Abstract
Objectives
Several different methods are currently used to detect antibodies to Japanese encephalitis virus (JEV) in serum samples or cerebrospinal fluid. These methods include the plaque reduction neutralization test (PRNT), the hemagglutination inhibition (HI) test, indirect immunofluorescence assay (IFA), and enzyme-linked immunosorbent assay (ELISA). The purpose of this study was to compare the performance of each method in detecting vaccine-induced antibodies to JEV.
Methods
The study included 29 children who had completed a primary immunization schedule with an inactivated vaccine against JEV derived from mouse brain (n = 15) or a live attenuated SA14-14-2 vaccine (n = 14). Serum samples were collected between 3 months and 47 months after the last immunization. The serum samples were tested by performing the PRNT, HI test, in-house IFA, and commercial ELISA. The antibody detection rates were compared between tests.
Results
All 29 serum samples were positive with the PRNT, showing antibody titers from 1:20 to 1:2560. The HI test showed positive rates of 86.7% (13/15) and 71.4% (10/14) in the inactivated and live attenuated vaccine groups, respectively. The results of the IFA for immunoglobulin (Ig)G were positive in 53.3% (8/15) of children in the inactivated vaccine group and 35.7% (5/14) in the live attenuated vaccine group. Neither the IFA nor ELISA detected JEV IgM antibodies in any of the 29 children.
Conclusion
These results show that detection rates of vaccine-induced antibodies to JEV have a wide range (0–100%) depending on the testing method as well as the time since immunization and individual differences between children. These findings are helpful in interpreting serological test results for the diagnosis of Japanese encephalitis in situations where vaccines are widely administered.
Keywords: enzyme-linked immunosorbent assay, hemagglutination inhibition, indirect immunofluorescence assay, Japanese encephalitis, plaque reduction neutralization test
1. Introduction
Japanese encephalitis (JE), a mosquito-borne disease, is caused by the JE virus (JEV), which belongs to the Flavivirus genus within the Flaviviridae family [1]. An estimated 67,900 cases of JE with 20–30% fatality occurred throughout the Asian and the Pacific regions in the early 2010s [2]. Vaccination is the most effective measure for disease control and extensive immunization programs have been implemented in Korea, Japan, and Taiwan [3–5]. Two types of vaccines have been used in Korea. Inactivated vaccines derived from mouse brain have been used since the late 1960s. Currently, a three-dose primary vaccination course at 1–3 years of age (0 day, 7–30 days at 12–23 months of age, and 1 year after the 2nd dose) and two booster immunizations at the ages of 6 years and 12 years are recommended by the National Immunization Program (NIP) [3]. A live attenuated SA 14-14-2 vaccine has been administered in the private sector since the late 1990s and was included in the NIP from 2013. The latest regimen is a one-dose primary vaccination at 12–23 months of age and one booster immunization 12 months after the first dose.
There are several methods used to detect the JEV antibodies induced by vaccination and natural infections; these tools have been used to evaluate the efficacy of the vaccine and to diagnose patients with the disease [6–8]. These include the plaque reduction neutralization test (PRNT), the hemagglutination inhibition (HI) test, an indirect immunofluorescence assay (IFA), and an enzyme-linked immunosorbent assay (ELISA). The PRNT has been considered to be the most reliable method for the evaluation of the efficacy of the vaccine or patient diagnosis, although its turnaround time is 5–7 days for most flaviviruses and quality control remains difficult [7,9]. The PRNT is advantageous in regions where two or more flaviviruses circulate together. Because strong cross-reactions occur between antibodies induced by flavivirus infections, it is hard to identify the exact pathogens with tools other than the PRNT [10]. However, more rapid and easier methods such as ELISA and IFA may be preferable when only one or two distantly related flavivirus species are transmitted. Serological tests other than PRNT may therefore be useful in Korea, where only JE has been reported, although tick-borne encephalitis virus (TBEV) has been isolated in ticks and rodents [11,12]. Although many papers have addressed the detection performance of each testing method for patient diagnosis [6,7,13–16], no standard or guideline has been established for choosing a methodology to detect vaccine-induced antibodies, although some investigators have demonstrated a high correlation of HI, ELISA, and IFA results with that of the PRNT [17,18].
In this study, we compared the performance of each test in detecting vaccine-induced antibodies to JEV in vaccinated children.
2. Materials and methods
2.1. Serum samples
A total of 29 serum samples was collected from healthy children who completed the primary JE vaccine schedule between June 2001 and August 2006. Fifteen children were vaccinated with an inactivated vaccine derived from mouse brain (3 doses) and 14 children were vaccinated with live attenuated SA14-14-2 vaccine (2 doses). Serum samples were collected from study participants between 3 months and 47 months after the last vaccine dose (between May 2006 and March 2007). The medical history of all participants showed no apparent history of infection with JEV or other flaviviruses during the study. This study was approved by the Institutional Review Board and written informed consent was obtained from the parents of the children (IUH IRB 06-390, Inha University).
2.2. PRNT
BHK-21 cells (ATCC, CCL-10) were initially inoculated at 4.5 × 105cells/well in six-well tissue culture plates and propagated for 48 hours at 37°C in a CO2 incubator. Serum samples were inactivated for 30 minutes in a 56°C water-bath and serially diluted two-fold from 1:5 to 1:1280 in minimum essential medium (MEM) containing 10% fetal bovine serum (FBS) and penicillin/streptomycin antibiotics (Gibco, Grand Island, NY, USA). A 100-μL aliquot of JEV (Nakayama strain) with 100 plaque-forming units (pfu) was mixed with equal volumes of diluted serum samples and incubated for 1 hour at 37°C. Each virus/serum mixture (total volume 200 μL) was inoculated onto the BHK-21 cell monolayer after draining the culture medium and was allowed to settle for 1 hour at 37°C in a CO2 incubator. The mixture was removed from the cell monolayer and each well washed once with phosphate-buffered saline (PBS). Then 4 mL of pre-warmed overlay medium consisting of 0.9% Noble agar, penicillin/streptomycin, and 2% FBS in MEM were poured onto each well. The plates were placed in a CO2 incubator and the overlay medium was removed 5 days after inoculation. Each well was fixed with 10% formalin for 30 minutes and stained with 1% crystal violet solution (resolved in 70% methanol). Plate wells were washed with tap water, dried, and the plaques were counted. The neutralizing antibody titer (PRNT50) was defined as the reciprocal of the last serum dilution that showed 50% or more plaque reduction compared with the plaque counts in the virus-only control well. PRNT50 titers ≥1:10 were considered positive.
2.3. HI test
The HI test was performed using goose erythrocyte and JEV (Nakayama strain) antigens derived from mouse brain according to the method described by Clarke and Casals [19]. Acetone-treated serum samples were serially diluted two-fold from 1:10 to 1:640. The HI antibody titer was expressed as the reciprocal of the highest serum dilution that showed complete inhibition of hemagglutination (8HA units). HI antibody titers ≥1:10 were considered positive.
2.4. IFA
Antigen slides were made using BHK-21 cells and JEV Nakayama strain. Cells were grown in MEM with 10% FBS (MEM-10) in T75 culture flasks until 70–80% confluence. A 1-mL aliquot of virus stock (2 × 106 pfu/mL) was mixed with 2 mL of medium, inoculated on the cell monolayer, and placed in a CO2 incubator for 1 hour. The cells were then rinsed once with PBS, detached with 2 mL of trypsin/EDTA and counted with a disposable hemocytometer. The cell density was adjusted to 1 × 102 cells/μL in MEM-10 and 30 μL of the diluted sample were added to wells of Teflon-coated slide glasses (75 × 25 × 1 mm, 12 wells, 5 mm diameter; Tekdon, Myakka, FL, USA) using an automatic dispenser (Biohit, Helsinki, Finland). The cells were allowed to attach to the wells in a CO2 incubator at 37°C for 15–17 hours. The media were then drained and the cells were fixed with 80% cold acetone for 20 minutes, dried at ambient temperature, and stored at −70°C until use.
For immunoglobulin (Ig)G antibody detection, serum samples were serially diluted two-fold from 1:16 to 1:1024 and 25 μL of diluted serum were applied to the antigen slide wells. After incubation in a moist chamber for 30 minutes at 37°C, the serum samples were drained and the antigen slides were washed in an agitating rack with PBS for 10 minutes (2 × 5 minutes). Fluorescein isothiocyanate-conjugated goat anti-human IgG antibodies (Jackson ImmunoResearch, West Grove, PA, USA) were diluted 1:200 in 0.0025% Evans blue solution. Next, 25 μL of the antibodies were added to the wells and the antigen slides were placed in a moist chamber for 30 minutes at 37°C. The slides were washed again in PBS for 10 minutes and a cover-glass with a drop of mounting medium (Sigma–Aldrich, St Louis, MO, USA) was applied. Finally, the slides were examined at 200 × magnification under a fluorescence microscope (Axioskop 2 plus, Carl Zeiss, Goettingen, Germany) in a dark room.
For IgM antibody detection, serum samples were treated with rheumatoid factor removal reagent (Chemicon, CA, USA). The treated sample (1:10 dilution) was applied to the antigen slides for 1 hour in a 37°C incubator. Fluorescein isothiocyanate-conjugated goat anti-human IgM (Jackson ImmunoResearch) was used as a secondary antibody under conditions identical to those used for IgG antibody detection. IFA (West Grove, PA, USA) antibody titers were expressed as the reciprocal of the last serum dilution that showed definite cytoplasmic fluorescence (an apple green color). Antibody titers ≥1:16 and titers ≥1:10 were considered IgG- and IgM-positive, respectively.
2.5. IgM capture ELISA
Samples were tested for JEV-specific IgM antibodies using the commercial JE-Dengue IgM Combo ELISA test (Panbio, Brisbane, QLD, Australia) according to the manufacturer's instructions. The kit was used for the differential screening of JE and dengue fever. Recombinant antigens produced in an insect cell system were used and 100 μL of serum samples diluted 1:100 were applied to each reaction well. Panbio units (PU) were calculated for each sample based on the absorbance at 450 nm. Samples with PU > 11 were considered positive, PU < 9 negative, and values of PU 9–11 were equivocal. When PU values for both JE and dengue fever were >11, a JE to dengue fever ratio was calculated for differential interpretation. A JE to dengue fever ratio ≥1 was considered to be JE and a JE to dengue fever ratio <1 was to be considered dengue fever.
2.6. IgG ELISA
The presence of JEV-specific IgG antibodies was tested using the commercial JE Detect IgG ELISA kit (InBios, Seattle, WA, USA) according to the manufacturer's protocol. Briefly, the micro-titer plate was coated with recombinant antigens consisting of peptides from different JEV genes. Fifty microliters of 1:300 diluted serum samples were applied to the reaction well. The immune status ratio was calculated for each sample based on the absorbance at 450 nm. Samples with an immune status ratio >5 were considered positive, while an immune status ratio <2 were considered to be negative. Samples with values of 2–5 were considered equivocal.
3. Results
The presence of antibodies to JEV after vaccination was assessed using four serological tests in 29 children who had completed the primary immunization schedule with either of two vaccine types (an inactivated vaccine derived from mouse brain or live attenuated vaccines). Table 1 gives information about the participants and sampling. The mean ± SD time from the last immunization to serum collection was 27.9 ± 15.6 months and 21.3 ± 15.8 months in the inactivated and live attenuated vaccine groups, respectively. The time difference between the two groups was not statistically significant (t = 1.139, p = 0.265).
Table 1.
Characteristics of the 29 children enrolled in the study.
| Characteristics | Group |
Total | |
|---|---|---|---|
| Inactivated vaccine | Live vaccine | ||
| Number of children | 15 | 14 | 29 |
| Number of doses in primary schedule | 3 | 2 | – |
| Mean (range) age at time of first dose (mo) | 16.4 (10.4–24.2) | 14.7 (11.9–24.3) | 15.6 (10.4–24.3) |
| Mean (range) age at time of last dose (mo) | 31 (19.6–67.7)a | 24.9 (18.4–31.6) | 28 (18.4–67.7) |
| Interval between sampling and last dose (mo) | 27.9 (3.2–47.0) | 21.3 (3.0–47.1) | 24.7 (3.0–47.1) |
Inactivated vaccine = inactivated vaccines derived from mouse brain; live vaccine = live attenuated vaccine.
Range was relatively wide because one child had a booster shot more than 1 year later than recommended in the schedule.
Positivity rates differed according to the testing method. All 29 (100%) children were positive for neutralizing antibodies to JEV using the PRNT regardless of the vaccine type (Table 2). The HI test was positive in 13/15 (86.6%) children in the inactivated vaccine group and in 10/14 (71.4%) children in the live attenuated vaccine group. The positive rates using the IgG IFA were 53.3% (8/15) and 35.7% (5/14), respectively. However, none of the 29 children was positive using the commercial IgG/IgM ELISA or the IgM IFA.
Table 2.
Rates of antibody detection obtained by four serological tests in children vaccinated for Japanese encephalitis.
| Test | Inactivated vaccine group (n = 15) |
Live attenuated vaccine group (n = 14) |
||
|---|---|---|---|---|
| No. (%) positive | GMT | No. (%) positive | GMT | |
| PRNT | 15 (100) | 1:292 | 14 (100) | 1:195 |
| HI | 13 (86.7) | 1:20 | 10 (71.4) | 1:17 |
| IFA (IgG) | 8 (53.3) | 1:99 | 5 (35.7) | 1:111 |
| IFA (IgM) | 0 | – | 0 | – |
| ELISA (IgG) | 0 | – | 0 | – |
| ELISA (IgM) | 0 | – | 0 | – |
ELISA = enzyme-linked immunosorbent assay; GMT = geometric mean titer; HI = hemagglutination inhibition test; IFA = indirect immunofluorescence assay; Ig = immunoglobulin; PRNT = plaque reduction neutralization test.
The geometric mean titer (GMT) of neutralizing antibodies in the inactivated vaccine group was higher than that in the live attenuated vaccine group (292 vs. 195). However, the difference was not statistically significant (p > 0.05). Similarly, the differences in the GMT of the HI test (20 vs. 17) and of the IgG IFA (99 vs. 111) between the groups were not statistically significant.
The different JEV antibody types observed in each child were plotted against the time since immunization (Figure 1). Neutralizing antibodies remained at detectable levels until at least 47 months after the last dose of vaccine. HI and IgG IFA antibodies were also detectable until 47 months post-immunization, although the detection rates were relatively low and showed individual differences (Table 2, Figure 1). However, the IgG/IgM ELISA and IgM IFA did not show any positive results in either vaccine group. There was no correlation in the antibody titers between the PRNT, HI test, and IgG IFA (data not shown).
Figure 1.
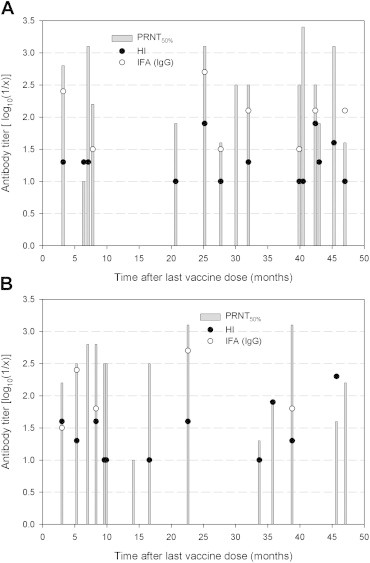
Comparison of antibody titers obtained using different tests in children who had completed a primary immunization schedule with mouse brain inactivated (A; n = 15) or live attenuated vaccines (B; n = 14). The PRNT and HI antibody titers ranged from 1:10 to 1:2560, whereas the IFA antibody titers started from 1:16 in two-fold serial dilutions. Test values: PRNT50% titers (closed boxes), HI titers (closed circles), IFA titers (open circles). Negative results (i.e., antibody titers less than 1:10 or 1:16) are not shown in the plots. 1/x = serum dilution factor (i.e., 80 or 160). HI = hemagglutination inhibition test; IFA = indirect immunofluorescence assay; PRNT = plaque reduction neutralization test.
4. Discussion
This study measured the duration for which vaccine-induced antibodies were detectable by currently used serological tests. This information is valuable for diagnostic laboratories because vaccine-induced antibodies can interfere with the interpretation of serological test results for patient diagnosis.
We compared the performance of four serological tests for detecting vaccine-induced antibodies in children who had completed the primary vaccination schedules recommended by the NIP. The PRNT detected neutralizing antibodies in all 29 children at least 47 months post-immunization, whereas the commercial ELISA tests showed no positive reaction. This means that the PRNT was the most reliable method for investigating the protective immunity against JEV; the HI test and IgG IFA showed lower positive rates of 35.7–87.5%. This result disagrees with the findings of other investigators. Venturi et al [17] examined humoral immunity and correlation between commercial IgG ELISA tests, HI, and PRNT in children vaccinated against TBEV. Serum samples were collected from 36 children 1–4 months (mean 123 days) after they had received a third immunization dose against TBEV. They found that all three tests detected antibodies at a maximum of 4 months after the last vaccine dose and that there was a good correlation between the antibody titers. Baldovin et al [20] investigated the persistence of TBEV antibodies in vaccinated (n = 126) and naturally infected (n = 66) groups. Blood samples from participants in the vaccinated and natural infection groups were sampled 3–8 years after the last immunization and 1.4–13.7 years after viral infection, respectively. Commercial IgG ELISA tests showed 79% and 100% positive results in the vaccination and natural infection groups, respectively. Litzba et al [18] evaluated commercial ELISA and IFA in 100 people immunized with two doses of inactivated Vero cell culture-derived JE vaccine (IXIARO) on Day 0 and Day 28. Among 78 serum samples collected 7–56 days after the final dose, 50 (64%) and 51 (65%) samples were positive using IgM ELISA (Panbio) and IgM IFA (Euroimmun, Lubeck, Germany), respectively. A positive rate ≥60% is fairly high compared with only 13% (9/68) of children being IgM ELISA-positive four weeks after receiving one dose of live attenuated vaccine, as reported by Park et al [21]. In the report of Litzba et al [18], serum samples collected 56 days after immunization were tested for IgG antibodies. The IgG ELISA test (InBios) showed a positive rate of 6.6% (6/91), whereas the IgG IFA a showed positive rate of 94% (91/97). This result was in good agreement with our finding that the IgG ELISA test (InBios) had a lower detection rate than the other tests. The low detection range observed for the InBios IgG ELISA test might be due to the antigens used in the kit, which consisted of a mixture of peptides produced from different parts of genes. The manufacturer states that the kit is not optimized for vaccine-induced seroconversion studies. We could not perform any further evaluation because no other commercial IgG ELISA kits are available. The use of the kit for diagnostic purposes would require an evaluation of patient samples. Differences in the reported performance of identical serological tests between studies may be caused by differences in vaccine type, immunization schedule, the efficacy of the vaccine itself, or sampling intervals.
It is well documented that natural flavivirus infections elicit greater immune responses and longer lasting immunity than vaccine-induced immunity [13,18,20,22]. Neutralizing antibodies may persist for years or over a lifetime in natural infections. HI antibodies in patients have been reported to persist at detectable levels for years or decades. IgG antibodies may be detectable in patients for years using IFA and ELISA. Sharma et al [23] reported that IgM ELISA antibodies were at detectable levels in JE patients for months and up to a year. Burk et al [14] provided results with more details and found that an in-house ELISA could be used to detect JEV IgM and IgG antibodies in patients at 180 days. Taken together, these studies confirm that the period of detectable JEV antibodies induced by vaccination or natural infection depends on the testing methods and individual differences.
In conclusion, this study shows that the PRNT is the most reliable method for detecting vaccine-induced JEV antibodies. We also found that IgM detection using IFA and ELISA is more suitable for diagnosis because the chance of confusion with vaccine-induced antibodies were relatively low in those tests. These findings may help to improve testing algorithms for JE diagnosis in situations where vaccines are widely administered.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors thank Y.J. Yu and S.K. Whang for their laboratory assistance. This study was funded in part by Control of Bacterial and Viral Diseases (4837-301-210-13) and National Immunization Program (4836-303-210-13) grants from the National Institute of Health, Korea Centers for Disease Control and Prevention.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Knipe D.M., Howley D.M. 5th ed. Lippincott-Raven; Philadelphia: 2007. Fields virology. (Flaviviridae). Ch 33. p. 1101–13. [Google Scholar]
- 2.Centers for Disease Control and Prevention (USA) Japanese encephalitis surveillance and immunization – Asia and the Western Pacific. MMWR Morb Mortal Wkly Rep. 2012 Aug;62(33):658–662. [PMC free article] [PubMed] [Google Scholar]
- 3.Korea Centers for Disease Control and Prevention (KCDC) 3rd ed. KCDC; Chungbuk: 2013. Epidemiology and control of vaccine preventable diseases; pp. 265–282. [Google Scholar]
- 4.Abe M., Okada K., Hayashida K. Duration of neutralizing antibody titer after Japanese encephalitis vaccination. Microbiol Immunol. 2007 Mar;51(6):609–616. doi: 10.1111/j.1348-0421.2007.tb03947.x. [DOI] [PubMed] [Google Scholar]
- 5.Yang S.E., Pan M.J., Tseng H.F. The efficacy of mouse-brain inactivated Nakayama strain Japanese encephalitis vaccine – results from 30 years experience in Taiwan. Vaccine. 2006 Mar;24(14):2669–2673. doi: 10.1016/j.vaccine.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 6.Prince H.E., Hogref W.R. Assays for detecting West Nile Virus antibodies in human serum, plasma, and cerebrospinal fluid. Clin Appl Immunol Rev. 2005;5:45–63. [Google Scholar]
- 7.Maeda A., Maeda J. Review of diagnostic plaque reduction neutralization tests for flavivirus infection. Vet J. 2013 Jan;195(1):33–40. doi: 10.1016/j.tvjl.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . WHO; Geneva: 2009. Dengue: guidelines for diagnosis, treatment, prevention and control. [PubMed] [Google Scholar]
- 9.Roehrig J.T., Hombach J., Barrett A.D. Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. Viral Immunol. 2008 Jun;21(2):123–132. doi: 10.1089/vim.2008.0007. [DOI] [PubMed] [Google Scholar]
- 10.Mansfield K.L., Horton D.L., Johnson N. Flavivirus-induced antibody cross-reactivity. J Gen Virol. 2011 Dec;92(12):2821–2829. doi: 10.1099/vir.0.031641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong Y.E., Kim Y.H., Cho J.E. Identification of dengue type 1 virus (DENV-1) in Koreans traveling abroad. Osong Public Health Res Perspect. 2011 Jun;2(1):34–40. doi: 10.1016/j.phrp.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S.Y., Jeong Y.E., Yun S.M. Molecular evidence for tick-borne encephalitis virus in ticks in South Korea. Med Vet Entomol. 2009 Mar;23(1):15–20. doi: 10.1111/j.1365-2915.2008.00755.x. [DOI] [PubMed] [Google Scholar]
- 13.Murray P.R., Baron E.J., Pfealler M.A. 7th ed. American Society for Microbiology; Washington: 1999. Manual of clinical microbiology. (Arboviruses). Ch 89, p. 1118–24. [Google Scholar]
- 14.Burke D.S., Nisalak A., Ussery M.A. Kinetics of IgM and IgG responses to Japanese encephalitis virus in human serum and cerebrospinal fluid. J Infect Dis. 1985 Jun;151(6):1093–1099. doi: 10.1093/infdis/151.6.1093. [DOI] [PubMed] [Google Scholar]
- 15.Niedrig M., Sonnenberg K., Steinhagen K. Comparison of ELISA and immunoassays for measurement of IgG and IgM antibody to West Nile virus in human sera against virus neutralisation. J Virol Methods. 2007 Jan;139(1):103–105. doi: 10.1016/j.jviromet.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Venturi G., Martelli P., Mazzolini E. Humoral immunity in natural infection by tick-borne encephalitis virus. J Med Virol. 2009 Apr;81(4):665–671. doi: 10.1002/jmv.21431. [DOI] [PubMed] [Google Scholar]
- 17.Venturi G., Mel R., Marchi A. Humoral immunity and correlation between ELISA, hemagglutination inhibition, and neutralization tests after vaccination against tick-borne encephalitis virus in children. J Virol Methods. 2006 Jun;134(1–2):136–139. doi: 10.1016/j.jviromet.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Litzba N., Klade C.S., Lederer S. Evaluation of serological diagnostic test systems assessing the immune response to Japanese encephalitis vaccination. PLoS Negl Trop Dis. 2010 Nov;4(11):e883. doi: 10.1371/journal.pntd.0000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke D.H., Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 20.Baldovin T., Mel R., Bertoncello C. Persistence of immunity to tick-borne encephalitis after vaccination and natural infection. J Med Virol. 2012 Aug;84(8):1274–1278. doi: 10.1002/jmv.23313. [DOI] [PubMed] [Google Scholar]
- 21.Park M., Rho H., Sohn Y. Immune response to SA14-14-2 live attenuated Japanese encephalitis vaccine. J Korean Pediatr Soc. 1999 Mar;43(3):351–359. [Google Scholar]
- 22.Tseng H.F., Tan H.F., Chang C.K. Seroepidemiology study of Japanese encephalitis neutralizing antibodies in southern Taiwan: a comparative study between urban city and country townships. Am J Infect Control. 2003 Nov;31(7):435–440. doi: 10.1067/mic.2003.73. [DOI] [PubMed] [Google Scholar]
- 23.Sharma S., Mathur A., Prakash V. Japanese encephalitis virus latency in peripheral blood lymphocytes and recurrence of infection in children. Clin Exp Immunol. 1991 Jul;85(1):85–89. doi: 10.1111/j.1365-2249.1991.tb05687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


