Abstract
Autophagy is an intracellular bulk degradation process involved in cell survival upon stress induction, but also with a newly identified function in myeloid differentiation. The autophagy-related (ATG)8 protein family, including the GABARAP and LC3 subfamilies, is crucial for autophagosome biogenesis. In order to evaluate the significance of the GABARAPs in the pathogenesis of acute myeloid leukemia, we compared their expression in primary AML patient samples, CD34+ progenitor cells and in granulocytes from healthy donors. GABARAPL1 and GABARAPL2/GATE-16, but not GABARAP, were significantly downregulated in particular AML subtypes compared to normal granulocytes. Moreover, the expression of GABARAPL1 and GATE-16 was significantly induced during ATRA-induced neutrophil differentiation of acute promyelocytic leukemia cells. Lastly, knocking down GABARAPL2/GATE-16 in APL cells attenuated neutrophil differentiation and decreased autophagic flux. In conclusion, low GABARAPL2/GATE-16 expression is associated with an immature myeloid leukemic phenotype and these proteins are necessary for neutrophil differentiation of APL cells.
Keywords: GABARAP, GABARAPL1, GATE-16, AML, APL, neutrophil differentiation
1. Introduction
Autophagy is a process of cellular self-digestion involved in the degradation of protein aggregates and cell organelles. Autophagy can be observed in a variety of normal cellular processes such as proliferation, cell death and differentiation, as well as in pathological cellular processes including neurodegeneration, inflammatory diseases and cancer [1,2]. Autophagy is a ubiquitous intracellular process in eukaryotes characterized by the formation of double-membraned vesicles, the so-called autophagosomes. Autophagosomes engulf dispensable or harmful components and finally fuse with lysosomes for the degradation of their contents [3]. Attenuation of autophagy results in genomic instability indicating a tumor suppressor function promoted by autophagy. Conversely, cancer therapy related induction of autophagy frequently supports tumor cell survival [4]. In addition, it has been described that autophagy promotes cancer cell metastasis but, may also promote cancer cell death and inhibit metastasis in certain cases [5]. Therefore, the role of autophagy in tumorigenesis is still controversial and clearly depends on the type of tumor and the stage of disease progression [6].
The autophagy-related (ATG) proteins are the major molecular components of the autophagy machinery [3]. In yeast a single ATG8 protein is essential in promoting expansion of the autophagosomal precursor membrane, the autophagophore [7]. In contrast, an ATG8 protein family exists in human that can be divided in three sub-families: (a) the microtubule-associated protein 1 light chain (MAP1LC3, often referred to as LC3), (b) the y-aminobutyric acid receptor-associated protein (GABARAP), and (c) the golgi-associated ATPase enhancer of 16 kDa GABARAPL2/GATE-16 (hereafter referred to as GATE-16) subfamily [8]. LC3 proteins have an essential function in elongating the autophagosomal membrane, whereas the GABARAP family members are required for closing the autophagosomal membrane [11]. Moreover, tissue or cell specific functions or different roles of ATG8 proteins in particular subtypes types of autophagy have been proposed [9,10].
The role of autophagy in hematopoietic development and function is still under investigation. Several reports showed a critical function for autophagy in the clearance of mitochondria during reticulocyte maturation [12,13]. Moreover, autophagy in macrophages and neutrophils is essential for effective innate immunity and contributes to the prevention of inflammatory diseases (reviewed in [4,14,15]). Recent findings highlighted an important role for autophagy during monocytic differentiation and acquisition of macrophage function [16]. A link to aberrant hematopoiesis was found in a hematopoietic tissue specific, ATG7 conditional knockout mouse model, where the mice developed invasive myeloproliferation, which strongly resembles acute myeloid leukemia (AML) [17]. Lastly, autophagy contributes to all-trans retinoic acid (ATRA) therapy-induced PML-RARA degradation in t(15;17) acute promyelocytic leukemia (APL) cell lines [18].
In this study, we aimed at analyzing ATG8 family expression in normal and leukemic myeloid tissue as well as during neutrophil differentiation of APL cells. Further, the requirement for ATG8 proteins for ATRA-induced neutrophil differentiation and autophagy was determined.
2. Materials and Methods
2.1 Patient samples and primary cells
A cohort of 98 AML patient samples (Supplementary Table 1), provided by Drs. P.J.M. Valk and. B. Löwenberg, was enrolled on HOVON/SAKK (Dutch-Belgian Hematology-Oncology/Swiss Group for Clinical Cancer Research Cooperative group) protocols -04, -04A, -29, and -42 (available at “http://www.hovon.nl” www.hovon.nl) between 1987 and 2006 [20-24].
Primary neutrophils from healthy donors were isolated using polymorphprep (AXIS-SHIELD Baden-Dattwil, Switzerland). In vitro differentiation of CD34+ progenitor cells was done as previously described [25].
2.2 Cell lines and culture conditions
NB4 APL cells and their all-trans retinoic acid (ATRA)-resistant NB4-R2 subclone and HEK-293T cells were cultured as described [26]. ATRA-induced autophagy was blocked using Bafilomycin A1 (BML-CM110, Enzo Life Science, Lausen, Switzerland) at a concentration of 100nM.
2.3 TaqMan low-density array (LDA) and quantitative real-time RT-PCR (qPCR)
RNA extraction, RT-PCR, LDA measurements as well as data analysis were performed as described [4,27]. Gene Expression Assays for GABARAPL1, GATE-16, MAP1LC3B and CSF3R used in a 96 well format on the ABI 7500 Sequence detection system were Hs00744468_s1, Hs00371854_m1, Hs00797944_s1 and Hs00167918_m1, respectively (Applied Biosystems, Rotkreuz, Switzerland). HMBS primer and probes have been described previously [28].
2.4 Western blotting
Westernblot was performed as described [26]. Primary antibodies used were anti-LC3B (NB600-1384; Novus Biologicals, Cambridge, England and anti-GAPDH (MAB374; Milipore, Zug, Switzerland).
2.5 Lentiviral transductions
pLKO.1 lentiviral vectors expressing small hairpin (sh)RNAs targeting GATE-16 (shGATE16_247: “NM_007285.6-247s1c1/TRCN0000048287” and shGATE16_359: NM_007285.6-359s1c1/TRCN0000048285) as well as a non-targeting shRNA control (SHCOO2) were purchased from Sigma-Aldrich, Buchs, Switzerland Lentiviral production and transduction of NB4 cells was done as described [29].
2.6 Fluorescent microscopy
Cells were fixed and permeabilized in methanol (-20°C) for 4 min, further washed once with PBS and incubated with the first antibody (LC3B, Cell signalling, Cat. no. 3686; ATG5, Cell signalling, Cat. no. 2630S) for one hour at room temperature. Then, cells were washed twice with PBS-Tween and once with PBS followed by the incubation with the secondary antibody (FITC conjugated anti-rabbit, Jackson Immunoresearch (Cat. no. 111-096-045) for 1 hour at room temperature. Fluorescence labeled cells were mounted (SlowFade® Gold Antifade Reagent with DAP, Invitrogen, Cat. no. S36938) and covered with a glass slide prior to analysis. Images were taken with an Olympus Fluoview FV1000-IX81 confocal laser scanning microscope, using a 60x oil immersion objective. Image processing and analysis were done with ImageJ 1.45s and Adobe Photoshop CS5.
2.7 Proteolysis assay
NB4 cells were treated with or without ATRA for 4 days. 0.3 uCi 14C-Valine (L-(U-14-C)Valine, Code CFB.75, Amersham) per ml/well was added after 2 days of ATRA treatment. Addition of BafilomycinA1 was performed 24h prior analysis. Assay was further performed as described in [30]. Exception: second incubation period which was performed during 5 hours.
3. Results
3.1 Regulation of the GABARAP subfamily in primary AML patient samples
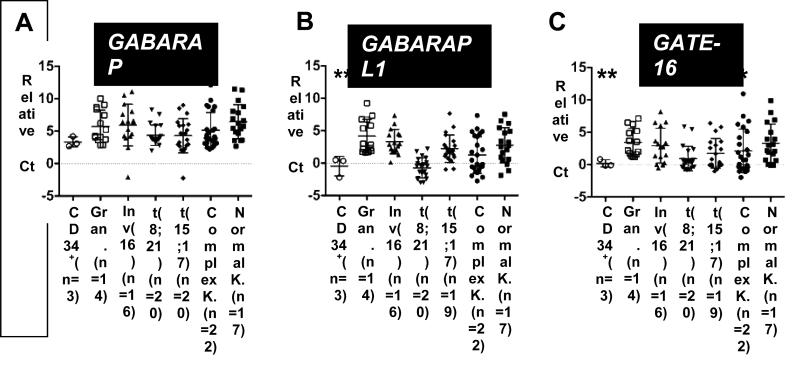
Based on the role of autophagy in normal myeloid development, we investigated whether autophagy gene expression is deregulated in primary AML patient samples. To this end, we first determined the mRNA expression levels of the GABARAP ATG8 subfamily (GABARAP, GABARAPL1 and GATE-16) in clinical AML samples, CD34+ progenitor cells and in mature neutrophils from healthy donors. We were able to detect GABARAP, GABARAPL1 and GATE-16 mRNA expression in 95/98, 95/98 and 94/97 AML patient samples, respectively. GABARAP family expression was found in all granulocyte (n=14) and CD34+ progenitor cell (n=3) samples. Compared to normal granulocytes GABARAPL1 was downregulated in the AML subtypes t(8;21), t(15;17) and complex karyotypes, whereas GATE-16 expression was significantly lower only in t(8;21) and t(15;17) AML. Interestingly, no significant differences in GABARAP expression were detected (Figs. 1A-C), suggesting that low expression of this GABARAP subfamily member is not associated with a differentiation block in AML. Together, GABARAPL1 and GATE-16 mRNA expression is significantly downregulated in particular AML subtypes.
Figure 1. Significantly decreased GABARAPL1 and GATE-16 expression in primary AML patient samples.
GABARAP (A), GABARAPL1 (B) and GATE-16 (C) mRNA levels of granulocytes from healthy donors, CD34+ progenitor cells and AML patient samples were quantified utilizing qPCR. The relative ∆Ct expression was calculated by the difference of GABARAP, GABARAPL1 and GATE-16 expression to the housekeeping genes HMBS and ABL. M.W.U, *p<0.01. **p<0.001, *** p<0.0001.
3.2 GABARAPL1 and GATE-16 expression is associated with ATRA-induced neutrophil differentiation of APL cells
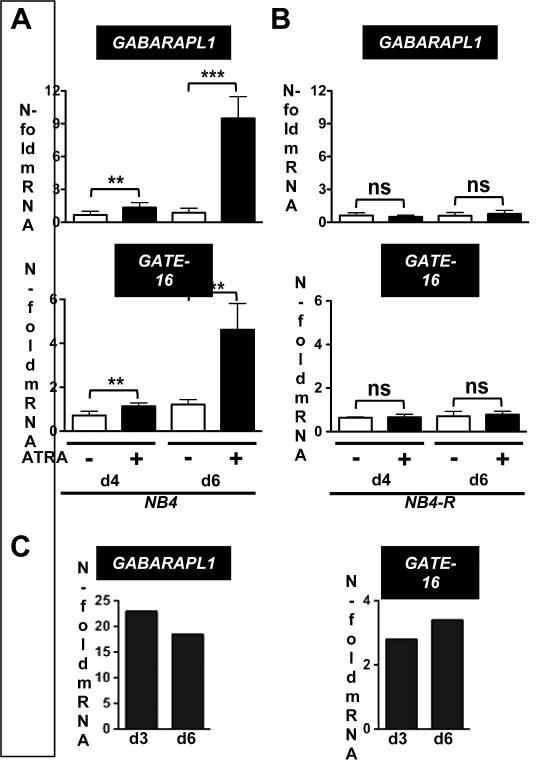
Given the low GABARABL1 and GATE-16 expression in AML blast cells we surmised that these genes are involved in myeloid differentiation of leukemic cells. Firstly, to determine if GABARAPL1 and GATE-16 are associated with neutrophil differentiation of APL cells, we measured mRNA expression levels during all-trans retinoic acid (ATRA)-induced neutrophil differentiation of NB4 cells (Fig. 2A and B). As a control we used the ATRA-resistant NB4-R2 APL cells to rule out a direct effect of ATRA on GABARAPL1 or GATE-16 mRNA expression. Successful neutrophil differentiation of NB4 cells was confirmed by increased CD11b surface and CSF3R mRNA expression (data not shown). A significant 2-fold increase in GABRAPL1 and GATE-16 mRNA levels in NB4 cells at day 4 of ATRA treatment compared to control cells was seen. At day 6, GABARAPL1 and GATE-16 expression increased 9- and 4-fold, respectively (Fig. 2A). In contrast, GABARAPL1 and GATE-16 mRNA levels did not significantly change in ATRA-resistant NB4-R2 cells upon neutrophil differentiation (Fig. 2B). Consistent with our findings in the NB4 neutrophil differentiation model, primary CD34+ progenitor cells showed markedly increased GABARAPL1 and GATE-16 mRNA levels upon in vitro neutrophil differentiation using G-CSF (Fig. 2C). Taken together, our data demonstrate that induction of GABARAPL1 and GATE-16 is clearly associated with neutrophil differentiation.
Figure 2. GABARAPL1 and GATE-16 expression during neutrophil differentiation of NB4 APL and CD34+ primary cells.
(A,B) mRNA levels of GABARAPL1 (upper panels) and GATE-16 (lower panels) were measured in control and ATRA-treated NB4 (A) and NB4-R2 APL (B) cell lines at day 4 and 6 using qPCR. Values were normalized to the housekeeping gene HMBS and are given as n-fold mRNA expression relative to the control of day 4 and 6, respectively. M.W.U, *p<0.01. **p<0.001, *** p<0.0001. (C) Primary CD34+ progenitor cells showed markedly increased GABARAPL1 and GATE-16 mRNA levels upon in vitro neutrophil differentiation using G-CSF over a 12 day period. Values were normalized to the housekeeping gene HMBS and given as n-fold mRNA expression relative to the levels at day 0 upon in vitro neutrophil differentiation of CD34+ using G-CSF.
3.3 Knocking down GATE-16 significantly impaired ATRA-induced neutrophil differentiation of APL cells
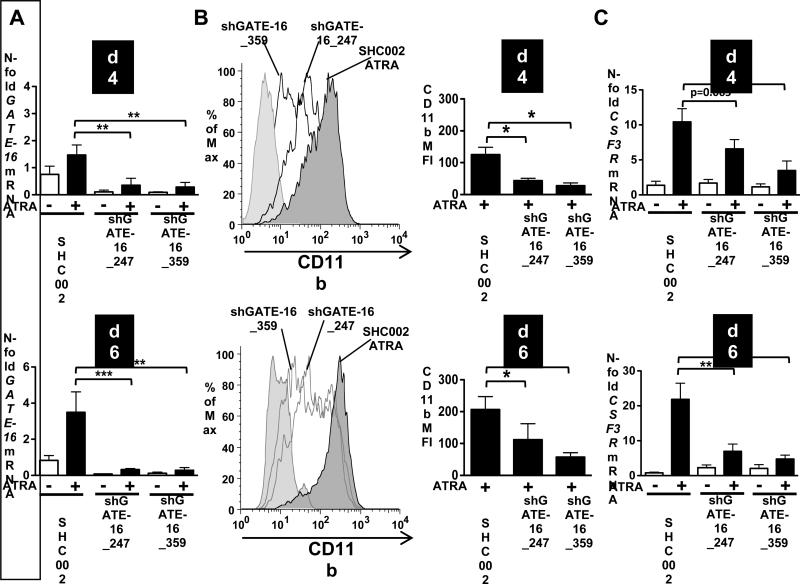
To evaluate whether GATE-16 is functionally involved in ATRA-induced neutrophil differentiation of APL cells, we inhibited GATE-16 expression in NB4 cells. We generated two different NB4 GATE-16 knockdown cell lines using lentiviral vectors expressing two independent small hairpin (sh) RNAs targeting GATE-16. GATE-16 knockdown efficiency compared to NB4 SHC002 control cells was 65% and 90% at day 4 and 6 of neutrophil differentiation, respectively (Fig. 3A). Knocking down GATE-16 resulted in impaired differentiation levels, as evidenced by a significant reduction of CD11b levels in NB4 shGATE-16_247 (50%) and NB4 shGATE-16_359 (70%) knockdown cell lines as compared to SHCOO2 control cells (Fig. 3B). Similarly, the expression of the neutrophil marker CSF3R was markedly downregulated in both NB4 shGATE-16 knockdown cells for both time points analyzed (Fig. 3C). These findings indicate that GATE-16 is functionally involved in ATRA-induced neutrophil differentiation of APL cells.
Figure 3. Impaired neutrophil differentiation in NB4 GATE-16 knockdown cells.
(A) GATE-16 mRNA expression was measured upon ATRA (1μM) administration in NB4 cells expressing non-targeting shRNA (SHC002) or shRNAs targeting GATE-16 (shGATE-16_247 and shGATE-16_359) at day 4 and 6, respectively. Values were calculated as described in Fig.1. (B) SHC002, shGATE-162_247 and shGATE-16_359 expressing NB4 cells were differentiated for 4 and 6 days and neutrophil differentiation was assessed by measuring CD11b surface expression. A representative CD11b histogram is shown in the upper panel. Bar graphs of the mean fluorescence intensity of 4 independent experiments are shown. (C) CSF3R mRNA was measured using qPCR in SHC002, shGATE-16_247 and shGATE-16_359 expressing NB4 cells upon 1μM ATRA treatment for 4 and 6 days, respectively. M.W.U, *p<0.01. **p<0.001, *** p<0.0001.
3.4 Inhibition of GATE-16 attenuates ATRA-induced autophagy in APL cells
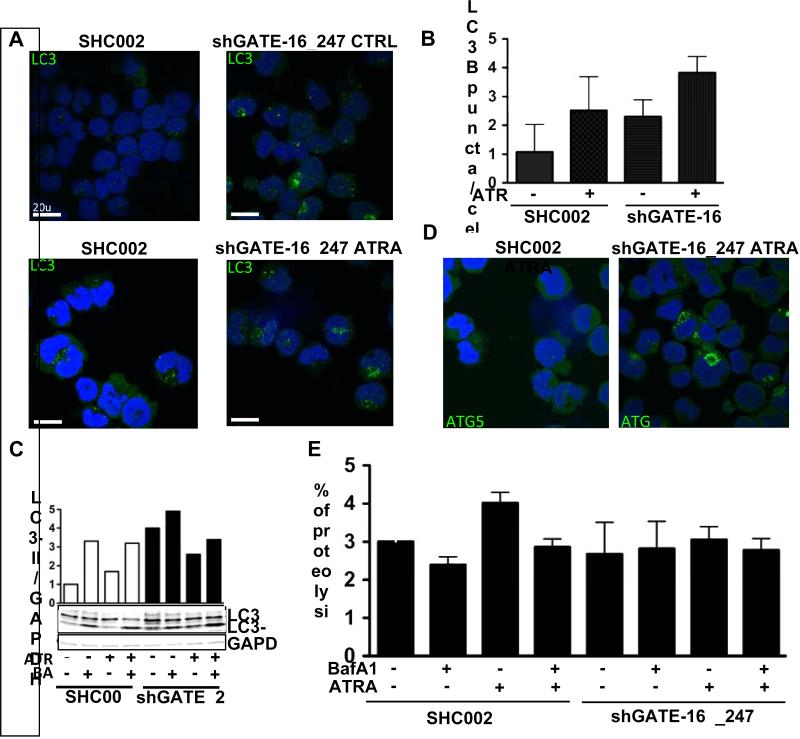
Next, we asked whether the reduced neutrophil differentiation in NB4 GATE-16 knockdown cells is linked to decreased autophagic activity. As a first indicator of reduced autophagy in NB4 GATE-16 knockdown cells, we found a significant 2-fold reduction of LC3B mRNA during ATRA-induced neutrophil differentiation in GATE-16 knockdown cells (Suppl. Fig.1). Moreover, we found increased LC3B puncta formation for control- as well as ATRA-treated GATE-16 knockdown cells as compared to SHC002 transduced control cells, suggesting either an increase, or a block in autophagic activity (Figs.4 A and B). Using the autophagosome-lysosome fusion inhibitor Bafilomycin A1 to determine autophagic flux, we found that BafilomycinA1 treatment resulted in slightly higher LC3-II levels in GATE-16 knockdown as compared to ATRA only treated cells (Fig.4 C). This is clearly not as pronounced as for SHC002 control cells, thus suggesting an autophagy block after GATE-16 knockdown. Supporting our above findings, reduced long-lived protein degradation was observed in ATRA-treated GATE-16 knockdown, as compared to SHC002 control cells, indicating a block in autophagy in GATE-16 APL knockdown cells during neutrophil differentiation (Fig.4 E). Furthermore, we observed increased ATG5 puncta formation in ATRA-treated GATE-16 NB4 knockdown cells. The accumulation of ATG5 puncta in these cells further indicates that the autophagosomes are not yet fused with the lysosome in GATE-16 impaired cells, since ATG5 dissociates from the membrane upon completion of autophagosome formation [33] (Fig. 4 D). In order to exclude an APL cell specific role for GATE-16 in autophagosomal membrane formation during ATRA-treatment, we knocked down GATE-16 in HEK 293T cells and treated them with ATRA. We observed an increase in LC3B puncta formation upon ATRA treatment in SHC002 control cells, which was paralleled by autophagic induction. This demonstrated for the first time that ATRA promotes autophagy in non-APL cells. Similar to NB4 cells, endogenous LC3B puncta accumulated in HEK293T GATE-16 knockdown cells in control as well as in ATRA-treated cells further supporting that attenuated GATE-16 expression blocks autophagy (Suppl. Figs. 2 A and B). Moreover, autophagic flux activation was seen in HEK293T cells upon ATRA treatment using the proteolysis assay (Suppl. Fig. 2C). In addition, we measured reduced proteolysis levels for GATE-16 knockdown cells upon starvation, a classical stimulus to induce autophagy, further supporting an essential role for GATE-16 in autophagy (Suppl. Fig. 2 D).
Figure 4. Knocking down GATE-16 impairs ATRA-induced autophagy in NB4 cells.
(A) Endogenous LC3B protein levels were analyzed using confocal microscopy at day 4 of ATRA only treatment or in in combination with Bafilomycin A1 in SHC002 control transfected cells or GATE-16 knockdown cells. (B) LC3B puncta were quantified using ImageJ software. (C) Western blot analysis of LC3-II expression in SHC002 or shGATE-16_247 expressing NB4 cells after 4 days of ATRA administration. Total protein was extracted and 30ng of protein was loaded. Immunoblots were incubated with LC3 antibody. GAPDH represents the loading control. (D) Endogenous ATG5 protein levels were analyzed in ATRA-treated NB4 cells using confocal microscopy at day 4. (E) The proteolysis rate in NB4 cells upon ATRA treatment was measured in 4 independent experiments. Cells were either incubated with complete medium or medium supplemented with ATRA, in the presence or absence of BafilomycinA1.
In summary, knocking down GATE-16 attenuates autophagic flux during ATRA-mediated differentiation of APL and in ATRA-treated 293T cells.
4. Discussion
Overall, we found that GABARAPL1 and GATE-16 mRNA levels were significantly lower expressed in APL, t(8;21) and complex karyotype AML than in mature neutrophils. The low levels of both autophagy genes in APL and t(8;21) positive AML samples may be attributed to transcriptional repression by the leukemic fusion proteins PML-RARA and AML1-ETO, respectively. Recently, the myeloid transcription factor GATA-1 was described as direct regulator of the ATG8 family in erythropoiesis [34] . We found that GABARAPL1 and GATE-16 expression is associated with neutrophil differentiation and that inhibiting GATE-16 expression attenuates development. We therefore speculate that particular ATG8 family members are involved in myeloid differentiation.
GABARAPL1 functions in selective autophagy as a cargo adaptor and may recruit harmful protein aggregates to the autophagic machinery for degradation [8,9]. Previous studies linked autophagy to early neutrophil differentiation of APL cells by contributing to PML-RARA degradation [18]. Together with our findings of low GABARAPL1 expression in APL cells we now propose a role for GABARAPL1 in recruiting aggregated PMLRARA proteins to the autophagosome. Furthermore, GABARAPL1 as well as GATE-16 associate with the heat shock protein (HSP) 90 resulting in protection from proteosomal degradation [35]. Recently, published findings revealed that heat shock protein inhibitors, already used in clinical trials for AML therapies, preferentially eliminate AML stem cells, [36]. One explanation for the killing of AML stem cells might be that these cells display increased autophagic activity [37] making them vulnerable to HSP90 inhibitors that attenuate autophagy via increased degradation of ATG8 proteins. Conversely, HSP90 inhibitors might interfere with differentiation therapy in APL patients due to the depletion of GABARAPL1 and GATE-16 proteins, possibly causes reduced differentiation. Our findings suggest that novel treatment strategies for AML need to take into consideration drugs that target autophagy function.
LC3 and GABARAP subfamilies have unique functions at different steps during autophagosomal membrane formation [11] and also differ in cargo recruitment [38]. We link for the first time GATE-16 to neutrophil differentiation of APL cells, presumably through an increase in autophagy. The observed increase in LC3B-II levels upon ATRA or starvation treatment in GATE-16 impaired APL cells, indicates a block in autophagic turnover rather than increased autophagy. The reduced turnover of 14C-labeled proteins in GATE-16 knockdown APL cells further supports our finding. This is in line with a report by Weidberg et. al. [11] describing that knocking down GATE-16 interferes with the autophagic machinery. Increased endogenous LC3B and the accumulation of endogenous ATG5 puncta in GATE-16 knockdown cells suggests that GATE-16 acts downstream of LC3. Moreover, GATE-16 may has a rather unique function in neutrophil differentiation that is not compensated by other GABARAP family members during autophagosomal biogenesis.
ATRA and other retinoid derivates are promising agents not only in the treatment of APL but also in solid cancers (reviewed in [39]. In breast cancer, for example, retinoids can trigger cell cycle arrest, apoptosis and differentiation (reviewed in [40-42]. Based on our findings that ATRA induces autophagy in solid cancer cells, the process needs to be studied in more detail in order to understand if retinoid therapies of solid cancers may profit from autophagy activation or inhibition.
In conclusion, we have found that decreased expression of the ATG8 family members GABARAPL1 and GATE-16 is associated with immature AML blast cells of particular subtypes. Furthermore, we found that GATE-16 expression is necessary for ATRA-induced differentiation and autophagy in APL cells.
Supplementary Material
Supplementary Figure 2. ATRA-induced autophagy in HEK293T cells. (A) Endogenous LC3B protein levels were analyzed using confocal microscopy after 5h of ATRA treatment. (B) LC3B puncta were quantified using ImageJ software. (C) The rate of proteolysis was measured in cells either incubated with complete medium or medium supplemented with ATRA, in the presence or absence of BafilomycinA1. (D) The rate of proteolysis was measured in cells either incubated with complete medium or EBSS medium supplemented with 0.1% FBS, in the presence or absence of BafilomycinA1.
Supplementary Figure 1. LC3 mRNA expression in GATE-16 knockdown NB4 cells. Endogenous LC3B mRNA expression was measured upon ATRA (1μM) administration in NB4 cells expressing non-targeting shRNA (SHC002) or shRNAs targeting GATE-16 (shGATE-16_247 and shGATE-16_359) at day 4 and 6, respectively. Values were calculated as described in Fig. 1.
Highlights.
Low GAPARAPL1 and GATE-16 mRNA expression is associated with immature AML blast cells of particular subtypes
A unique role for GATE-16 during ATRA-mediated neutrophil differentiation APL cells
Gate-16 contributes to autophagosomal membrane formation downstream of LC3 during ATRA-induced autophagy
ATRA induces autophagy in non-hematopoietic cells
Acknowledgments
We gratefully acknowledge Dr. P.J.M. Valk and Dr. B. Löwenberg and the HOVON (Dutch-Belgian Hematology-Oncology) cooperative group for providing primary AML patient samples. Expert technical assistance by D. Shan was appreciated. We thank Dr. V. Heussler and N. Eickel for their help with the fluorescence microscopy.
This study was supported by grants from the Foundation Cancer Research Switzerland (KFS-02486-08-2009 to MPT), the Marlies-Schwegler Foundation, the Ursula-Hecht-Foundation for Leukemia Research, the Bernese Foundation of Cancer Research (to MFF), the Werner and Hedy Berger-Janser Foundation of Cancer Research (to MFF and MPT), the Bern University Research Foundation (to MPT), NIH 5T32 HL007195-34 (JC), and the Joyce Klein Stock Gift and NIH R01HL091219 (to BET).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship
DB performed the experimental research and drafted the article. BET and JC provided primary cells and revised the article. MPT and MFF designed the project, wrote the manuscript and gave final approval of the submitted manuscript.
References
- 1.Cecconi F, Levine B. The Role of Autophagy in Mammalian Development: Cell Makeover Rather than Cell Death. Developmental Cell. 2008;15:344–357. doi: 10.1016/j.devcel.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanida I. Autophagosome Formation and Molecular Mechanism of Autophagy. Antioxidants & Redox Signaling. 2011;14:2201–2214. doi: 10.1089/ars.2010.3482. [DOI] [PubMed] [Google Scholar]
- 4.Tschan MP, Simon HU. The role of autophagy in anticancer therapy: promises and uncertainties. Journal of Internal Medicine. 2010;268:410–418. doi: 10.1111/j.1365-2796.2010.02266.x. [DOI] [PubMed] [Google Scholar]
- 5.Pan H. Active autophagy in the tumor microenvironment: A novel mechanism for cancer metastasis (Review) Oncol Lett. 2012 doi: 10.3892/ol.2012.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoare M, Young AR, Narita M. Autophagy in cancer: Having your cake and eating it. Seminars in Cancer Biology. 2011:1–8. doi: 10.1016/j.semcancer.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Xie Z, Nair U, Klionsky DJ. Atg8 Controls Phagophore Expansion during Autophagosome Formation. Mol Biol Cell. 2008:3290–3298–. doi: 10.1091/mbc.E07-12-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shpilka T, Weidberg H, Pietrokovski S, Elazar Z. Atg8: an autophagy-related ubiquitin-like protein family. Genome Biol. 2011 doi: 10.1186/gb-2011-12-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Grand JN, Chakrama F-Z, Seguin-Py S, Fraichard A, Delage-Mourroux R, Jouvenot M, et al. GABARAPL1 (GEC1) Autophagy. 2011;7:1098–1107. doi: 10.4161/auto.7.10.15904. [DOI] [PubMed] [Google Scholar]
- 10.Elazar Z, Scherz-Shouval R, Shorer H. Involvement of LMA1 and GATE-16 family members in intracellular membrane dynamics. Biochimica Et Biophysica Acta (BBA) - Molecular Cell Research. 2003;1641:145–156. doi: 10.1016/s0167-4889(03)00086-7. [DOI] [PubMed] [Google Scholar]
- 11.Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, Elazar Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. Embo J. 2010;29:1792–1802. doi: 10.1038/emboj.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mortensen M, Ferguson DJP, Edelmann M, Kessler B, Morten KJ, Komatsu M, et al. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proceedings of the National Academy of Sciences. 2010;107:832–837. doi: 10.1073/pnas.0913170107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kundu M, Lindsten T, Yang CY, Wu J, Zhao F, Zhang J, et al. Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood. 2008;112:1493–1502. doi: 10.1182/blood-2008-02-137398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mihalache CC, Simon H-U. Autophagy regulation in macrophages and neutrophils. Experimental Cell Research. 2012;318:1187–1192. doi: 10.1016/j.yexcr.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Yousefi S, Simon H-U. Autophagy in cells of the blood. Biochimica Et Biophysica Acta (BBA) - Molecular Cell Research. 2009;1793:1461–1464. doi: 10.1016/j.bbamcr.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Jacquel A, Obba S, Boyer L, Dufies M, Robert G, Gounon P, et al. Autophagy is required for CSF-1-induced macrophagic differentiation and acquisition of phagocytic functions. Blood. 2012;119:4527–4531. doi: 10.1182/blood-2011-11-392167. [DOI] [PubMed] [Google Scholar]
- 17.Mortensen M, Soilleux EJ, Djordjevic G, Tripp R, Lutteropp M, Sadighi-Akha E, et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. Journal of Experimental Medicine. 2011;208:455–467. doi: 10.1084/jem.20101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isakson P, Bjørås M, Bøe SO, Simonsen A. A new oncoprotein catabolism pathway. 2010;116:2200–2201. doi: 10.1182/blood-2010-07-294025. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Hou J-K, Chen T-T, Zhao X-Y, Yan Z-W, Zhang J, et al. PML-RARα enhances constitutive autophagic activity through inhibiting the Akt/mTOR pathway. Autophagy. 2011;7:1132–1144. doi: 10.4161/auto.7.10.16636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breems DA, Boogaerts MA, Dekker AW, Van Putten WLJ, Sonneveld P, Huijgens PC, et al. Autologous bone marrow transplantation as consolidation therapy in the treatment of adult patients under 60 years with acute myeloid leukaemia in first complete remission: a prospective randomized Dutch-Belgian Haemato-Oncology Co-operative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) trial. Br J Haematol. 2005;128:59–65. doi: 10.1111/j.1365-2141.2004.05282.x. [DOI] [PubMed] [Google Scholar]
- 21.Ossenkoppele GJ. The value of fludarabine in addition to ARA-C and G-CSF in the treatment of patients with high-risk myelodysplastic syndromes and AML in elderly patients. Blood. 2004;103:2908–2913. doi: 10.1182/blood-2003-07-2195. [DOI] [PubMed] [Google Scholar]
- 22.Lowenberg B, MD W, van Putten, MSc M, Theobald MD, et al. Effect of Priming with Granulocyte Colony-Stimulating Factor on the Outcome of Chemotherapy for Acute Myeloid Leukemia. The New England Journal of Medicine. 2003;349:743–752. doi: 10.1056/NEJMoa025406. [DOI] [PubMed] [Google Scholar]
- 23.Lowenberg B, Boogaerts M, Daenen S, Verhoef G, Hagenbeek A, Vellenga E, et al. Value of Different Modalities of Granulocyte-Macrophage Colony-Stimulating Factor Applied During or After Induction Therapy of Acute Myeloid Leukemia. Journal of Clinical Oncology. 1997;15:3496–3506. doi: 10.1200/JCO.1997.15.12.3496. [DOI] [PubMed] [Google Scholar]
- 24.Federzoni EA, Valk PJM, Torbett BE, Haferlach T, Lowenberg B, Fey MF, et al. PU.1 is linking the glycolytic enzyme HK3 in neutrophil differentiation and survival of APL cells. Blood. 2012;119:4963–4970. doi: 10.1182/blood-2011-09-378117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Britschgi A, Simon H-U, Tobler A, Fey MF, Tschan MP. Epigallocatechin-3-gallate induces cell death in acute myeloid leukaemia cells and supports all-transretinoic acid-induced neutrophil differentiation via death-associated protein kinase 2. Br J Haematol. 2010;149:55–64. doi: 10.1111/j.1365-2141.2009.08040.x. [DOI] [PubMed] [Google Scholar]
- 26.Humbert M, Mueller C, Fey MF, Tschan MP. Inhibition of damage-regulated autophagy modulator-1 (DRAM-1) impairs neutrophil differentiation of NB4 APL cells. Leukemia Research. 2012 doi: 10.1016/j.leukres.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Tschan MP, Shan D, Laedrach J, Eyholzer M, Leibundgut EO, Baerlocher GM, et al. NDRG1/2 expression is inhibited in primary acute myeloid leukemia. Leukemia Research. 2010;34:393–398. doi: 10.1016/j.leukres.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 28.Britschgi A, Trinh E, Rizzi M, Jenal M, Ress A, Tobler A, et al. DAPK2 is a novel E2F1/KLF6 target gene involved in their proapoptotic function. Oncogene. 2008;27:5706–5716. doi: 10.1038/onc.2008.179. [DOI] [PubMed] [Google Scholar]
- 29.Tschan MP. Alternative Splicing of the Human Cyclin D-binding Myb-like Protein (hDMP1) Yields a Truncated Protein Isoform That Alters Macrophage Differentiation Patterns. J Biol Chem. 2003;278:42750–42760. doi: 10.1074/jbc.M307067200. [DOI] [PubMed] [Google Scholar]
- 30.Ogier-Denis E, Houri J-J, Bauvy C, Codogno P. Guanine Nucleotide Exchange on Heterotrimeric Gi3 Protein Controls Autophagic Sequestration in HT-29 Cells*. J Biol Chem. 1996;271:28593–28600. doi: 10.1074/jbc.271.45.28593. [DOI] [PubMed] [Google Scholar]
- 31.Isakson P, Bjoras M, Boe SO, Simonsen A. Autophagy contributes to therapy-induced degradation of the PML/RARA oncoprotein. Blood. 2010;116:2324–2331. doi: 10.1182/blood-2010-01-261040. [DOI] [PubMed] [Google Scholar]
- 32.Klionsky DJ. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:1–100. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizushima N, Yoshimori T, Ohsumi Y. Role of the Apg12 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2003;35:553–561. doi: 10.1016/s1357-2725(02)00343-6. [DOI] [PubMed] [Google Scholar]
- 34.Kang YA, Sanalkumar R, O'Geen H, Linnemann AK, Chang CJ, Bouhassira EE, et al. Autophagy Driven by a Master Regulator of Hematopoiesis. Mol Cell Biol. 2011;32:226–239. doi: 10.1128/MCB.06166-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seguin-Py S, Lucchi G, Croizier S, Chakrama FZ, Despouy G, Le Grand JN, et al. Identification of HSP90 as a new GABARAPL1 (GEC1)-interacting protein. Biochimie. 2012;94:748–758. doi: 10.1016/j.biochi.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Rao R, Nalluri S, Fiskus W, Balusu R, Joshi A, Mudunuru U, et al. Heat Shock Protein 90 Inhibition Depletes TrkA Levels and Signaling in Human Acute Leukemia Cells. Molecular Cancer Therapeutics. 2010;9:2232–2242. doi: 10.1158/1535-7163.MCT-10-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salemi S, Yousefi S, Constantinescu MA, Fey MF, Simon H-U. Autophagy is required for self-renewal and differentiation of adult human stem cells. Cell Res. 2011;22:432–435. doi: 10.1038/cr.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shvets E, Abada A, Weidberg H, Elazar Z. Dissecting the involvement of LC3B and GATE-16 in p62 recruitment into autophagosomes. Autophagy. 2011;7:683–688. doi: 10.4161/auto.7.7.15279. [DOI] [PubMed] [Google Scholar]
- 39.Mamede AC, Tavares SD, Abrantes AM, Trindade J, Maia JM, Botelho MF. The Role of Vitamins in Cancer: A Review. Nutrition & Cancer. 2011;63:479–494. doi: 10.1080/01635581.2011.539315. [DOI] [PubMed] [Google Scholar]
- 40.Simeone AM, Tari AM. How retinoids regulate breast cancer cell proliferation and apoptosis. Cell. Mol. Life Sci. 2004;61 doi: 10.1007/s00018-004-4002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connolly RM, Nguyen NK, Sukumar S. Molecular Pathways: Current Role and Future Directions of the Retinoic Acid Pathway in Cancer Prevention and Treatment. Clinical Cancer Research. 2013;19:1651–1659. doi: 10.1158/1078-0432.CCR-12-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang X-H, Gudas LJ. Retinoids, Retinoic Acid Receptors, and Cancer. Annu. Rev. Pathol. Mech. Dis. 2011;6:345–364. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 2. ATRA-induced autophagy in HEK293T cells. (A) Endogenous LC3B protein levels were analyzed using confocal microscopy after 5h of ATRA treatment. (B) LC3B puncta were quantified using ImageJ software. (C) The rate of proteolysis was measured in cells either incubated with complete medium or medium supplemented with ATRA, in the presence or absence of BafilomycinA1. (D) The rate of proteolysis was measured in cells either incubated with complete medium or EBSS medium supplemented with 0.1% FBS, in the presence or absence of BafilomycinA1.
Supplementary Figure 1. LC3 mRNA expression in GATE-16 knockdown NB4 cells. Endogenous LC3B mRNA expression was measured upon ATRA (1μM) administration in NB4 cells expressing non-targeting shRNA (SHC002) or shRNAs targeting GATE-16 (shGATE-16_247 and shGATE-16_359) at day 4 and 6, respectively. Values were calculated as described in Fig. 1.