 12-lead electrocardiogram. The blood samples were analyzed for metabolic and lipid profiles. The study physician who determined medical eligibility reviewed findings from the screening visit; 339 individuals were excluded, 121 declined an invitation to participate, and the remaining 612 individuals were randomized to one of four study conditions (Figure 1).
Figure 1.
Participant flow through screening, randomization, and follow up
Primary and Secondary Outcomes
Percent change in body weight from Month 0 (baseline) to Month 24 represented the primary outcome for the trial. The study nurse who was masked to participants’ randomized assignments measured body weight. Weight was measured with a digital scale (Tanita Model BWB-800S, Arlington Heights, IL) at Months 0, 6, and 24. Secondary outcomes included (a) the percentage of participants in each group who achieved weight reductions ≥ 5% from baseline to Month 24 and (b) the cost and cost effectiveness of each treatment.
Interventionists
The interventionists for all conditions were Cooperative Extension Service Family and Consumer Sciences Agents or individuals with bachelors or masters degrees in nutrition, exercise science, or psychology (hired on behalf of the local Extension office by the University of Florida). Agents have a minimum of a bachelor’s degree in one of its component fields (e.g., nutrition, family economics, etc.). Agents who teach nutrition education programs receive ongoing training in nutrition from state Extension specialists. The interventionists were provided with training in lifestyle treatment and nutrition education that included ten bimonthly workshops (six hours each) plus weekly supervisory contacts by phone (one hour each). The interventionists delivered treatment in groups of 6 to 15 participants at local Extension offices.
Content of Lifestyle Treatment
The contents of the lifestyle program employed in the LOW, MOD, and HIGH conditions were modeled after the Diabetes Prevention Program (DPP) (18,19) and included the following components: (a) a low-calorie eating pattern (1200 kcal/day for participants weighing italic>114 kg, 1500 kcal/day for those weighing 114–136 kg, and 1800 kcal/day for those weighing >136 kg); (b) increased physical activity in the form of 30 min/day of walking above baseline levels; and (c) training in behavior modification strategies including goal setting, self-monitoring, stimulus control, cognitive restructuring, and problem solving. Modifications to the DPP approach included group rather than individual counseling (20) and home-based rather than center-based exercise (21). Also included were topics that pilot testing suggested were issues of special concern to residents of the rural community in Florida, such as cooking demonstrations to illustrate low-calorie preparation of Southern dishes and strategies for coping with a lack of family support for weight loss (12).
Doses of Lifestyle Treatment
The intervention content and the accompanying written materials provided to participants was the same for the LOW, MOD, and HIGH conditions, but the time available for discussion varied according to the dose of treatment. In each of the three lifestyle conditions, the program was delivered in two phases: Phase 1, initial weight-loss induction, and Phase 2, extended care. Phase 1 consisted of weekly sessions (8 for LOW, 16 for MOD, and 24 for HIGH). Phase 2 targeted maintenance of behavior change (22,23) and was conducted on a faded schedule, using a combination of scheduled telephone sessions and office-based “campaign sessions.” Telephone sessions were used to reduce the travel burden for participants. Periodic campaigns (clusters of five weekly sessions) were employed to enhance motivation by setting specific weight-loss targets (e.g., 1.82 kg in one month) and providing motivational incentives (e.g., water bottles, caps, tee shirts, etc.) for the achievement of campaign objectives. Both the number of sessions allocated for extended care and the number of scheduled campaigns was carried out in proportion to the dosing schedules for the LOW, MOD, and HIGH conditions (i.e., 8, 16, and 24 extended care sessions, and 1, 2 and 3 campaigns, respectively).
Nutrition Education Condition
The nutrition education (CONTROL) condition served as a control for staff attention and for the delivery of appropriate information regarding proper diet and exercise for weight management. Each session included a lecture on a topic relevant to nutrition, physical activity, or weight control, followed by a group discussion of how the information was relevant to health and weight management. The information presented in the lectures was derived from resources available from U.S. government agencies, including the National Institutes of Health (24) and the USDA (25). The schedule of sessions provided to participants in the CONTROL condition was identical to that of the LOW dose lifestyle condition.
Statistical Analysis
The sample size was selected to provide a statistical power of .80 to detect a 2.5 kg difference in weight change at 24 months among groups assuming a within-group standard deviation of 5.5 kg (two-tailed test with Bonferroni adjustments). We used a replicated Latin square design with county and session time as factors. Data were analyzed using WinBUGS (26) and R (27).
Preliminary analyses using chi-squared tests and ANOVA F-tests indicated that there were no significant differences between conditions at baseline (Table 1). Differences in attendance (Table 2) across groups was tested using a likelihood ratio test based on fitting beta-binomial models.
Table 1.
Baseline Characteristics of Participants According to Randomized Assignment
| Characteristic | Control n =169 |
Low Dose n=148 |
Moderate Dose n=134 |
High Dose n=161 |
Total N=612 |
|---|---|---|---|---|---|
| Sex, N (%) | |||||
| Men | 31 (18.3) | 36 (24.3) | 25 (18.7) | 41 (25.5) | 133 (21.7) |
| Women | 138 (81.7) | 112 (75.7) | 109 (81.3) | 120 (74.5) | 479 (78.3) |
|
| |||||
| Age, years, mean (SD) | 52.0 (10.8) | 51.5 (12.3) | 52.8 (10.6) | 53.2 (12.0) | 52.3 (11.5) |
|
| |||||
| Race/Ethnicity, N (%) | |||||
| Black, Non-Hispanic | 29 (17.2) | 25 (16.9) | 16 (11.9) | 25 (15.5) | 95 (15.5) |
| Hispanic | 10 (5.9) | 3 (2.0) | 4 (3.0) | 6 (3.7) | 23 (3.7) |
| White, Non-Hispanic | 124 (73.4) | 118 (79.7) | 112 (83.6) | 122 (75.8) | 476 (77.7) |
| Other/multiple | 6 (3.6) | 2 (1.4) | 2 (1.5) | 8 (5.0) | 18 (2.9) |
|
| |||||
| Education, highest level completed, N (%) | |||||
| < high school | 28 (16.6) | 25 (16.9) | 24 (17.9) | 32 (19.9) | 109 (17.8) |
| high school | 87 (51.5) | 64 (43.2) | 75 (56.0) | 80 (49.7) | 306 (50.0) |
| associate’s degree | 18 (10.7) | 20 (13.5) | 18 (13.4) | 23 (14.3) | 79 (12.9) |
| bachelor’s degree | 16 (9.5) | 23 (15.5) | 8 (6.0) | 19 (11.8) | 66 (10.8) |
| advanced degree | 20 (11.8) | 16 (10.8) | 9 (6.7) | 7 (4.3) | 52 (8.5) |
|
| |||||
| Annual household income, N (%) | |||||
| <$20,000 | 29 (17.2) | 13 (8.8) | 15 (11.2) | 17 (10.5) | 74 (12.1) |
| $20,000–$34,999 | 31 (18.3) | 30 (20.3) | 22 (16.4) | 27 (16.8) | 110 (18.0) |
| $35,000–$49,999 | 31 (18.3) | 26 (17.6) | 28 (20.9) | 37 (23.0) | 122 (19.9) |
| $50,000–$74,000 | 31 (18.3) | 33 (22.3) | 33 (24.6) | 38 (23.6) | 135 (22.1) |
| >$75,000 | 43 (25.4) | 38 (25.7) | 28 (20.9) | 36 (22.4) | 145 (23.7) |
| Unknown/refused | 4 (2.4) | 8 (5.5) | 8 (5.9) | 6 (3.6) | 26 (4.2) |
|
| |||||
| Body weight, kg, mean (SD) | 100.1 (14.4) | 102.0 (16.6) | 98.6 (15.6) | 101.6 (14.8) | 100.6 (15.3) |
|
| |||||
| Body mass index, kg/m2, mean (SD) | 36.3 (3.9) | 36.1 (4.2) | 36.2 (3.8) | 36.7 (4.0) | 36.3 (4.0) |
Table 2.
Attendance at Treatment Sessions According to Randomized Assignment
| Time Period | Control n =169 |
Low Dose n=148 |
Moderate Dose n=134 |
High Dose n=161 |
P-value |
|---|---|---|---|---|---|
| Phase 1 (months 0–6) | 87.80 (17.78) | 86.32 (21.05) | 83.77 (20.74) | 77.36 (25.26) | 0.126 |
| Phase 2 (months 7–27) | 53.40 (36.64) | 51.27 (37.93) | 54.89 (32.30) | 52.95 (31.02) | 0.189 |
Data are given as mean (SD). P values are based on likelihood ratio tests.
We used Bayesian models for the analyses of weight changes and the percent achieving 5% weight loss by group. For Bayesian analyses, we defined statistical significance as the 99.2% credible interval excluding the null value (e.g., for a treatment difference, excluding zero) or posterior probabilities for a difference larger than the null greater than .996; these choices were based on Bonferroni corrections to each based on the six pairwise comparisons between groups. Raw changes in weight from Month 0 (baseline) to Months 6 and 24 were analyzed using pattern mixture models (28). Percent changes in weight from Month 0 (baseline) to Months 6 and 24 were also analyzed using pattern-mixture models. Missingness was monotone, with dropout status as the sole consideration. For the pattern-mixture model, we used the model proposed by Daniels and Hogan (29) in “Case Study 1,” which assumes (restricted) multivariate normal distributions in each pattern. The analysis allows sensitivity parameters, an approach essential in clinical trials with missing data (30). After fitting the model, we converted the raw weights to percent changes using a simple Monte Carlo procedure.
We considered three different specifications for the sensitivity parameter, which correspond to different missing data assumptions for participants who were lost to follow-up. The first (missing not at random, MNAR) corresponds to regaining (on average) 0.3 kg per month after leaving the study, and the second corresponds to a missing at random (MAR) assumption. The first scenario is based on the documented pattern of weight regain following lifestyle treatment (31,32). The second scenario is a common one for dealing with missing data and assumes that missingness can be predicted based on the observed data. For a third scenario, we consider a combination of MAR and MNAR; in particular, those who withdrew due to cancer or serious illness, due to pregnancy, due to moving out of state, and due to death from terminal cancer were considered MAR, and those who dropped out for other reasons, such as lack of time or loss of interest, were consider MNAR. All three approaches revealed the same pattern of significant findings. Because the rate of weight regain following lifestyle treatment has been well documented, we present the weight change outcomes according to MAR/MNAR mixture scenario.
Examination of Costs
Costs for each condition were estimated over the two years required for participants to complete all sessions. Cost categories included the value of staff time for program activities (training and program delivery), facility rental for live sessions, toll-free telephone service for phone sessions, program manuals that were distributed to all participants, and intervention supplies materials that varied according to condition. Wage rates included an average fringe benefit rate of 30% and costs were calculated using constant 2007 dollars. Twenty-four month weight changes, based on the MAR/MNAR analysis described above, were used in calculating cost per kg lost per participant.
RESULTS
Attendance
In Phase 1, participants completed 83.8% of scheduled treatment sessions. The rates of attendance did not vary significantly by condition (Table 2). In Phase 2, the overall rate of attendance was 53.1%, and no significant between-group differences were observed in the percentage of sessions attended.
Weight Changes
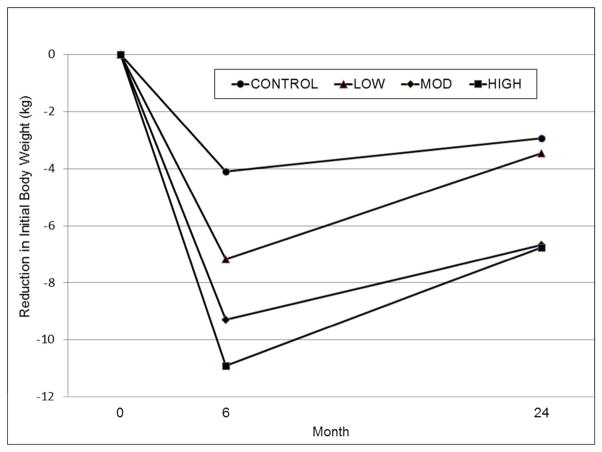
Six-month changes generally followed a dose response relationship for the CONTROL, LOW, MOD, and HIGH conditions, respectively (Table 3). The 99.2% credible intervals for the differences between the conditions resulted in intervals excluding zero for the following comparisons: CONTROL versus all other conditions; LOW versus MOD; and LOW versus HIGH. At 24 months, the 99.2% credible intervals for the differences between conditions resulted in intervals excluding zero for the following comparisons: CONTROL versus MOD, CONTROL versus HIGH, LOW versus MOD, and LOW versus HIGH. Trajectories for changes in initial body weights, expressed in kg, are presented in Figure 2.
Table 3.
Percent Reductions in Initial Body Weight According to Treatment Condition
| Control n =169 |
Low Dose n=148 |
Moderate Dose n=134 |
High Dose n=161 |
|
|---|---|---|---|---|
| Month 6 | 4.1a (3.1, 5.1) | 7.2b (6.1, 8.3) | 9.3c (8.2, 10.3) | 10.9c (9.8,11.9) |
| Month 24 | 2.9a (1.7, 4.3) | 3.5a (2.0, 4.8) | 6.7b (5.3, 7.9) | 6.8b (5.5, 8.1) |
Data are expressed as means (95% CI). At each time point, means that do not share a superscript are significantly different from each other.
Figure 2.
Weight trajectories showing mean reductions (kg) in initial body weight at months 6 and 24. At 6 months, all conditions differed significantly from each other (posterior probability > .996) except for MOD vs. HIGH. At 24 months, the MOD and HIGH conditions had significantly larger reductions than the LOW and CONTROL conditions (posterior probability > .996), and the differences between CONTROL and LOW and between MOD and HIGH were not significantly different.
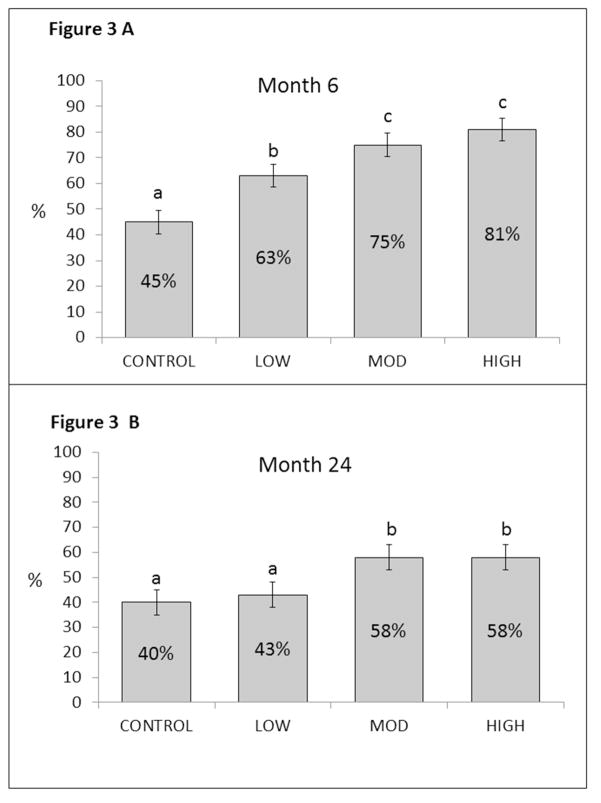
The percent of individuals achieving ≥ 5% weight loss at 6 months were 45%, 63%, 75%, and 81% for the CONTROL, LOW, MOD, and HIGH conditions, respectively, with the 99.2% credible intervals for between-group difference excluding zero for all pairwise comparisons, except for MOD versus HIGH (Figure 3, Panel A). Those achieving 5% weight loss at 24 months by condition were 40%, 43%, 58%, and 58% for the CONTROL, LOW, MOD, and HIGH conditions, respectively, with the 99.2% credible intervals for between-group difference excluding zero for CONTROL versus MOD and HIGH and, LOW versus MOD, and LOW versus HIGH (Figure 3, Panel B).
Figure 3.
Percentage of participants in each treatment condition achieving body weight reductions ≥ 5% at months 6 and 24. At each time point, conditions that do not share a superscript are significantly different from each other.
Costs
We computed the average cost per kg decrease in weight under each condition (Table 4). Both total program costs and cost per participant were lowest in the control group and were higher in the LOW, MOD, and HIGH conditions in dose-response sequence. However, when examined in terms of cost per kg of weight lost per participant, the analyses favored the MOD dose over all other conditions.
Table 4.
Costs According to Treatment Condition
| Cost | Control n =169 |
Low Dose n=148 |
Moderate Dose n=134 |
High Dose n=161 |
|---|---|---|---|---|
| Total program cost | $13,233 | $16,351 | $19,426 | $26,630 |
| Cost per participant | $78 | $111 | $145 | $165 |
| Cost per kg lost per participant | $28 | $33 | $22 | $25 |
DISCUSSION
Behavioral treatment of obesity produces clinically meaningful changes in body weight, but the high dose of treatment commonly employed in efficacy trials represents a barrier to dissemination and implementation in community settings (7). The key findings from the Rural LITE Trial demonstrate that the dose of treatment commonly used in efficacy studies can be reduced substantially without lessening benefit with respect to the long-term (two-year) mean change in body weight or the percentage of participants who achieve clinically meaningful reductions in body weight. Moreover, the data from this trial document that the benefits achieved with a moderate versus high dose of treatment can be achieved more efficiently with respect to program costs and participant time.
Analyses of the weight changes at 6 months showed that all three behavioral interventions achieved larger mean weight losses than the nutrition education control group. The superior performance of the LOW dose of behavioral treatment compared with the CONTROL group, (mean reductions of 7.2% versus 4.1%, respectively) highlights the benefits of incorporating behavior modification strategies such as written self-monitoring into weight management programs centered on changes in diet and physical activity (33). Nonetheless, it is important to note that a substantial percentage of the CONTROL group participants (45%) evidenced clinically meaningful reductions (≥ 5%) in initial body weight. Even though this figure was lower than that for the LOW group (63%), it suggests that an 8-session program of education regarding proper methods for weight change produces meaningful benefits for a substantial portion of participants (13–15).
The initial weight changes in the LOW, MOD, and HIGH conditions generally followed a dose-response relationship. Increasing the length of initial behavioral treatment from 8 to 16 weekly sessions produced a significantly larger reduction in body weight (means = 7.2% versus 9.3%, respectively). However, extending initial treatment from 16 to 24 sessions did not result in significantly greater benefit (9.3% versus 10.9%, respectively, for the MOD and HIGH conditions). This result stands in contrast to the findings of Perri et al. (34) who showed that increasing treatment length from 20 to 40 sessions produced significantly greater weight loss. Because the rate of weight loss slows over the course of treatment, more than 8 additional sessions may be required to produce significantly larger reductions than what is achieved with 16 weekly sessions of treatment. Nonetheless, the two-year mean weight reduction of 6.7% in the MOD condition in this study, implemented in Cooperative Extension offices in underserved rural communities, compares favorably with the two-year mean weight reductions observed in efficacy trials conducted in academic medical centers located in urban and suburban settings (e.g., 5.4% in the DPP [35] and 6.4% in Look AHEAD [36]).
At 24 months, the MOD and HIGH dose treatments demonstrated superior mean weight-loss outcomes than the LOW and CONTROL groups with to respect to both mean body weight reductions and percentages of participants achieving net reductions ≥ 5%. The difference in mean reductions was equivalent for the LOW and CONTROL groups as was the percentage of participants in each condition with clinically meaningful changes. These findings suggest that the incremental benefits associated with the inclusion of the behavioral strategies in the LOW condition diminished over time and were no longer evident at 24 months. The level of extended care in the LOW group may have been insufficient to reinforce the behavioral changes needed for sustaining the weight differential versus the education control group.
The differences in weight reductions between the MOD and HIGH conditions were not significant with respect to either the primary or secondary outcomes at either 6 or 24 months. This finding suggests that high doses of treatment, such as those delivered in the TOURS Study (12) and the Look AHEAD Study (16), can be thinned by one-third without significantly compromising mean weight loss or the percent of participants achieving clinically meaningful weight reductions.
Both total program costs and cost per participant followed the expected pattern with the CONTROL group having the lowest costs followed by the LOW, MOD, and HIGH conditions respectively. However, when calculated as cost per kg lost per participant, the findings showed that the MOD treatment was more cost efficient than the HIGH, LOW, and CONTROL conditions. Increasing treatment length from 8 to 16 sessions produces significantly greater weight loss and improved cost effectiveness. However, lengthening initial treatment from 16 to 24 sessions does not produce a large enough increase in weight reduction to offset the greater cost associated with longer treatment.
Several limitations of this study are worth noting. First, over the course of two years, 19.6% of the sample was lost to follow-up. Participants who drop out may have poorer outcomes than treatment completers (30,32). To address this concern, we employed statistical methods to correct for the likely poorer performance of participants lost to follow-up (29). Nonetheless, we are unable to determine whether a lower attrition rate might have affected the pattern of findings. In addition, a low questionnaire return rate at 24 months precluded examination of specific changes in diet and exercise behaviors. Second, while the treatment in this trial was delivered by bachelors- and masters-level interventionists, the degree of training and ongoing supervision that they received exceeds what is commonly available in rural community settings. Third, our cost-effectiveness analyses did not include both societal (payer) and participant perspectives. Finally, following initial treatment, the participants in all conditions regained weight. While weight regain is common in obesity treatment, it is unclear whether the manner in which extended care contacts were delivered affected the pattern of weight regain. Distributing extended care contacts on a fixed interval basis (e.g., monthly or bimonthly) might have been more effective than the use of “campaigns” consisting of clusters of five weekly sessions that entailed longer intervals without interventionist contact during the follow-up period (12,23).
In the context of obesity treatment, there are two major barriers to research translation and dissemination to underserved rural populations. The first entails the lack of an infrastructure to support treatment access for residents of medically underserved locales. The second involves the absence of an empirical database indicating the dose of treatment required to provide clinically meaningful benefits for the majority of participants who undergo treatment. The findings from the current trial provide important steps toward overcoming each of these barriers.
To our knowledge, no prior RCT has attempted to address the dose-response issue in the context of community-based, lifestyle interventions for obesity. The results in this trial demonstrate that delivering lifestyle interventions via the existing infrastructure of Cooperative Extension represents a potentially effective means of research translation and dissemination into underserved rural communities (12). The findings show that low-dose treatment, the most common level of care provided in community settings (13–15), is less effective and less cost-efficient than moderate-dose treatment. Moreover, the results also demonstrate that a moderate dose of behavioral treatment can produce clinically meaningful, two-year reductions in body weight comparable to high-dose treatment, but at a lower cost. These findings, which highlight the benefits of RCTs in dissemination research (37), have important policy implications regarding the design of obesity interventions in low-resource communities (6,38,39).
What is already known about this subject?
Rural communities in the U.S. have higher rates of obesity and obesity-related chronic diseases than urban areas.
The high dose of treatment commonly employed in behavioral weight-loss interventions represents a barrier to implementation in rural communities with limited resources.
What does this study add?
A moderate-dose of behavioral weight–loss treatment can produce 2-year reductions in body weight that are similar to high-dose treatment and significantly larger than low-dose treatment.
Moderate-dose treatment is more cost-effective than both low- and high-dose treatments.
Acknowledgments
This study was supported by NIH/NHLBI, R18HL87800. We are grateful to Data and Safety Monitoring Board Members, Drs. Marcia Stefanick, Ronald Prineas, and John Foreyt, and to staff of the University of Florida Weight Management Program and Cooperative Extension Offices in Baker, Bradford, Clay, Dixie, Flagler, Lafayette, Levy, Putnam, Suwannee, and Union Counties. MGP, MCL, DMJ, LBB, and ADM conceived and designed the study. KvCR, PED, and VAM were involved in data acquisition/management. MCL served as Medical Director. MGP, KvCR, VAM, and LBB trained and supervised the interventionists. All authors were involved in writing the paper.
Footnotes
The authors declared no conflict of interest.
References
- 1.Befort CA, Nazir N, Perri MG. Prevalence of obesity among adults from rural and urban areas of the United States: Findings from NHANES (2005–2008) J Rural Health. 2012;28:392–397. doi: 10.1111/j.1748-0361.2012.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eberhardt MS, Freid VM, Harper S, Pamuk E, Ingram DD, Makuc DM. Health, United States, 2001: Urban and rural health chartbook. National Center for Health Statistics; Hyattsville, MD: 2001. [Google Scholar]
- 3.Cossman JS, James WL, Cosby AG, Cossman RE. Underlying causes of the emerging nonmetropolitan mortality penalty. Am J Public Health. 2010;100(8):1417–1419. doi: 10.2105/AJPH.2009.174185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patterson PD, Moore CG, Probst JC, Shingle JA. Obesity and physical inactivity in rural America. J Rural Health. 2004;20:151–159. doi: 10.1111/j.1748-0361.2004.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 5.Gamm LD, Hutchison LL, Dabney BJ, Dorsey AM, editors. Rural Healthy People 2010: A Companion Document to Healthy People 2010. 1–3. The Texas A&M University System Health Science Center, School of Rural Public Health, Southwest Rural Health Research Center; College Station, TX: 2003. [Google Scholar]
- 6.Kerner J, Rimer B, Emmons K. Introduction to the special section on dissemination: Dissemination research and research dissemination: how can we close the gap? Health Psychol. 2005;24:443–6. doi: 10.1037/0278-6133.24.5.443. [DOI] [PubMed] [Google Scholar]
- 7.Wadden TA, Osei SY. The treatment of obesity: an overview. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. Guilford; New York: 2002. pp. 229–248. [Google Scholar]
- 8.Befort CA, Donnelly JE, Sullivan DK, Ellerbeck EF, Perri MG. Group versus individual phone-based obesity treatment for rural women. Eating Behaviors. 2010;11(1):11–17. doi: 10.1016/j.eatbeh.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.USDA: National Institute of Food and Agriculture. Extension: About Us. 2014 [WWW document]. URL http://www.csrees.usda.gov/qlinks/extension.html.
- 10.Melvin CL, Corbie-Smith G, Kumanyika S, Pratt CA, Nelson C, Walker ER, et al. Developing an agenda for cardiovascular disease prevention in high-risk rural communities: guiding principles and recommendations. Am J Public Health. 2013;103(6):1011–1021. doi: 10.2105/AJPH.2012.300984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rejeski WJ, Brubaker PH, Goff DC, Jr, Bearon LB, McClelland JW, Perri MG, et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011;17:880–886. doi: 10.1001/archinternmed.2010.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perri MG, Limacher MC, Durning PE, Janicke DM, Lutes LD, Bobroff LB, et al. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch Intern Med. 2008;168(21):2347–2354. doi: 10.1001/archinte.168.21.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med. 2009;24(9):1073–1079. doi: 10.1007/s11606-009-1042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson LM, Quinn TA, Glanz K, Ramirez G, Kahwati LC, Johnson DB, et al. The effectiveness of worksite nutrition and physical activity interventions for controlling employee overweight and obesity: a systematic review. Am J Prev Med. 2009;37(4):340–357. doi: 10.1016/j.amepre.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 15.AARP. Fat to Fit Challenge. 2011 [WWW document] URL http://www.aarp.org/health/fitness/fat2fit1/
- 16.Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services: Health Resources and Services Administration. Health professional shortage areas. 2014 [WWW document[ URL http://www.hrsa.gov/shortage/find.html.
- 18.The Diabetes Prevention Program (DPP) Research Group. Description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer MK, Kriska AM, Venditti EM, Miller RG, Brooks MM, Burke LE, et al. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am J Prev Med. 2009;37(6):505–511. doi: 10.1016/j.amepre.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Renjilian DA, Perri MG, Nezu A, McKelvey WF, Shermer RL, Anton SD. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol. 2001;69:717–721. [PubMed] [Google Scholar]
- 21.Perri MG, Martin AD, Leermakers EA, Sears SF, Notelovitz M. Effects of group-versus home-based exercise in the treatment of obesity. J Consult Clin Psychol. 1997;65:278–285. doi: 10.1037//0022-006x.65.2.278. [DOI] [PubMed] [Google Scholar]
- 22.Perri MG, Nezu AM, Viegener BJ. Improving the long-term management of obesity: theory research, and clinical guidelines. Wiley; New York: 1992. [Google Scholar]
- 23.Perri MG, Nezu AM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem-solving therapy in the long-term management of obesity. J Consult Clin Psychol. 2001;69:722–726. [PubMed] [Google Scholar]
- 24.DHHS, NIDDK. NIH Publication 2008: 06–3680. Understanding adult overweight and obesity; pp. 1–6. [Google Scholar]
- 25.USDA, Center for Nutrition Policy and Promotion. Choose MyPlate: 10 tips to a great plate. 2011 [WWW document] URL http://www.choosemyplate.gov/food-groups/downloads/TenTips/DGTipsheet1ChooseMyPlate.pdf.
- 26.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS: a Bayesian modelling framework: concepts, structure, and extensibility. Statistics and Computing. 2000;10(4):325–337. [Google Scholar]
- 27.R Core Group. The R project for statistical computing. 2014 [WWW document] URL http://www.r-project.org/
- 28.Little RJA. A class of pattern-mixture models for incomplete data. Biometrika. 1994;81(3):471–483. [Google Scholar]
- 29.Daniels MJ, Hogan JW. Missing data in longitudinal studies: strategies for Bayesian modeling and sensitivity analysis. CRC Press; Boca Raton: 2008. [Google Scholar]
- 30.National Research Council. The prevention and treatment of missing data in clinical trials. National Academies Press; Washington, DC: 2010. Panel on Handling Missing Data in Clinical Trials. [PubMed] [Google Scholar]
- 31.Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, et al. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19(1 Suppl):5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 32.Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353(20):2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 33.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111(1):92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perri MG, Nezu AM, Patti ET, McCann KL. Effect of length of treatment on weight loss. J Consult Clin Psychol. 1989;57(3):450–452. [PubMed] [Google Scholar]
- 35.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity. 2014;22(1):5–13. doi: 10.1002/oby.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zerhouni EA. Translational and clinical science – time for a new vision. N Engl J Med. 2005;353(15):1621–1623. doi: 10.1056/NEJMsb053723. [DOI] [PubMed] [Google Scholar]
- 38.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Obesity. 2013;21(S3) doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Perri MG. Effects of behavioral treatment on long-term weight loss: Lessons learned from the Look AHEAD trial. Obesity. 2014;22(1):3–4. doi: 10.1002/oby.20672. [DOI] [PubMed] [Google Scholar]