Abstract
Objective
To describe objective measures of kidney function and analyze factors associated with kidney dysfunction in severely obese adolescents undergoing weight loss surgery.
Design and Methods
We analyzed cross-sectional data from 242 adolescent participants in the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study before weight loss surgery. Kidney status was assessed by measuring urine albumin creatinine ratio to determine microalbuminuria and by calculating serum cystatin C-based estimated glomerular filtration rate (eGFR) to assess kidney function.
Results
Mean age and median body mass index (BMI) were 17.1 years and 50.5kg/m2, respectively; 76% were females and 65% were non-Hispanic white race. Fourteen percent of the cohort had microalbuminuria, and 3% had macroalbuminuria; 3% had eGFR <60 ml/min/1.73m2, and 7.1% had eGFR >150 ml/min/1.73m2. In adjusted analyses, female gender and increasing ferritin levels were significantly associated with the presence of microalbuminuria/macroalbuminuria. Increasing BMI and HOMA-IR values were significantly associated with lower eGFR.
Conclusions
A significant number of severely obese adolescents undergoing weight loss surgery have evidence of early kidney dysfunction. Longitudinal studies following weight loss surgery in these individuals are needed to determine whether these kidney abnormalities are reversible following weight loss therapy.
Keywords: obesity, children, adolescents, kidney function, microalbuminuria
Introduction
The most recent data suggest that the overall prevalence of obesity among U.S. youth might have reached its peak.1 In contrast, the prevalence of severe obesity, defined as an absolute BMI >35 kg/m2 or > 120th percent of the 95th percentile, is increasing and now affects 4–6% of U.S. children and adolescents.2,3 Recently, the American Heart Association issued a scientific statement on associated risk factors and treatment approaches for severely obese children.4 The statement specifically focused on immediate and long-term risks including cardiovascular disease, metabolic complications, obstructive sleep apnea, nonalcoholic liver disease, musculoskeletal and behavior problems. Notably, it did not address the issue of obesity associated kidney dysfunction.
Obesity, and particularly severe obesity, has important pathophysiologic consequences for the kidney. Obesity-associated focal segmental glomerular sclerosis has been well described in adolescents and adults.5–7 It is also well-documented that obesity during adolescence is associated with a higher prevalence of chronic kidney disease (CKD) in adulthood.8–10
The Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study aims to answer questions related to the safety and effectiveness of bariatric surgery in adolescence. As part of the baseline evaluation of this cohort of 242 patients, detailed clinical phenotyping included kidney function data, specifically urinary albumin and serum cystatin C-based glomerular filtration rate (eGFR). In this report, the baseline, preoperative kidney status of the Teen-LABS cohort was analyzed to describe the prevalence and factors associated with kidney abnormalities in this carefully studied population of severely obese adolescents.
Methods and procedures
Teen-LABS was designed as an ancillary study to the Longitudinal Assessment of Bariatric Surgery (LABS, NCT00465829). The standardized methodology developed for the LABS-2 study was modified for this adolescent cohort.11 Briefly, 277 consecutive adolescents (age ≤19 years) undergoing bariatric surgery at each of five Teen-LABS centers between March 2007 and February 2012 were offered enrollment. However, 13 declined participation and 22 did not undergo the operation by the end of the enrollment period, leaving a final cohort of 242 subjects. The study protocol, assent/consent forms, data and safety monitoring plans were approved by the IRBs of each institution and by an independent Data and Safety Monitoring Board prior to study initiation. Pre-operative data were collected within 30 days of operation by trained study personnel. A Teen-LABS-trained clinical research coordinator or surgical investigator followed standardized procedures to determine the presence or absence of co-morbid conditions using medical records, physical exam, patient interview, and laboratory values. Detailed descriptions of study definitions, including hypertension, dyslipidemia, and diabetes have been described elsewhere.11 All laboratory assays were performed at the central laboratory, the Northwest Lipid Research Laboratories at the University of Washington, Seattle, WA. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as (fasting glucose (mg/dL) × insulin (uU/ml)) / 405. Kidney function was assessed by calculating cystatin C-based eGFR, where eGFR = 77.24 × (Cystatin-C)−1.2623 according to the Larsson formula as recommended by the assay manufacturer (Dade Behring, Deerfield, Illinois).12 Microalbuminuria was defined as having a urine Albumin to Creatinine Ratio (ACR) ≥30 mg/gm and <300 mg/gm; macroalbuminuria was defined as ACR ≥300 mg/gm. Abnormal kidney function was defined based on Kidney/Dialysis Outcome Quality Initiative (KDOQI) guidelines.13
Statistical Analyses
Descriptive statistics were calculated to summarize subject characteristics. Frequencies and percentages are reported for categorical measures. Means and standard deviations or medians and interquartile ranges were calculated for continuous variables. Scatterplots and Pearson correlation coefficients were also generated to describe select variables. Among the 16 variables evaluated in the statistical models, 1.6% of data values were missing. Most subjects (85.5%) had complete data, while 14.5% were missing at least one value. Missing values ranged from 1.7% (n=4) for lab values (transferrin, ferritin, serum albumin, HOMA-IR, hsCRP, LDL, HDL, triglycerides) to 6.6% (n=16) for the microalbuminuria outcome. Multivariate imputation by chained equations was performed to address these missing data. IVEware software (version 0.2) in SAS (version 9.3) was used to generate 20 imputed data sets for use in multivariable modeling analyses. A single, confirmed outlier insulin value (1314.2 uU/mL) was replaced with multiply imputed values for these analyses. To evaluate predictors of elevated ACR (i.e., ≥ 30 mg/gm), we calculated crude and adjusted prevalence ratios (PR) and 95% confidence intervals by fitting modified Poisson regression models with robust error estimates (SAS Proc GENMOD). Multivariable linear regression (SAS Proc GLM) models were used to evaluate predictors of cystatin C-based eGFR. SAS Proc MiAnalyze was used to generate all estimates from the multiply imputed datasets. All aforementioned descriptive and clinical variables were considered for inclusion in the final models. Multicollinearity diagnostics were reviewed to confirm appropriateness of these variables. All reported p-values are two-sided and considered statistically significant at ≤ 0.05.
Results
Demographic and clinical characteristics of the cohort are presented in Table 1. Of the 242 subjects, 75.6% were female and 64.9% were non-Hispanic white race. Mean age at surgery was 17.1 years, while the median body mass index (BMI) was 50.5 kg/m2 (range: 34.0 to 87.7). Seventy-four percent had dyslipidemia, 13.6% were diagnosed with diabetes, and 4.3% had a history of kidney stones. Forty-five percent were hypertensive. Forty-six (19%) subjects were taking anti-hypertensive medications at baseline. Twenty-four (9.9%) subjects were taking angiotensin converting enzyme inhibitors (ACEs) and 2 (0.8%) were taking angiotensin receptor blockers (ARBs) to control blood pressure.
Table 1.
Demographic and Clinical Characteristics
| N=242 | |
|---|---|
| Age at surgery (years) – x̄ (SD) | 17.1 (1.56) |
| Female – n (%) | 183 (75.6%) |
| Race/Ethnicity – n (%) | |
| Non-Hispanic White | 157 (64.9%) |
| Non-Hispanic Black | 54 (22.3%) |
| Hispanic | 17 (7.0%) |
| Non-Hispanic Other races | 14 (5.8%) |
| Body Mass Index (kg/m2) – median (Q1,Q3) | 50.5 (45.2, 58.3) |
| Cystatin C-based eGFR (ml/min/1.73m−2) – x̄ (SD)* | 107.6 (26.77) |
| Urine Albumin Creatinine Ratio – median (Q1,Q3)† | 6.70 (3.93, 16.18) |
| Microalbuminuria – n (%)‡ | 32 (14.2%) |
| Macroalbuminuria – n (%)‡ | 7 (3.1%) |
| Hypertension – n (%) | 109 (45.0%) |
| Diabetes – n (%) | 33 (13.6%) |
| LDL (mg/dL) – x̄ (SD)* | 93.0 (25.99) |
| HDL (mg/dL) – x̄ (SD)* | 37.6 (9.05) |
| Triglycerides (mg/dL) – median (Q1,Q3)* | 113.0 (82.0, 162.0) |
| Dyslipidemia – n (%) | 180 (74.4%) |
| History of Kidney Stones – n (%)+ | 10 (4.3%) |
| Transferrin (mg/dL) – x̄ (SD)* | 271.6 (38.06) |
| Ferritin (µg/L) – median (Q1,Q3)* | 37.0 (23.0, 66.0) |
| Serum albumin (g/L) – x̄ (SD)* | 4.1 (0.34) |
| HOMA-IR – median (Q1,Q3)* | 5.91 (3.64, 9.84) |
| hsCRP (mg/dL) – median (Q1,Q3)* | 0.63 (0.30, 1.17) |
| HbA1c – median (Q1,Q3)++ | 5.2 (5.0, 5.5) |
n = 4 missing.
n = 12 missing.
n = 16 missing.
n = 10 missing.
n = 11 missing.
Thirty-nine (17.3%) subjects had elevated ACR, of which 32 (14.1%) had microalbuminuria (ACR range: 31–283 mg/gm) and 7 (3.1%) had macroalbuminuria (ACR range: 489–1758mg/gm). Based on crude analyses, increasing levels of ferritin, HOMA-IR, and HbA1c were significantly associated with the presence of elevated ACR (each p < 0.05; Table 2). The fully adjusted model demonstrated that increasing ferritin value (PR 1.07, 95% CI 1.03–1.10) and female gender (PR 2.34, 95% CI 1.02 – 5.34) were significantly associated with elevated ACR.
Table 2.
Crude and adjusted associations with elevated albumin/creatinine ratio
| Elevated albumin/creatinine ratio | ||||
|---|---|---|---|---|
| Crude | Adjusted | |||
| PR (95% CI) | p-value | PR (95% CI) | p-value | |
| Age at surgery (years) | 1.02 (0.84, 1.23) | 0.87 | ||
| Sex | ||||
| Female | 1.83 (0.81, 4.14) | 0.15 | 2.34 (1.02,5.34) | 0.04 |
| Male | <Ref> | <Ref> | ||
| Race/ethnicity | ||||
| NH White | <Ref> | |||
| NH Black | 1.55 (0.77, 3.09) | 0.22 | ||
| Hispanic | 2.28 (0.89, 5.82) | 0.09 | ||
| NH Other | 1.84 (0.62, 5.51) | 0.27 | ||
| BMI (per 10 units) | 1.12 (0.81, 1.54) | 0.49 | ||
| Hypertension | ||||
| Yes | 1.19 (0.65, 2.19) | 0.58 | ||
| No | <Ref> | |||
| Diabetes | ||||
| Yes | 1.70 (0.80, 3.60) | 0.17 | ||
| No | <Ref> | |||
| Dyslipidemia | ||||
| Yes | 1.19 (0.53, 2.64) | 0.68 | ||
| No | <Ref> | |||
| Kidney stones | ||||
| Yes | 0.74 (0.12, 4.42) | 0.74 | ||
| No | <Ref> | |||
| Transferrin (mg/dL) | 1.00 (0.99, 1.01) | 0.83 | ||
| Ferritin (µg/L)/per 10units | 1.06 (1.02, 1.10) | < 0.01 | 1.07 (1.03,1.10) | < 0.01 |
| Serum Albumin (g/L) | 0.50 (0.21, 1.20) | 0.12 | ||
| HOMA-IR | 1.04 (1.00, 1.07) | 0.04 | ||
| hsCRP (mg/dL) | 1.12 (0.99, 1.27) | 0.07 | ||
| HbA1c | 1.32 (1.08, 1.61) | < 0.01 | ||
PR = Prevalence ratio. CI = Confidence interval.
Elevated albumin/ creatinine ratio (ACR): microalbuminuria with ACR ≥ 30 mg/gm and <300 mg/gm and macroalbuminuria with ACR ≥300 mg/gm.
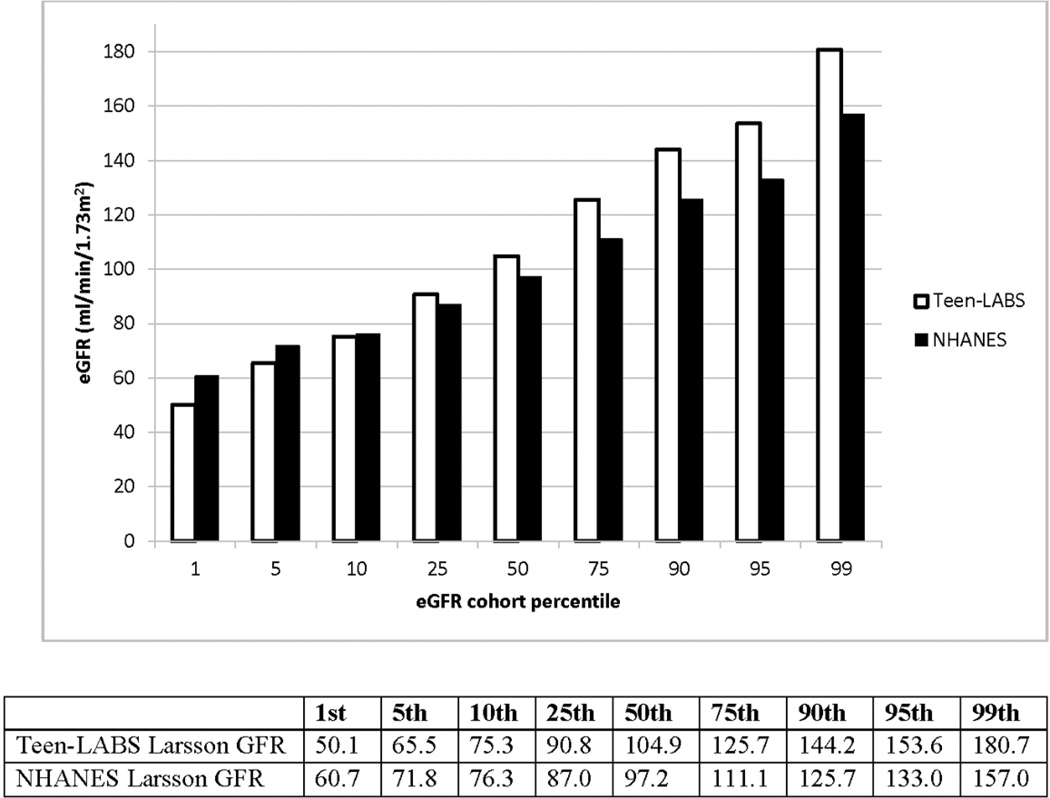
The mean cystatin C-based eGFR was 107.6 ml/min/1.73m2. Most of the Teen-LABS participants (68.5%) had a normal eGFR (90–150 ml/min/1.73m2) However, 51 (21.4%) had an eGFR of 60–90 ml/min/1.73m2; 7 (3.0%) had an eGFR <60 ml/min/1.73m2; and 17 (7.1%) had an eGFR >150 ml/min/1.73m2. Percentile distribution of eGFR from the Teen-LABS cohort and from published NHANES data is shown in Figure 1. There were more subjects in low percentile and high percentile brackets in the Teen-LABS cohort than in the NHANES participants.14
Figure 1. The percentile distribution of cystatin C-based eGFR from the Teen-LABS cohort and from published NHANES data (reference 14).
Cystatin C was measured using the same methodology in both Teen-LABS and NHANES (Dade Behring, Deerfield, Illinois).39
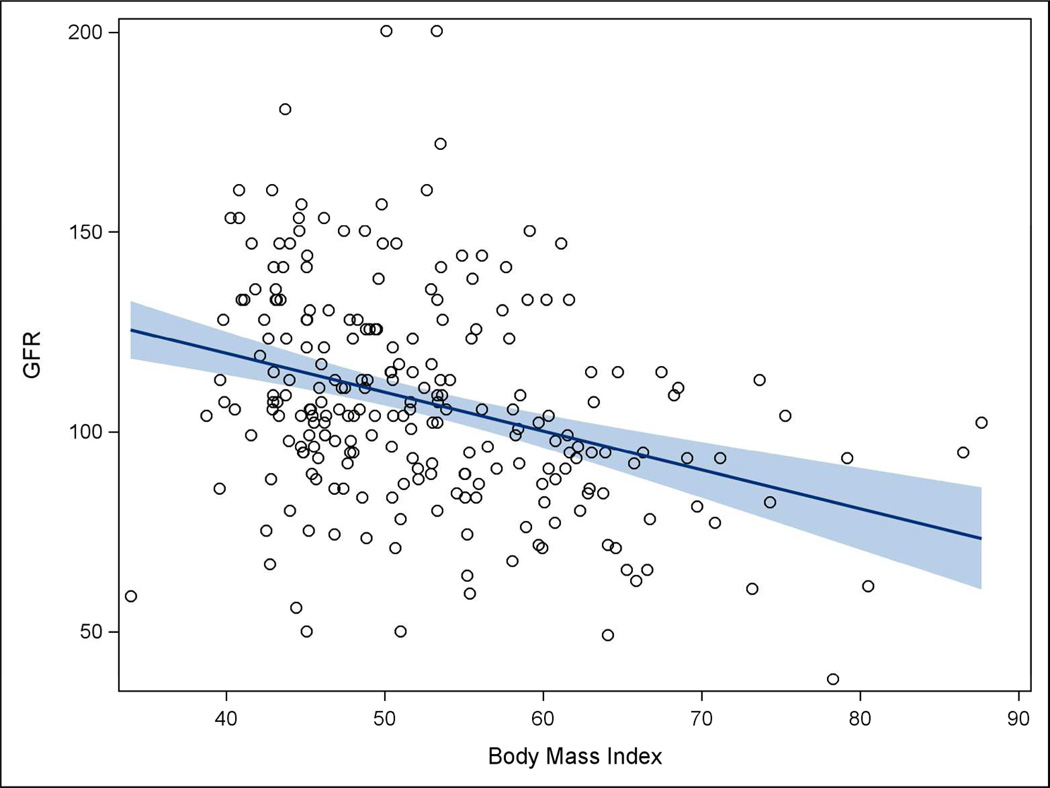
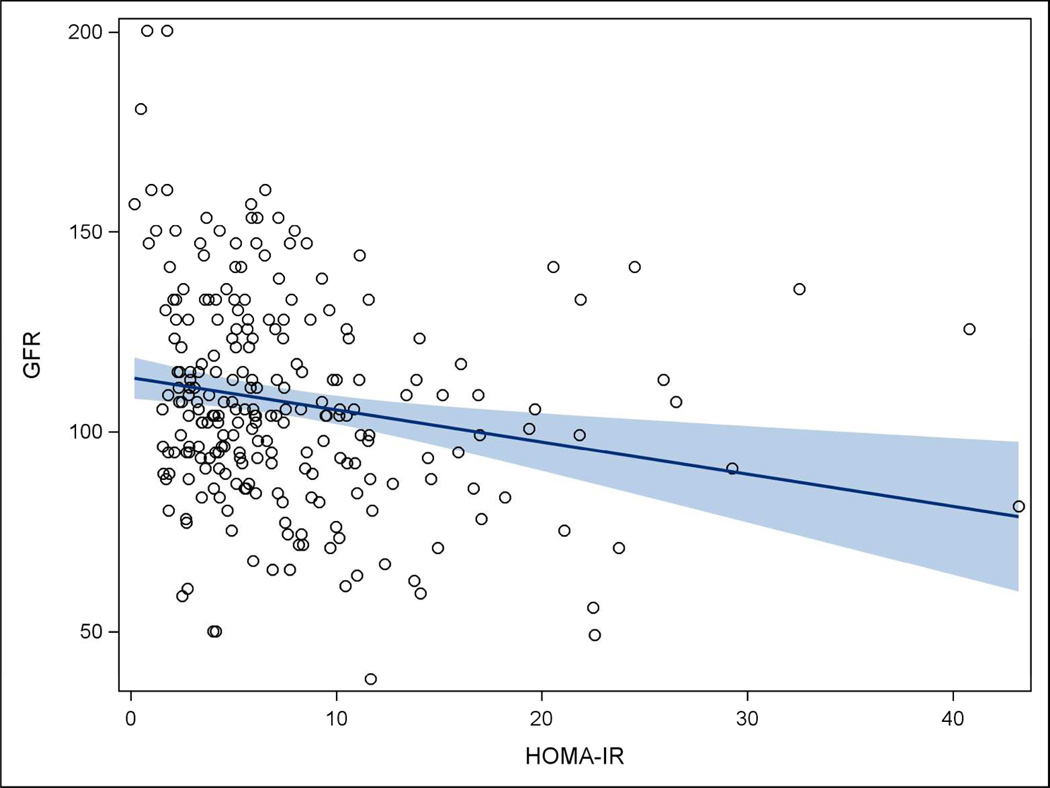
Table 3 displays crude and adjusted associations between clinical features and eGFR. Crude results indicate female gender, BMI, transferrin, serum albumin, and HOMA-IR were associated with eGFR (each p < 0.05). However, after adjustment, increasing BMI and HOMA-IR values were the only factors that were significantly associated with decreased eGFR (each p < 0.05). Analyses indicated that for each 10-unit increase in BMI, eGFR decreased by nearly 8 mL/min/1.73m2, while for each 1-unit increase in HOMA-IR, eGFR decreased by only 0.57 mL/min/1.73m2. Figures 2 and 3 show the inverse relationships of BMI and HOMA-IR with eGFR.
Table 3.
Crude and adjusted associations with cystatin c-based eGFR
| Cystatin c-based eGFR | ||||
|---|---|---|---|---|
| Crude | Adjusted | |||
| β (95% CI) | p-value | β (95% CI) | p-value | |
| Age at surgery (years) | −0.04 (−2.26, 2.18) | 0.97 | ||
| Sex | ||||
| Female | 8.80 (0.93, 16.66) | 0.03 | 6.87 (−1.09, 14.82) | 0.09 |
| Male | <Ref> | |||
| Race/ethnicity | ||||
| NH White | <Ref> | |||
| NH Black | −0.91 (−9.35, 7.52) | 0.83 | ||
| Hispanic | −2.16 (−15.64, 11.32) | 0.75 | ||
| NH Other | 11.70 (−3.01, 26.41) | 0.12 | ||
| BMI (per 10 units) | −9.29 (−12.87, −5.70) | < 0.01 | −7.90 (−11.60,−4.20) | < 0.01 |
| Hypertension | ||||
| Yes | −6.10 (−12.95, 0.74) | 0.08 | ||
| No | <Ref> | |||
| Diabetes | ||||
| Yes | 2.03 (−7.98, 12.03) | 0.69 | ||
| No | <Ref> | |||
| Dyslipidemia | ||||
| Yes | −4.20 (−12.45, 4.04) | 0.32 | ||
| No | <Ref> | |||
| Kidney stones | ||||
| Yes | −8.66 (−25.61, 8.29) | 0.32 | ||
| No | <Ref> | |||
| Transferrin (mg/dL) | 0.13 (0.04, 0.21) | < 0.01 | 0.08 (−0.004, 0.173) | 0.06 |
| Ferritin (µg/L) (per 10 units) | −0.32 (−0.99, 0.34) | 0.34 | ||
| Serum Albumin (g/L) | 10.55 (0.55, 20.56) | 0.04 | ||
| HOMA-IR | −0.72 (−1.26, −0.19) | < 0.01 | −0.57 (−1.09,−0.06) | 0.03 |
| hsCRP (mg/dL) | −0.30 (−2.72, 2.12) | 0.81 | ||
| HbA1c | −0.20 (−3.88, 3.48) | 0.91 | ||
CI = Confidence interval.
Figure 2. Scatterplot of BMI and eGFR, Teen-LABS subjects.
Figure 3. Scatterplot of HOMA-IR and eGFR, Teen-LABS subjects.
Discussion
This study represents the first attempt to characterize kidney status in a large cohort of severely obese adolescents. The most important findings were the high proportion of severely obese adolescents with microalbuminuria/macroalbuminuria and the significant association of increasing BMI with declining GFR.
The percent of Teen-LABS participants with microalbuminuria/macroalbuminuria was higher than anticipated (17.3%) based on previous reports. Previous studies analyzing NHANES data in obese and non-obese adolescents reported microalbuminuria prevalence values ranging from 8.9 to 10.4%.14–16 Furthermore, Nguyen et al. reported that microalbuminuria was less prevalent in overweight (0.3%) compared with normal weight (8.7%) adolescents, likely due to orthostatic proteinuria in lean children.16 In their study, despite overall lower prevalence of microalbuminuria among obese adolescents, there was a strong association between microalbuminuria and cardiovascular risk factors, suggesting that proteinuria may be predictive of future kidney and cardiovascular disease. In this context, our results indicate that in addition to a higher prevalence of microalbuminuria, the frequency of macroalbuminuria (3.1%) was more than twice as high than the 1.3% which was reported from the NHANES unselected pediatric population.14 These findings are worrisome and suggest that severely obese adolescents are at increased risk for future CKD.
Our data show that obese subjects with elevated ferritin were more likely to have elevated ACR. Ferritin has well-known associations with many cardiovascular risk factors including inflammation, obesity, insulin resistance, and the metabolic syndrome.17–19 Furthermore, Hsu et al. recently demonstrated an independent association of ferritin with microalbuminuria in a cohort of adults with diabetes.20 The similar association observed in our cohort is consistent with the concept that obesity is a chronic, mild-inflammatory process with alterations of iron metabolism. Iron causes cellular damage by the formation of reactive oxygen species21 and promotes oxidative stress and inflammation in both murine adipocytes and human aortic endothelial cells.22,23 Glomerular endothelial injury mediated by iron-induced oxidative stress may therefore contribute to the development of microalbuminuria and proteinuria in severely obese patients. Multivariate analysis also revealed an association of elevated ACR with female gender. Epidemiologic studies of NHANES data have demonstrated increased prevalence of microalbuminuria among female adolescents and young adults.15,16 However, Nguyen et al. reported that this gender discrepancy was not observed in obese adolescents.16 The relevance of this finding in our cohort of severely obese females warrants additional investigation.
When we compared kidney function among the severely obese adolescents in this cohort and children participating in the NHANES survey14, we observed a consistent trend of lower eGFR among the bottom 10% and higher eGFR among the upper quartile of GFR distribution in severely obese adolescents (Figure 1). The presence of a higher eGFR may represent an increased prevalence of hyperfiltration in morbidly obese adolescents. Hyperfiltration is a proposed mechanism of early glomerular injury occurring in a number of conditions, including diabetes, hypertension, and obesity.24 Furthermore, hyperfiltration precedes the development of proteinuria in patients with diabetes and hypertension.25,26 Prospective studies of obese adolescents will be needed to evaluate if obese adolescents with hyperfiltration represent a group with early glomerular injury at risk for the development of future microalbuminuria and CKD.
One of the strongest relationships we found in adjusted analyses was that higher BMI was associated with lower eGFR in the Teen-LABS participants. This is in contrast to some adult studies that have reported an association of higher BMI with hyperfiltration.26–28 These studies, however, were conducted in research participants with a broad BMI distribution, including lean and overweight (mean BMI 25–28 kg/m2), whereas the current cohort of exclusively severely obese youth (median BMI of 50.5 kg/m2) is a uniquely homogeneous study group. It has been postulated that hyperfiltration associated with obesity precedes a subsequent decline in GFR24. It is possible that as obesity progresses from mild to severe in the relatively short timeframe of childhood, nephron damage occurs and the cumulative effect is a reduced GFR in those with highest BMI values at an earlier time-point. This study was not designed to address this question but the results are intriguing and warrant further inquiry. The association of increased BMI with decreased GFR in our study population is concerning and suggests an increased risk for CKD in adolescents with severe obesity. Our results are also supported by several studies that have identified obesity as an independent risk factor for progression to CKD.29–31
In our study, we found a weak but statistically significant negative association between eGFR and HOMA-IR, suggesting that those with the greatest insulin resistance had the greatest kidney dysfunction. Other studies examining this relationship have shown inconsistent results.32–35 Nerpin et al32 analyzed data from a community-based cohort of 1070 elderly men and demonstrated a direct association between insulin resistance and eGFR after adjusting for demographic, metabolic and cardiovascular risks. The study also reported that higher insulin resistance was associated with a higher risk of impaired kidney function during a 7-year follow up period. In contrast, in a large study of 145,865 adults, Park et al35 found no significant differences in the HOMA-IR values among eGFR groups when groups were stratified by the metabolic syndrome components. The association of both BMI and abnormal glucose metabolism with impaired kidney function in this cohort further emphasizes the complexity of the metabolic dysfunction that should be expected in these severely obese individuals, and provides the rationale for further data collection and analysis of longitudinal trends in kidney function over time, since both BMI and insulin resistance are significantly impacted by bariatric surgery.
Our study has a few limitations that deserve consideration. We used serum cystatin-C to estimate kidney function and did not have a direct measurement of GFR. Various creatinine-based estimations of kidney function have demonstrated significant variability in performance among obese patients.36 While some feel that cystatin C-based formulas may provide a more sensitive and accurate quantitation of kidney function irrespective of body composition14,37, others argue that cystatin C might underestimate eGFR in both normal and obese populations.38 Therefore we cannot exclude that the performance of cystatin C-based eGFR was affected by body composition. However, median eGFR values in our study were comparable to those obtained in healthy NHANES subjects14, which suggests that there was not a systematic bias of cystatin-C based GFR estimation in the Teen-LABS cohort. It is important to note that cystatin C determination in our study and in NHANES utilized the same methodology.39 Another limitation was the evaluation of proteinuria using random samples (rather than first morning void), which precluded the identification of patients with orthostatic proteinuria. However, previous studies using a random sample in children showed lower prevalence of microalbuminuria.16,40 Therefore we suspect this had little effect on the study results. It is also possible that we did not fully control for all confounding variables affecting the measured outcomes (e.g. birth weight, duration of obesity, maternal diabetes) since Teen-LABS study does not collect this information.
Conclusion
A significant number of severely obese adolescents undergoing bariatric surgery demonstrate evidence of significant kidney abnormalities. This comorbidity has previously not been recognized in this population. Even more concerning, for those with impaired kidney function, progressive deterioration is predicted in the absence of effective weight management.10 Longitudinal analyses of kidney function are necessary to determine if kidney abnormalities can be reversed in obese adolescents following weight loss.
What is already known on this subject
Obesity during adolescence is associated with a higher prevalence of chronic kidney disease (CKD) in adulthood.
What this study adds
This is the most comprehensive analysis of kidney status in severely obese adolescents undergoing weight loss surgery.
The study determined that before surgery a large number of these patients have micro- and macroalbuminuria and decreased kidney function.
Acknowledgements
The authors gratefully acknowledge the expertise and assistance of the clinical research coordinators at each Teen-LABS study site, and the coordinators and data processing and data management staff at the Teen-LABS Data Coordinating Center at Cincinnati Children’s Medical Center and the University of Cincinnati. In addition, the authors appreciate the expertise of the dedicated staff at the Northwest Lipid Research Laboratory, the NIDDK project scientist Dr. Mary Horlick, and members Longitudinal Assessment of Bariatric Surgery (LABS) consortium and data center for their continued support and insightful input.
Funding Source: The Teen-LABS consortium was funded by cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), through grants: U01DK072493 (PI, Dr. Thomas Inge, CCHMC), UM1DK072493 (PI, Dr. Thomas Inge, CCHMC), and UM1DK095710 (PI, Dr. Ralph Buncher, University of Cincinnati). The study was also supported by grants UL1 TR000077-04 (Cincinnati Children’s Hospital Medical Center), UL1RR025755 (Nationwide Children’s Hospital), M01-RR00188 (Texas Children’s Hospital/Baylor College of Medicine), UL1 RR024153 and UL1TR000005 (University of Pittsburgh), UL1 TR000165 (University of Alabama, Birmingham).
Dr Inge was the recipient of an Ethicon Endosurgery investigator initiated research grant award. Dr. Courcoulas has received research grants from Covidien, Nutrisystem, Endogastric solutions, and is on the Scientific Advisory Board of Ethicon Endosurgery.
Abbreviations
- ACR
albumin to creatinine ratio
- eGFR
estimated glomerular filtration rate
Footnotes
Potential Conflicts of Interest: All authors have indicated they have no relationships relevant to this article to disclose
Financial Disclosure: The other authors have indicated they have no financial relationships relevant to this article to disclose.
Author contributions:
Nianzhou Xiao made substantial contributions to analysis and interpretation of data, drafting the manuscript, and approved the final version
Todd M Jenkins made substantial contributions to study design, analysis and interpretation of data, revising the article critically for important intellectual content, and approved the final version
Edward Nehus made substantial contributions to analysis and interpretation of data, drafting the manuscript, and approved the final version
Thomas H. Inge made substantial contributions to study conception and design, recruitment of participants, collection of data, analysis and interpretation of data, critical revision of the article for important intellectual content, and approved the final version
Marc P. Michalsky made substantial contributions to recruitment of participants, collection of data, critical revision of the article for important intellectual content, and approved the final version
Carroll M. Harmon made substantial contributions to recruitment of participants, collection of data, critical revision of the article for important intellectual content, and approved the final version
Michael A. Helmrath made substantial contributions to recruitment of participants, collection of data, critical revision of the article for important intellectual content, and approved the final version
Mary L. Brandt made substantial contributions to recruitment of participants, collection of data, critical revision of the article for important intellectual content, and approved the final version
Anita Courcoulas made substantial contributions to recruitment of participants, collection of data, and critical revision of the article for important intellectual content, and approved the final version
Marva Moxey-Mims made substantial contributions to study conception and design, critical revision of the article for important intellectual content, and approved the final version
Mark M Mitsnefes made substantial contributions to analysis and interpretation of data, drafting the manuscript, and approved the final version
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA : the journal of the American Medical Association. 2012 Feb 1;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claire Wang Y, Gortmaker SL, Taveras EM. Trends and racial/ethnic disparities in severe obesity among US children and adolescents, 1976–2006. International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2011 Feb;6(1):12–20. doi: 10.3109/17477161003587774. [DOI] [PubMed] [Google Scholar]
- 3.Koebnick C, Smith N, Coleman KJ, et al. Prevalence of extreme obesity in a multiethnic cohort of children and adolescents. The Journal of pediatrics. 2010 Jul;157(1):26–31. e22. doi: 10.1016/j.jpeds.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013 Oct 8;128(15):1689–1712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- 5.Fowler SM, Kon V, Ma L, Richards WO, Fogo AB, Hunley TE. Obesity-related focal and segmental glomerulosclerosis: normalization of proteinuria in an adolescent after bariatric surgery. Pediatr Nephrol. 2009 Apr;24(4):851–855. doi: 10.1007/s00467-008-1024-6. [DOI] [PubMed] [Google Scholar]
- 6.Chen HM, Li SJ, Chen HP, Wang QW, Li LS, Liu ZH. Obesity-related glomerulopathy in China: a case series of 90 patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008 Jul;52(1):58–65. doi: 10.1053/j.ajkd.2008.02.303. [DOI] [PubMed] [Google Scholar]
- 7.Kambham N, Markowitz GS, Valeri AM, Lin J, D'Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney international. 2001 Apr;59(4):1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 8.Serra A, Romero R, Lopez D, et al. Renal injury in the extremely obese patients with normal renal function. Kidney international. 2008 Apr;73(8):947–955. doi: 10.1038/sj.ki.5002796. [DOI] [PubMed] [Google Scholar]
- 9.Vivante A, Golan E, Tzur D, et al. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Archives of internal medicine. 2012 Nov 26;172(21):1644–1650. doi: 10.1001/2013.jamainternmed.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inge TH, King WC, Jenkins TM, et al. The effect of obesity in adolescence on adult health status. Pediatrics. 2013 Dec;132(6):1098–1104. doi: 10.1542/peds.2013-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inge TH, Zeller M, Harmon C, et al. Teen-Longitudinal Assessment of Bariatric Surgery: methodological features of the first prospective multicenter study of adolescent bariatric surgery. Journal of pediatric surgery. 2007 Nov;42(11):1969–1971. doi: 10.1016/j.jpedsurg.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64(1):25–30. doi: 10.1080/00365510410003723. [DOI] [PubMed] [Google Scholar]
- 13.Eckardt KU, Berns JS, Rocco MV, Kasiske BL. Definition and classification of CKD: the debate should be about patient prognosis--a position statement from KDOQI and KDIGO. Am J Kidney Dis. 2009 Jun;53(6):915–920. doi: 10.1053/j.ajkd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Fadrowski JJ, Neu AM, Schwartz GJ, Furth SL. Pediatric GFR estimating equations applied to adolescents in the general population. Clinical journal of the American Society of Nephrology : CJASN. 2011 Jun;6(6):1427–1435. doi: 10.2215/CJN.06460710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones CA, Francis ME, Eberhardt MS, et al. Microalbuminuria in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002 Mar;39(3):445–459. doi: 10.1053/ajkd.2002.31388. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen S, McCulloch C, Brakeman P, Portale A, Hsu CY. Being overweight modifies the association between cardiovascular risk factors and microalbuminuria in adolescents. Pediatrics. 2008 Jan;121(1):37–45. doi: 10.1542/peds.2007-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng HL, Bryant C, Cook R, O'Connor H, Rooney K, Steinbeck K. The relationship between obesity and hypoferraemia in adults: a systematic review. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2012 Feb;13(2):150–161. doi: 10.1111/j.1467-789X.2011.00938.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim CH, Kim HK, Bae SJ, Park JY, Lee KU. Association of elevated serum ferritin concentration with insulin resistance and impaired glucose metabolism in Korean men and women. Metabolism. 2011 Mar;60(3):414–420. doi: 10.1016/j.metabol.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Sun L, Franco OH, Hu FB, et al. Ferritin concentrations, metabolic syndrome, and type 2 diabetes in middle-aged and elderly chinese. J Clin Endocrinol Metab. 2008 Dec;93(12):4690–4696. doi: 10.1210/jc.2008-1159. [DOI] [PubMed] [Google Scholar]
- 20.Hsu YH, Huang MC, Chang HY, et al. Association between serum ferritin and microalbuminuria in Type 2 diabetes in Taiwan. Diabet Med. 2013 Nov;30(11):1367–1373. doi: 10.1111/dme.12257. [DOI] [PubMed] [Google Scholar]
- 21.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999 Dec 23;341(26):1986–1995. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- 22.Tajima S, Ikeda Y, Sawada K, et al. Iron reduction by deferoxamine leads to amelioration of adiposity via the regulation of oxidative stress and inflammation in obese and type 2 diabetes KKAy mice. American journal of physiology. Endocrinology and metabolism. 2012 Jan 1;302(1):E77–86. doi: 10.1152/ajpendo.00033.2011. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Frei B. Prolonged exposure to LPS increases iron, heme, and p22phox levels and NADPH oxidase activity in human aortic endothelial cells: inhibition by desferrioxamine. Arterioscler Thromb Vasc Biol. 2009 May;29(5):732–738. doi: 10.1161/ATVBAHA.108.183210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palatini P. Glomerular hyperfiltration: a marker of early renal damage in pre-diabetes and pre-hypertension. Nephrol Dial Transplant. 2012 May;27(5):1708–1714. doi: 10.1093/ndt/gfs037. [DOI] [PubMed] [Google Scholar]
- 25.Amin R, Turner C, van Aken S, et al. The relationship between microalbuminuria and glomerular filtration rate in young type 1 diabetic subjects: The Oxford Regional Prospective Study. Kidney Int. 2005 Oct;68(4):1740–1749. doi: 10.1111/j.1523-1755.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 26.Palatini P, Mormino P, Dorigatti F, et al. Glomerular hyperfiltration predicts the development of microalbuminuria in stage 1 hypertension: the HARVEST. Kidney Int. 2006 Aug;70(3):578–584. doi: 10.1038/sj.ki.5001603. [DOI] [PubMed] [Google Scholar]
- 27.Wuerzner G, Pruijm M, Maillard M, et al. Marked association between obesity and glomerular hyperfiltration: a cross-sectional study in an African population. Am J Kidney Dis. 2010 Aug;56(2):303–312. doi: 10.1053/j.ajkd.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Tomaszewski M, Charchar FJ, Maric C, et al. Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int. 2007 Apr;71(8):816–821. doi: 10.1038/sj.ki.5002160. [DOI] [PubMed] [Google Scholar]
- 29.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyren O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006 Jun;17(6):1695–1702. doi: 10.1681/ASN.2005060638. [DOI] [PubMed] [Google Scholar]
- 30.Gelber RP, Kurth T, Kausz AT, et al. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005 Nov;46(5):871–880. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis. 2005 Oct;46(4):587–594. doi: 10.1053/j.ajkd.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Nerpin E, Riserus U, Ingelsson E, et al. Insulin sensitivity measured with euglycemic clamp is independently associated with glomerular filtration rate in a community-based cohort. Diabetes care. 2008 Aug;31(8):1550–1555. doi: 10.2337/dc08-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basturk T, Unsal A. Is insulin resistance a risk factor for the progression of chronic kidney disease? Kidney & blood pressure research. 2011;34(2):111–115. doi: 10.1159/000323904. [DOI] [PubMed] [Google Scholar]
- 34.Mohteshamzadeh M, Wong C, Whiticar R, Thomas S. Is there a link between insulin resistance and chronic kidney disease in men with treated hypertension? Analysis of 5-year data. American journal of nephrology. 2009;29(2):116–122. doi: 10.1159/000151490. [DOI] [PubMed] [Google Scholar]
- 35.Park JH, Oh SW, Ahn SY, et al. Decreased estimated glomerular filtration rate is not directly related to increased insulin resistance. Diabetes research and clinical practice. 2013 Mar;99(3):366–371. doi: 10.1016/j.diabres.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Verhave JC, Gansevoort RT, Hillege HL, De Zeeuw D, Curhan GC, De Jong PE. Drawbacks of the use of indirect estimates of renal function to evaluate the effect of risk factors on renal function. J Am Soc Nephrol. 2004 May;15(5):1316–1322. [PubMed] [Google Scholar]
- 37.Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C--a new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998 May;101(5):875–881. doi: 10.1542/peds.101.5.875. [DOI] [PubMed] [Google Scholar]
- 38.Vupputuri S, Fox CS, Coresh J, Woodward M, Muntner P. Differential estimation of CKD using creatinine- versus cystatin C-based estimating equations by category of body mass index. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009 Jun;53(6):993–1001. doi: 10.1053/j.ajkd.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finney H, Newman DJ, Gruber W, Merle P, Price CP. Initial evaluation of cystatin C measurement by particle-enhanced immunonephelometry on the Behring nephelometer systems (BNA, BN II) Clinical chemistry. 1997 Jun;43(6 Pt 1):1016–1022. [PubMed] [Google Scholar]
- 40.Mueller PW, Caudill SP. Urinary albumin excretion in children: factors related to elevated excretion in the United States population. Ren Fail. 1999 May-Jul;21(3–4):293–302. doi: 10.3109/08860229909085091. [DOI] [PubMed] [Google Scholar]