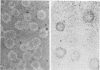
Abstract
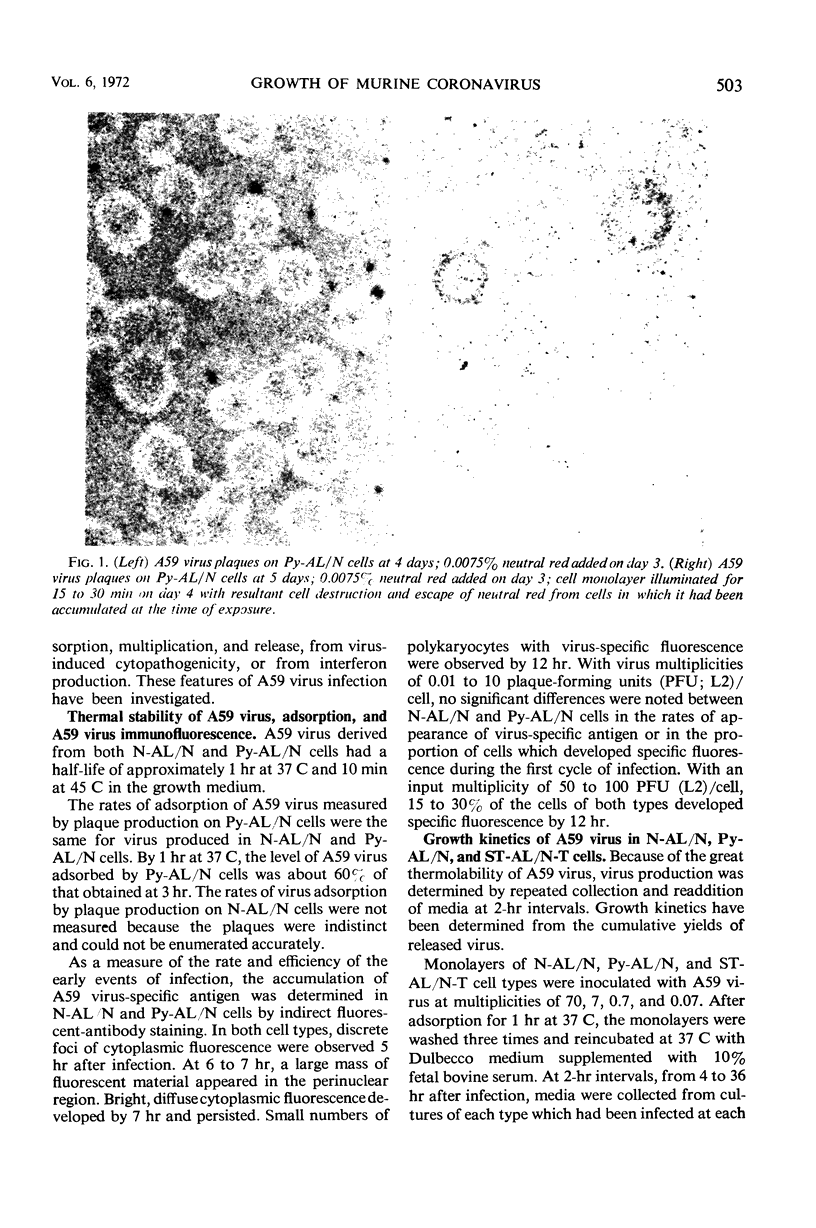
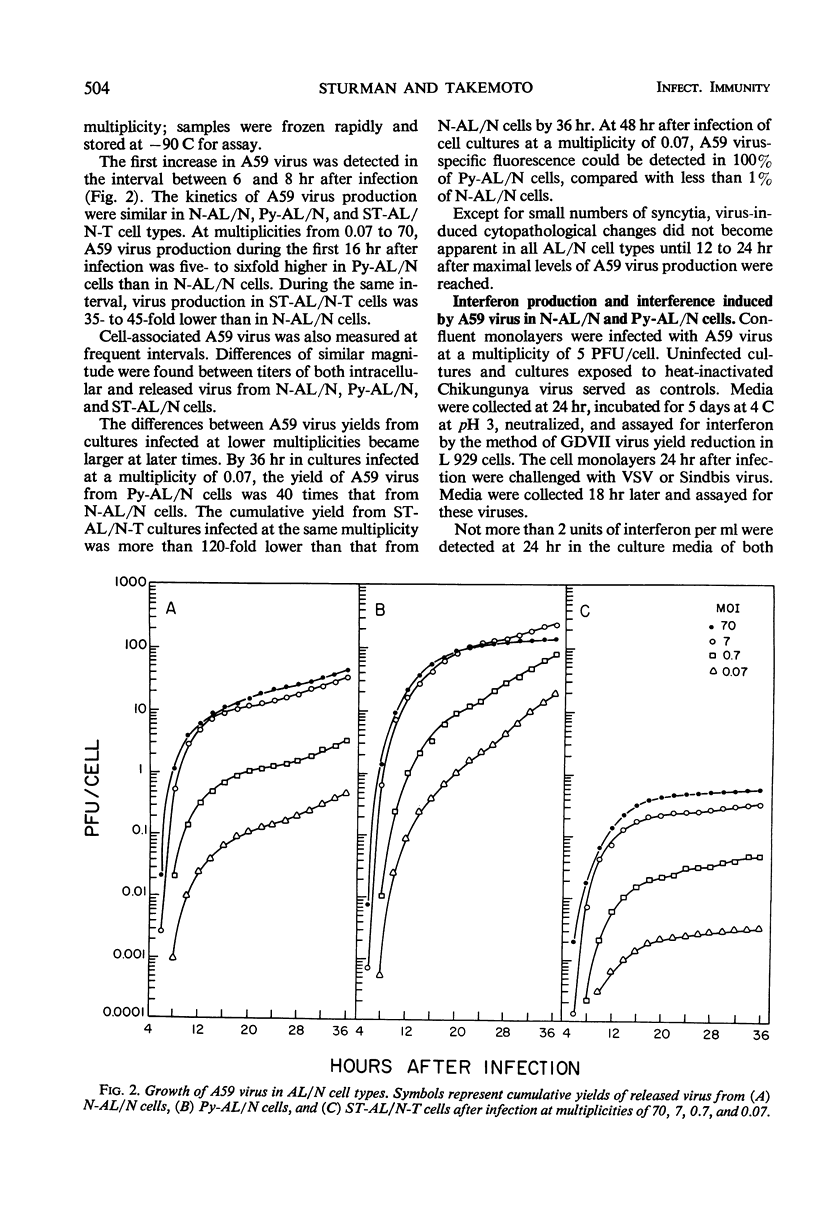
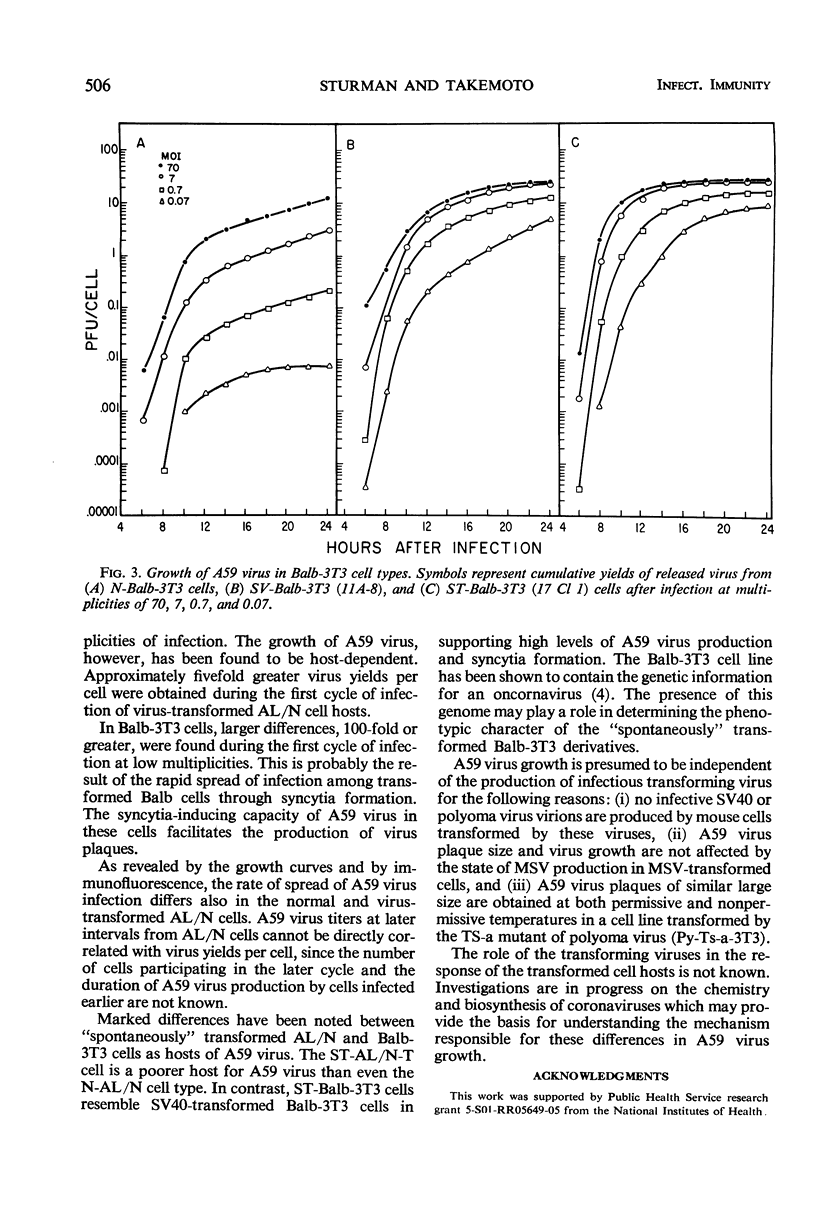
Plaque formation by A59 virus, a murine coronavirus, was facilitated in AL/N and Balb mouse cells transformed by polyoma virus, simian virus 40, murine sarcoma virus, or mammary tumor virus. In these virus-transformed cells, A59 virus plaques were larger, they appeared earlier, and plaquing efficiencies were higher than in normal, untransformed cells. “Spontaneously” transformed AL/N cells behaved similarly to untransformed cells, whereas “spontaneously” transformed Balb cells resembled virus-transformed cell hosts. Both untransformed and transformed AL/N and Balb cells were permissive hosts for A59 virus. However, multiplication of A59 virus was enhanced at least fivefold in the virus-transformed AL/N cell hosts. Larger differences (100-fold or greater) in A59 virus production were obtained during the first cycle of infection in Balb cells at low multiplicities and in AL/N cells after multiple cycles of virus growth. In virus-transformed and “spontaneously” transformed Balb cells, A59 virus induced extensive syncytia formation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Hartley J. W., Todaro G. J. Mouse leukemia virus: "spontaneous" release by mouse embryo cells after long-term in vitro cultivation. Proc Natl Acad Sci U S A. 1969 Sep;64(1):87–94. doi: 10.1073/pnas.64.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Rowe S. P. Nonproducer clones of murine sarcoma virus transformed BALB-3T3 cells. Virology. 1970 Sep;42(1):9–19. doi: 10.1016/0042-6822(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradburne A. F., Tyrrell D. A. The propagation of "coronaviruses" in tissue-culture. Arch Gesamte Virusforsch. 1969;28(2):133–150. doi: 10.1007/BF01249379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucková M., McIntosh K., Kapikian A. Z., Chanock R. M. The adaptation of two human coronavirus strains (OC38 and OC43) to growth in cell monolayers. Proc Soc Exp Biol Med. 1970 Nov;135(2):431–435. doi: 10.3181/00379727-135-35068. [DOI] [PubMed] [Google Scholar]
- Coria M. F. Intracellular avian infectious bronchitis virus: detection by fluorescent antibody techniques in nonavian kidney cell cultures. Avian Dis. 1969 Aug;13(3):540–547. [PubMed] [Google Scholar]
- Cunningham C. H. Avian infectious bronchitis. Adv Vet Sci Comp Med. 1970;14:105–148. [PubMed] [Google Scholar]
- Hamre D., Procknow J. J. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966 Jan;121(1):190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- Lukert P. D. Immunofluorescence of avian infectious bronchitis virus in primary chicken embryo kidney, liver, lung, and fibroblast cell cultures. Arch Gesamte Virusforsch. 1966;19(3):265–272. doi: 10.1007/BF01241849. [DOI] [PubMed] [Google Scholar]
- McIntosh K., Dees J. H., Becker W. B., Kapikian A. Z., Chanock R. M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci U S A. 1967 Apr;57(4):933–940. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shif I., Bang F. B. In vitro interaction of mouse hepatitis virus and macrophages from genetically resistant mice. I. Adsorption of virus and growth curves. J Exp Med. 1970 Apr 1;131(4):843–850. doi: 10.1084/jem.131.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Tamm I. Host dependence of GDVII virus: complete or abortive multiplication in various cell types. J Immunol. 1966 Dec;97(6):885–896. [PubMed] [Google Scholar]
- TYRRELL D. A., BYNOE M. L. CULTIVATION OF A NOVEL TYPE OF COMMON-COLD VIRUS IN ORGAN CULTURES. Br Med J. 1965 Jun 5;1(5448):1467–1470. doi: 10.1136/bmj.1.5448.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Ting R. C., Ozer H. L., Fabisch P. Establishment of a cell line from an inbred mouse strain for viral transformation studies: simian virus 40 transformation and tumor production. J Natl Cancer Inst. 1968 Dec;41(6):1401–1409. [PubMed] [Google Scholar]