Abstract
OBJECTIVE: The prognostic value of inflammation indexes in esophageal cancer was not established. In this study, therefore, both prognostic values of Glasgow prognostic score (GPS) and combination of platelet count and neutrophil lymphocyte ratio (COP-NLR) in patients with esophageal squamous cell carcinoma (ESCC) were investigated and compared. METHODS: This retrospective study included 375 patients who underwent esophagectomy for ESCC. The cancer-specific survival (CSS) was calculated by the Kaplan-Meier method, and the difference was assessed by the log-rank test. The GPS was calculated as follows: patients with elevated C-reactive protein (> 10 mg/l) and hypoalbuminemia (< 35 g/l) were assigned to GPS2. Patients with one or no abnormal value were assigned to GPS1 or GPS0, respectively. The COP-NLR was calculated as follows: patients with elevated platelet count (> 300 × 109/l) and neutrophil lymphocyte ratio (> 3) were assigned to COP-NLR2. Patients with one or no abnormal value were assigned to COP-NLR1 or COP-NLR0, respectively. RESULTS: The 5-year CSS in patients with GPS0, 1, and 2 was 50.0%, 27.0%, and 12.5%, respectively (P < .001). The 5-year CSS in patients with COP-NLR0, 1, and 2 was 51.8%, 27.0%, and 11.6%, respectively (P < .001). Multivariate analysis showed that both GPS (P = .003) and COP-NLR (P = .003) were significant predictors in such patients. In addition, our study demonstrated a similar hazard ratio (HR) between COP-NLR and GPS (HR = 1.394 vs HR = 1.367). CONCLUSIONS: COP-NLR is an independent predictive factor in patients with ESCC. We conclude that COP-NLR predicts survival in ESCC similar to GPS.
Introduction
Esophageal cancer (EC) is the eighth most common cancer worldwide and the sixth leading cause of death from cancer [1]. Squamous cell carcinoma (SCC) comprises about 80% of all ECs worldwide [2]. In China, SCC is the most common pathologic type of ECs, in contrast to the predominance of adenocarcinoma in the Western countries [3], [4]. There are important biologic differences between China and Western countries regarding ECs; therefore, a prognostic study that takes into account SCC in China is necessary.
Recently, systemic inflammatory response plays an important role in the progression of cancer [5], [6]. Previous studies have shown that serum C-reactive protein (CRP) influenced the prognosis in patients with gastrointestinal cancers [7]. Moreover, the Glasgow prognostic score (GPS) combines serum CRP and hypoalbuminemia and has been demonstrated to be a predictive factor in various cancers, including ECs [8], [9], [10]. In addition, there is an increasing evidence that platelet count and neutrophil lymphocyte ratio (NLR) can be used for prognostication in several cancers [11], [12]. Recently, Ishizuka et al. [13] evaluated a novel inflammation-based prognostic system, termed as the combination of platelet count and NLR (COP-NLR). They demonstrated that COP-NLR is a useful predictor of postoperative survival in patients with colorectal cancer [13]. However, to the best of our knowledge, no studies regarding COP-NLR in patients with EC are available. Therefore, the aim of this study was to investigate and compare the prognostic values of COP-NLR and GPS in patients with esophageal squamous cell carcinoma (ESCC).
Methods
Patients
From January 2006 to December 2008, a retrospective analysis was conducted in 375 patients with ESCC who underwent curative esophagectomy at Zhejiang Cancer Hospital. All of the patients included in the analysis fit the following criteria: 1) ESCC confirmed by histopathology, 2) surgery with curative esophagectomy, 3) at least six lymph nodes were examined for pathologic diagnosis, and 4) surgery was neither preceded nor followed by adjuvant chemotherapy and/or radiotherapy.
On the basis of the medical records, the following data were collected for each patient: age, gender, laboratory examination, differentiation, tumor length and location, depth of invasion, nodal metastasis, and other miscellaneous characteristics. Ethical approval was obtained from the Ethical Committees of Zhejiang Cancer Hospital. All of the patients included in the study were staged according to the seventh edition of the American Joint Committee on Cancer Cancer Staging Manual [14].
In our institute, patients were followed up in the outpatient department. X-ray or computed tomography of the chest was performed during the follow-up. As this study described the prognosis of patients with ESCC, therefore, a cancer-specific survival (CSS) analysis would be more appropriate. Therefore, the CSS was ascertained in this study. The last follow-up time was November 2011.
GPS and COP-NLR Evaluation
Routine laboratory measurements including the serum levels of CRP, albumin, and blood cell counts were extracted in a retrospective fashion from the medical records. GPS was calculated as follows: patients with elevated CRP (> 10 mg/l) and hypoalbuminemia (< 35 g/l) were assigned to GPS2. Patients with one or no abnormal value were assigned to GPS1 or GPS0, respectively [8]. COP-NLR was calculated as follows: patients with elevated platelet count level (> 300 × 109/l) and NLR (> 3) were assigned to COP-NLR2. Patients with one or no abnormal value were assigned to COP-NLR1 or COP-NLR0, respectively [13].
Statistical Analysis
Statistical evaluation was conducted with SPSS 17.0 (Chicago, IL). The Pearson Chi-squared test was used to determine the significance of differences. Correlation analysis was performed by Pearson and Spearman correlation analyses. CSS was calculated by the Kaplan-Meier method, and the difference was assessed by the log-rank test. A univariate analysis was used to examine the association between various prognostic predictors and CSS. Possible prognostic factors associated with CSS on univariate analysis were considered in a multivariable Cox proportional hazards regression analysis with the enter method. Moreover, the Akaike information criterion (AIC) and Bayesian information criteria (BIC) were used to identify the statistical model [15], [16]. AIC was defined as AIC = − 2log(maximum likelihood) + 2 × (the number of parameters in the model). BIC was defined as BIC = − 2log(maximum likelihood) + (the number of parameters in the model) × log(sample size). A smaller AIC or BIC value indicates a more desirable model for predicting the outcome. A P value less than .05 was considered to be statistically significant.
Results
Patient Characteristics
Among the 375 patients with ESCC, 49 (13.1%) were women and 326 (86.9%) were men. The mean age was 59.1 ± 7.8 years, with an age range from 36 to 80 years. All of the clinicopathologic characteristics were comparable between patients grouped by GPS and COP-NLR, as shown in Table 1, Table 2. There were significant differences between the GPS and COP-NLR groups in tumor length (P < .001), depth of invasion (P < .001), and nodal metastasis (P < .001). In addition, an elevated COP-NLR was also associated with higher differentiation (P = .006).
Table 1.
The Characteristics of the 375 SCCE Patients Grouped by GPS
| GPS0 (n) | GPS1 (n) | GPS2 (n) | P Value | |
|---|---|---|---|---|
| Age (years) | .697 | |||
| ≤ 60 | 125 | 63 | 26 | |
| > 60 | 87 | 52 | 22 | |
| Gender | .245 | |||
| Female | 32 | 14 | 3 | |
| Male | 180 | 101 | 45 | |
| Tumor length (cm) | < .001 | |||
| ≤ 3 | 71 | 25 | 3 | |
| > 3 | 141 | 90 | 45 | |
| Tumor location | .193 | |||
| Upper | 9 | 7 | 4 | |
| Middle | 93 | 61 | 26 | |
| Lower | 110 | 47 | 18 | |
| Vessel involvement | .101 | |||
| Negative | 183 | 90 | 37 | |
| Positive | 29 | 25 | 11 | |
| Perineural invasion | .226 | |||
| Negative | 177 | 92 | 35 | |
| Positive | 35 | 23 | 13 | |
| Differentiation | .273 | |||
| Well | 32 | 13 | 7 | |
| Moderate | 144 | 75 | 27 | |
| Poor | 36 | 27 | 14 | |
| Depth of invasion | < .001 | |||
| T1 | 49 | 13 | 1 | |
| T2 | 41 | 17 | 4 | |
| T3 | 108 | 67 | 35 | |
| T4 | 14 | 18 | 8 | |
| Nodal metastasis | < .001 | |||
| Negative | 128 | 56 | 15 | |
| Positive | 84 | 59 | 33 |
Table 2.
The Characteristics of the 375 SCCE Patients Grouped by COP-NLR
| COP-NLR0 (n) | COP-NLR1 (n) | COP-NLR2 (n) | P Value | |
|---|---|---|---|---|
| Age (years) | .576 | |||
| ≤ 60 | 107 | 83 | 24 | |
| > 60 | 88 | 54 | 19 | |
| Gender | .287 | |||
| Female | 30 | 13 | 6 | |
| Male | 165 | 124 | 37 | |
| Tumor length (cm) | < .001 | |||
| ≤ 3 | 74 | 21 | 4 | |
| > 3 | 121 | 116 | 39 | |
| Tumor location | .785 | |||
| Upper | 10 | 9 | 1 | |
| Middle | 92 | 68 | 20 | |
| Lower | 93 | 60 | 22 | |
| Vessel involvement | .688 | |||
| Negative | 164 | 112 | 34 | |
| Positive | 31 | 25 | 9 | |
| Perineural invasion | .531 | |||
| Negative | 161 | 107 | 36 | |
| Positive | 34 | 30 | 7 | |
| Differentiation | .006 | |||
| Well | 23 | 24 | 5 | |
| Moderate | 142 | 82 | 22 | |
| Poor | 30 | 31 | 16 | |
| Depth of invasion | < .001 | |||
| T1 | 48 | 12 | 3 | |
| T2 | 38 | 19 | 5 | |
| T3 | 96 | 86 | 28 | |
| T4 | 13 | 20 | 7 | |
| Nodal metastasis | < .001 | |||
| Negative | 119 | 68 | 12 | |
| Positive | 76 | 69 | 31 |
Cancer-Specific Survival
The 5-year CSS was 38.1% in our study. The 5-year CSS in patients with GPS0, 1, and 2 was 50.0%, 27.0%, and 12.5%, respectively (GPS0 vs GPS1, P < .001; GPS1 vs GPS2, P = .035; Figure 1). The 5-year CSS in patients with COP-NLR0, 1, and 2 was 51.8%, 27.0%, and 11.6%, respectively (COP-NLR0 vs COP-NLR1, P < .001; COP-NLR1 vs COP-NLR2, P = .005; Figure 2).
Figure 1.
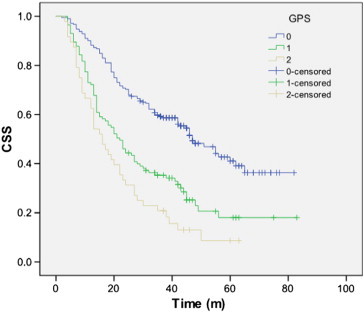
The 5-year CSS in patients with GPS0, 1, and 2 was 50.0%, 27.0%, and 12.5%, respectively (GPS0 vs GPS1, P < .001; GPS1 vs GPS2, P = .035).
Figure 2.
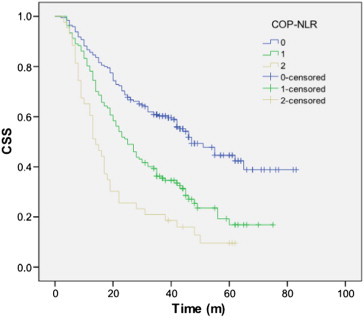
The 5-year CSS in patients with COP-NLR0, 1, and 2 was 51.8%, 27.0%, and 11.6%, respectively (COP-NLR0 vs COP-NLR1, P < .001; COP-NLR1 vs COP-NLR2, P = .005).
Prognostic Factors
By univariate analysis, we found that seven clinicopathologic variables had significant associations with CSS (Table 3). Then, all of the seven significant variables above were included in a multivariate Cox proportional hazards model. In that model, we demonstrated that both the GPS (P = .003) and the COP-NLR (P = .003) were significant independent predictors of CSS (Table 4). In addition, our study showed a similar hazard ratio (HR) between COP-NLR and GPS (HR = 1.394 vs HR = 1.367).
Table 3.
Univariate Analyses in Patients with ESCC
| 5-Year CSS (%) | Chi-Square | P Value | HR (95% CI) | P Value | |
|---|---|---|---|---|---|
| Age (years) | 0.074 | .785 | .787 | ||
| ≤ 60 | 38.3 | 1.000 | |||
| > 60 | 37.9 | 1.036 (0.799-1.344) | |||
| Gender | 0.761 | .383 | .389 | ||
| Female | 44.9 | 1.000 | |||
| Male | 37.1 | 1.193 (0.799-1.782) | |||
| Tumor length (cm) | 16.001 | < .001 | < .001 | ||
| ≤ 3 | 53.5 | 1.000 | |||
| > 3 | 32.6 | 1.903 (1.376-2.632) | |||
| Tumor location | 0.327 | .568 | .572 | ||
| Upper/middle | 40.0 | 1.000 | |||
| Lower | 36.0 | 1.077 (0.833-1.394) | |||
| Vessel involvement | 11.874 | .001 | .001 | ||
| Negative | 41.6 | 1.000 | |||
| Positive | 21.5 | 1.709 (1.251-2.334) | |||
| Perineural invasion | 6.453 | .011 | .013 | ||
| Negative | 41.4 | 1.000 | |||
| Positive | 25.4 | 1.479 (1.088-2.011) | |||
| Differentiation | 3.777 | .052 | .055 | ||
| Well/moderate | 39.2 | 1.000 | |||
| Poor | 32.9 | 1.355 (0.993-1.850) | |||
| Depth of invasion | 27.016 | < .001 | < .001 | ||
| T1-2 | 58.2 | 1.000 | |||
| T3-4 | 28.5 | 2.216 (1.623-3.024) | |||
| Nodal metastasis | 60.200 | < .001 | < .001 | ||
| Negative | 54.8 | 1.000 | |||
| Positive | 19.3 | 2.735 (2.094-3.573) | |||
| GPS | 51.088 | < .001 | < .001 | ||
| 0 | 50.0 | 1.000 | |||
| 1 | 27.0 | 2.075 (1.556-2.768) | |||
| 2 | 12.5 | 3.107 (2.166-4.456) | |||
| COP-NLR | 46.603 | < .001 | < .001 | ||
| 0 | 51.8 | 1.000 | |||
| 1 | 27.0 | 1.909 (1.439-2.532) | |||
| 2 | 11.6 | 3.261 (2.230-4.767) |
Table 4.
Multivariate Analyses in Patients with ESCC
| HR (95% CI) | P Value | |
|---|---|---|
| Tumor length (> 3 cm vs ≤ 3 cm) | 1.033 (0.716-1.490) | .862 |
| Vessel involvement (positive vs negative) | 1.035 (0.742-1.442) | .841 |
| Perineural invasion (positive vs negative) | 1.128 (0.821-1.550) | .458 |
| Depth of invasion (T3-4a vs T1-2) | 1.459 (1.018-2.091) | .040 |
| Nodal metastasis (positive vs negative) | 2.109 (1.567-2.837) | < .001 |
| GPS (1-2 vs 0) | 1.367 (1.114-1.677) | .003 |
| COP-NLR (1-2 vs 0) | 1.394 (1.120-1.735) | .003 |
Correlation Analysis
There were significant positive correlations between COP-NLR and GPS (r = 0.494, P < .001). Our results showed significant negative correlations between CRP and albumin (r = − 0.300, P < .001; Figure 3A), NLR and albumin (r = − 0.148, P = .004; Figure 3E), and platelet count and albumin (r = − 0.210, P < .001; Figure 3F). There were significant positive correlations between CRP and NLR (r = 0.157, P = .002; Figure 3B) and CRP and platelet count (r = 0.138, P = .007; Figure 3C). However, there were no correlation between NLR and platelet count (r = 0.079, P = .125; Figure 3D).
Figure 3.
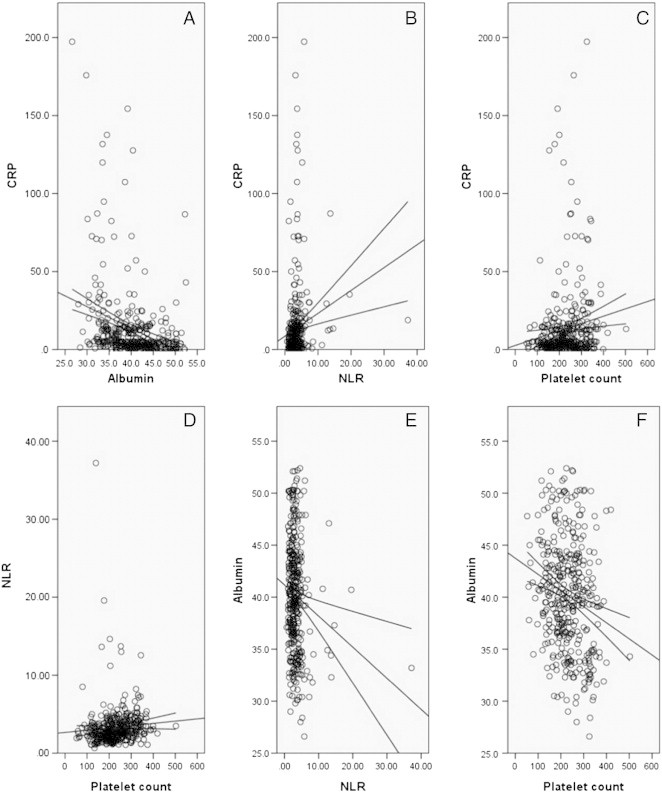
Negative correlations between CRP and albumin (A), NLR and albumin (E), and platelet count and albumin (F). Positive correlations between CRP and NLR (B) and CRP and platelet count (C). No correlation between NLR and platelet count (D).
AIC and BIC Analyses
AIC and BIC values were calculated by using logistic regression according to the survival status of patients when the follow-up was over. The AIC and BIC values were similar between COP-NLR and GPS, indicating that COP-NLR predicts survival in ESCC similar to GPS (Table 5).
Table 5.
AIC and BIC Analyses in Patients with ESCC
| AIC | BIC | − 2Log(Maximum Likelihood) | |
|---|---|---|---|
| GPS | 472.120 | 487.808 | 464.120 |
| COP-NLR | 468.824 | 484.532 | 460.824 |
Discussion
There is strong linkage between inflammation and cancer [5], [6]. In our study, we analyze the potential prognostic values of COP-NLR and GPS in ESCC patients without adjuvant chemoradiotherapy mainly because chemotherapy or radiation will have an important impact on the systemic inflammation. To the best of our knowledge, this is the first study to show COP-NLR as an independent prognostic factor in patients with ESCC. Our study showed that both GPS (P = .003) and COP-NLR (P = .003) were significantly associated with CSS in multivariate analysis. We conclude that COP-NLR is an independent predictive factor in patients with ESCC, and it predicts survival similar to GPS.
There are now a number of well-established systemic inflammation-based prognostic indexes for patients with EC. In particular, the GPS has been well validated. Several previous studies have shown that GPS is associated with survival in various cancers, including ECs [8], [9], [10]. Our study showed that GPS was associated with tumor size, depth of invasion, and nodal metastasis. This observation is in line with data from Vashist et al. [8] but is contrary to the result of Kobayashi et al. [9], who suggested that GPS has no significant correlation with the above clinicopathologic factors. Moreover, our study demonstrated that COP-NLR is an independent predictive factor in patients with ESCC, and the result was consistent with previous studies [8], [9]. However, some of the previous reports using the GPS had several problems [17], [18], [19]. Therefore, the GPS may be considered insufficient for prognostication.
There is an increasing evidence that platelet count and NLR can be used for prognostication in patients with several types of cancer [11], [12]. Recently, Ishizuka et al. [13] showed that COP-NLR is considered to be a useful predictor of postoperative survival in patients with colorectal cancer. They showed that COP-NLR is easy to measure routinely because of its low cost and convenience [13]. Therefore, we conducted a study to determine whether COP-NLR is useful for predicting long-term survival in patients with ESCC. In our study, we demonstrated that COP-NLR (P = .003) was significantly associated with CSS. Moreover, our study showed a similar HR between COP-NLR and GPS. In addition, the AIC and BIC values were similar between COP-NLR and GPS, indicating that COP-NLR predicts survival in ESCC similar to GPS.
The potential limitations of the present study include the use of a retrospective analysis and the short duration of the mean follow-up duration. In addition, we excluded patients who had adjuvant chemotherapy and/or radiotherapy, which may have influenced our analysis. Furthermore, AIC and BIC values were not correct if follow-up differed between patients, and the results of the study should therefore be regarded with caution. Thus, larger prospective studies will need to be performed to confirm these preliminary results.
Conclusion
In summary, our study showed that both GPS and COP-NLR are associated with tumor progression and can be considered as independent markers in patients with ESCC. We conclude that COP-NLR predicts survival in ESCC similar to GPS. However, larger prospective studies will need to be performed to confirm these preliminary results.
Competing Interests
The authors declare that they have no competing interests.
Contributor Information
Ji-Feng Feng, Email: Jifzhejiang@gmail.com.
Ying Huang, Email: Huangyingzj@yeah.net.
Qi-Xun Chen, Email: Chenqix@yeah.net.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2897. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Corley DA, Buffler PA. Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the cancer incidence in five continents database. Int J Epidemiol. 2001;30:1415–1425. doi: 10.1093/ije/30.6.1415. [DOI] [PubMed] [Google Scholar]
- 3.Feng JF, Huang Y, Zhao Q. Tumor length in elderly patients with esophageal squamous cell carcinoma: Is it a prognostic factor? Ups J Med Sci. 2013;118:145–152. doi: 10.3109/03009734.2013.792887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vizcaino AP, Moreno V, Lambert R, Parkin DM. Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973–1995. Int J Cancer. 2002;99:860–868. doi: 10.1002/ijc.10427. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 6.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 7.Crumley AB, McMillan DC, McKernan M, Going JJ, Shearer CJ, Stuart RC. An elevated C-reactive protein concentration, prior to surgery, predicts poor cancer-specific survival in patients undergoing resection for gastro-oesophageal cancer. Br J Cancer. 2006;94:1568–1571. doi: 10.1038/sj.bjc.6603150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vashist YK, Loos J, Dedow J, Tachezy M, Uzunoglu G, Kutup A, Yekebas EF, Izbicki JR. Glasgow prognostic score is a predictor of perioperative and long-term outcome in patients with only surgically treated esophageal cancer. Ann Surg Oncol. 2011;18:1130–1138. doi: 10.1245/s10434-010-1383-7. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi T, Teruya M, Kishiki T, Endo D, Takenaka Y, Tanaka H, Miki K, Kobayashi K, Morita K. Inflammation-based prognostic score, prior to neoadjuvant chemoradiotherapy, predicts postoperative outcome in patients with esophageal squamous cell carcinoma. Surgery. 2008;144:729–735. doi: 10.1016/j.surg.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg. 2007;246:1047–1051. doi: 10.1097/SLA.0b013e3181454171. [DOI] [PubMed] [Google Scholar]
- 11.Sato H, Tsubosa Y, Kawano T. Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg. 2012;36:617–622. doi: 10.1007/s00268-011-1411-1. [DOI] [PubMed] [Google Scholar]
- 12.Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, Altorki NK, Abrams JA. Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18:3362–3369. doi: 10.1245/s10434-011-1754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer. 2013;109:401–407. doi: 10.1038/bjc.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice TW, Rusch VW, Ishwaran H, Blackstone EH, Worldwide Esophageal Cancer Collaboration Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763–3773. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 15.Roberts SA, Hirst WM, Brison DR, Vail A, towardSET collaboration Embryo and uterine influences on IVF outcomes: an analysis of a UK multi-centre cohort. Hum Reprod. 2010;25:2792–2802. doi: 10.1093/humrep/deq213. [DOI] [PubMed] [Google Scholar]
- 16.Stylianou C, Pickles A, Roberts SA. Using Bonferroni, BIC and AIC to assess evidence for alternative biological pathways: covariate selection for the multilevel Embryo-Uterus model. BMC Med Res Methodol. 2013;13:73. doi: 10.1186/1471-2288-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishizuka M, Nagata H, Takagi K, Kubota K. Influence of inflammation-based prognostic score on mortality of patients undergoing chemotherapy for far advanced or recurrent unresectable colorectal cancer. Ann Surg. 2009;250:268–272. doi: 10.1097/SLA.0b013e3181b16e24. [DOI] [PubMed] [Google Scholar]
- 18.Nozoe T, Iguchi T, Egashira A, Adachi E, Matsukuma A, Ezaki T. Significance of modified Glasgow prognostic score as a useful indicator for prognosis of patients with gastric carcinoma. Am J Surg. 2011;201:186–191. doi: 10.1016/j.amjsurg.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 19.Hwang JE, Kim HN, Kim DE, Choi HJ, Jung SH, Shim HJ, Bae WK, Hwang EC, Cho SH, Chung IJ. Prognostic significance of a systemic inflammatory response in patients receiving first-line palliative chemotherapy for recurred or metastatic gastric cancer. BMC Cancer. 2011;11:489. doi: 10.1186/1471-2407-11-489. [DOI] [PMC free article] [PubMed] [Google Scholar]


