Abstract
Tumor associated carbohydrate antigens (TACAs) are overexpressed on tumor cells, which renders them attractive targets for anti-cancer vaccines. To overcome the poor immunogenicity of TACAs, we designed a polymer platform for antigen presentation by co-delivering TACA and helper T (Th) cell epitope on the same chain. The block copolymer was synthesized by cyanoxyl-mediated free radical polymerization followed by conjugation with a TACA Tn antigen and a mouse Th-cell peptide epitope derived from polio virus (PV) to afford the vaccine construct. The glycopolymer vaccine elicited an anti-Tn immune response with significant titers of IgG antibodies, which recognized Tn-expressing tumor cells.
Introduction
The stimulation of immune systems through the use of a construct that can elicit a specific immune response against cancer is the basis of anti-cancer vaccines.1 Cancer cells often bear characteristic carbohydrate structures on their cell surface.2,3 These tumor associated carbohydrate antigens (TACAs) are shared by a variety of cancer cell types, which make them attractive for anti-cancer vaccine development.4–11 However, serious challenges exist in order to elicit powerful anti-TACA immunity. Direct vaccination with TACA alone typically can only induce weak activation of antibody secreting B cells with no cooperation from Th cells.12 As a result, the antibodies secreted are mainly the low affinity IgM type. Since T cells typically recognize peptide epitopes, conjugating TACA to a Th cell peptide epitope should allow the stimulation of both B cells and Th cells. The matched Th cells provide stimulatory signals that can induce the B cells to undergo isotype switching leading to high affinity IgG antibodies.13 Many innovative carriers have been developed to co-deliver TACAs with Th epitopes. The most common type of carrier is immunogenic proteins such as keyhole limpet haemocyanin,14–17 tetanus toxoid,18,19 and Bacillus Calmette–Guerin.20 Other antigen presenting platforms include dendrimers,21,22 regioselectively addressable functionalized templates,23 nanomaterials,24,25 liposomes and proteoliposomes26,27 polysaccharides28 and virus-like particles.29,30
Polymers are a class of synthetic carrier that has multiple potential advantages for TACA delivery. A polymer chain can carry many TACA molecules, which can enhance the avidities between the antigen and B cell receptors (BCRs) through the polyvalency effect and lead to strong activation of B cells. Furthermore, Th epitopes can be introduced into the glycopolymer to potentiate Th cells generating a long lasting humoral immune response. Although synthetic glycopolymers have been utilized in a variety of applications31,32 including biosensing,33 delivery of therapeutic,34,35 modulation of natural killer cell function36 and cellular signaling,37 it is only recently that they have been explored as a TACA carrier.38,39 Herein, we present our results on using water soluble block copolymers as a platform to codeliver TACA and a Th epitope as a potential anti-cancer vaccine.
Results and discussion
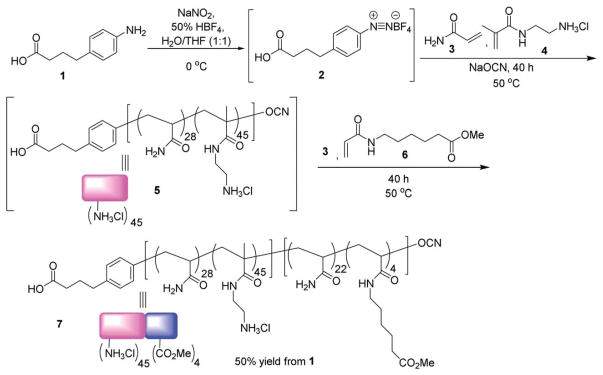
We selected the cyanoxyl-mediated free radical polymerization method40–43 for polymer construction due to the mild reaction condition. In order to incorporate both TACAs and Th epitope, the copolymer was designed to contain a block with multiple ammonium moieties followed by a methyl ester block (see polymer 7 in Scheme 1). The polymerization was initiated by the treatment of aniline 1 with sodium nitrite and fluoroboric acid, which was followed by the addition of a mixture of sodium cyanate, acrylamide 3 and methacrylamide amine 4 and heating at 50 °C for 40 hours leading to intermediate polymer 5 (Scheme 1). Subsequently, acrylamide 3 and acrylamide methyl ester monomer 6 were added to the reaction mixture with further heating for another 40 hours. The resulting mixture was dialyzed in water to obtain copolymer 7 in 50% yield. Based on integrations of 1H-NMR peaks from the polymers using the aromatic peaks from the terminal phenyl ring as the internal standard, there were on average 45 ammonium ion and 4 of methyl esters per polymer chain of 7. Gel permeation chromatography analysis showed that polymer 7 has a molecular weight (Mn) of 13 800 with a polydispersity index of 1.14.
Scheme 1.
Synthesis of polymer 7.
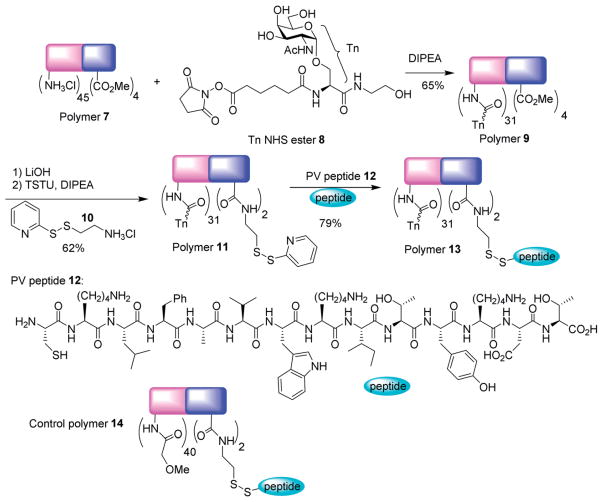
To test the efficiency of TACA delivery, a representative TACA, i.e., the Tn antigen was introduced into the polymer. The Tn antigen, found over-expressed on a variety of cancer cell surface including 90% of breast cancer carcinoma, is an appealing target for TACA based anti-cancer vaccine development.44–46 A flexible amide linker was designed to conjugate Tn with the polymer to avoid potential humoral responses to the linker.47 In order to accomplish this, Tn derivative 8 in the form of N-hydroxysuccinimide (NHS) activated ester was synthesized48 and linked with the amines in polymer 7 promoted by N,N-diisopropyl ethyl amine (DIPEA) leading to glycopolymer 9 in 65% yield (Scheme 2). On average, 31 copies of Tn were introduced per chain based on 1H-NMR analysis. Polymer 9 was then treated with LiOH to hydrolyze all the methyl esters, which was supported by 1H-NMR analysis showing the complete disappearance of the methyl groups. The resulting polymer was functionalized with pyridyl disulfide 10, which then reacted with a cysteine modified oligopeptide from polio virus (PV) to introduce the helper T cell epitope26,49 through the formation of disulfide bonds. The number of PV peptide per glycopolymer 13 was determined to be 2 peptides per chain by cleaving the disulfide linkage between the peptide and the glycopolymer followed by HPLC quantification. As a control, polymer 14 was synthesized by capping the amine groups of polymer 7 with methoxyacetic acid, which was followed by introduction of two PV peptides per chain utilizing a similar protocol as in the construction of polymer 13.
Scheme 2.
Synthesis of glycopolymer 13.
With the peptidic glyco-copolymer 13 in hand, its ability to elicit antibodies was evaluated. Mice were injected with glyco-polymer 13 (4 μg Tn per dose) subcutaneously with Freund’s adjuvant (CFA) followed by two booster injections at intervals of two weeks (days 14 and 28). Sera were drawn from mice one week after the final injection (day 35). The control group was vaccinated with polymer 14 following an identical protocol. To test anti-Tn antibody levels in sera, enzyme linked immunosorbent assay (ELISA) was performed using Tn functionalized bovine serum albumin (BSA) immobilized on microtiter plates. Analysis showed that the main antibodies induced by 13 were the IgG type with an IgG titer of 4432 (Fig. 1a. For IgM titers, see Fig. S2†). In comparison, the sera from mice receiving control polymer 14 contained extremely low titers of anti-Tn IgG antibodies (mean titer = 100) (Fig. 1a). The fact that high titers of IgG antibodies were induced by 13 implies the Tn-specific B cells have undergone isotype switching. Another important characteristic of a successful vaccine is the maintenance of immune responses. To evaluate this, mice immunized with 13 were bled on day 89. ELISA analysis showed that the anti-Tn IgG titers remained at a similar level (Fig. 1a, mean titer = 4369) suggesting long lasting humoral immunity was generated.
Fig. 1.
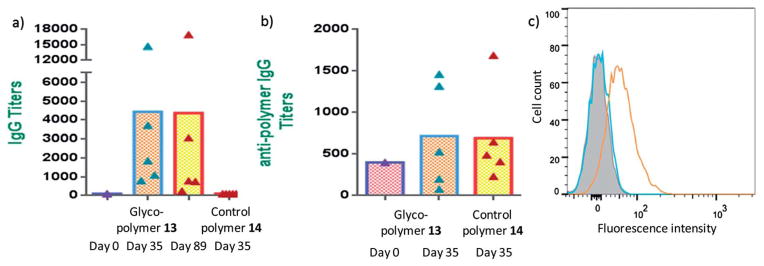
(a) Anti-Tn IgG titers on days 0, 35 and 89 from mice immunized with glyco-polymer 13 and the anti-Tn IgG titers on day 35 from mice receiving the control polymer 14. (b) Anti-polymer backbone IgG titers on days 0 and 35 from mice immunized with glyco-polymer 13 and anti-polymer backbone IgG titers on day 35 from mice receiving control polymer 14. (c) Flow cytometry analysis of Jurkat cell binding by IgG antibodies in sera from representative mice immunized with glycopolymer 13 (orange curve) and control polymer 14 (blue curve). The shaded curve was from pre-immune serum binding with Jurkat cells.
For many TACA constructs with highly immunogenic protein carriers, antibodies specific against the carrier are induced as well, the titers of which can be hundreds times higher than that against the desired TACA.47,50 The strong anti-carrier responses can potentially interfere with the generation of glycan specific antibodies due to antigen competition.51–53 The antibodies generated against the polymer backbone were analyzed. As shown in Fig. 1b, immunization with glycopolymer 13 or control polymer 14 elicited similar amounts of anti-polymer IgG antibodies with titers around 700 as compared to a titer of 400 pre-immunization. The relatively low anti-polymer titers induced suggest the polymer backbone most likely does not compete significantly for B cell interactions.
As ELISA tests antibody binding to an artificial BSA–Tn construct, it is important to determine whether the antibodies elicited can recognize Tn expressed in its native environment, i.e., cancer cells. Jurkat cells are known to express large amounts of Tn antigen on their surfaces.54 The post-immune serum from mice immunized with glycopolymer 13 exhibited significant binding with Jurkat cells, while those from the control polymer did not react with the cells (Fig. 1c).
In conclusion, a fully synthetic glycopolymer vaccine incorporating multiple Tn antigen and Th cell peptide epitope has been prepared, which elicited significant and long-lasting anti-Tn IgG antibody titers. The antibodies recognized Tn antigens on tumor cells. Compared with other delivery platforms such as virus like particles,29,30,48 the anti-Tn antibody titers generated by the glycopolymer constructs were modest. However, the polymer platform offers great flexibilities to adjust antigen densities and valency as well as the ratio of TACA vs. Th epitope. In addition, the immunogenicity of the polymer backbone is not high, which likely will not compete significantly with the desired TACA for B cell activation. These attributes bode well for further optimization of the glycopolymer construct to enhance the humoral responses against the TACAs.
Supplementary Material
Acknowledgments
We are grateful to the National Cancer Institute for generous financial support of our work (R01CA149451).
Footnotes
Electronic supplementary information (ESI) available: Full experimental details including: chemical synthesis, NMR spectra and immunological analysis. See DOI: 10.1039/c4md00103f
References
- 1.Finn OJ. Nat Rev Immunol. 2003;3:630. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 2.Hakomori S. Adv Exp Med Biol. 2001;491:369. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 3.Hakomori S, Zhang Y. Chem Biol. 1997;4:97. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- 4.Liu CC, Ye XS. Glycoconjugate J. 2012;29:259. doi: 10.1007/s10719-012-9399-9. [DOI] [PubMed] [Google Scholar]
- 5.Yin Z, Huang X. J Carbohydr Chem. 2012;31:143. doi: 10.1080/07328303.2012.659364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heimburg-Molinaro J, Lum M, Vijay G, Jain M, Almogren A, Rittenhouse-Olson K. Vaccine. 2011;29:8802. doi: 10.1016/j.vaccine.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morelli L, Poletti L, Lay L. Eur J Org Chem. 2011;2011:5723. [Google Scholar]
- 8.Zhu JL, Warren JD, Danishefsky SJ. Expert Rev Vaccines. 2009;8:1399. doi: 10.1586/erv.09.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo ZW, Wang QL. Curr Opin Chem Biol. 2009;13:608. doi: 10.1016/j.cbpa.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freire T, Bay S, Vichier-Guerre S, Lo-Man R, Leclerc C. Mini-Rev Med Chem. 2006;6:1357. doi: 10.2174/138955706778992996. [DOI] [PubMed] [Google Scholar]
- 11.Kuberan B, Linhardt RJ. Curr Org Chem. 2000;4:653. [Google Scholar]
- 12.Mond JJ, Lees A, Snapper CM. Annu Rev Immunol. 1995;13:655. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 13.Goldsby RA, Kindt TJ, Osborne BA. Kuby Immunology. 4. W. H. Freeman and Company; New York: 2000. p. 461. [Google Scholar]
- 14.Holmberg LA, Guthrie KA, Sandmaier BM. In: Chemical Glycobiology (ACS Symposium Series, 990) Chen X, Halcomb RL, Wang PG, editors. Washington, D. C: 2008. p. 197. [Google Scholar]
- 15.Sabbatini PJ, Ragupathi G, Hood C, Aghajanian CA, Juretzka M, Iasonos A, Hensley ML, Spassova MK, Ouerfelli O, Spriggs DR, Tew WP, Konner J, Clausen H, Abu Rustum N, Dansihefsky SJ, Livingston PO. Clin Cancer Res. 2007;13:4170. doi: 10.1158/1078-0432.CCR-06-2949. [DOI] [PubMed] [Google Scholar]
- 16.Danishefsky SJ, Allen JR. Angew Chem, Int Ed. 2000;39:836. doi: 10.1002/(sici)1521-3773(20000303)39:5<836::aid-anie836>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Helling FS, Shang A, Calves M, Zhang S, Ren S, Yu RK, Oettgen HE, Livingston PO. Cancer Res. 1994;54:197. [PubMed] [Google Scholar]
- 18.Hoffmann-Roder A, Kaiser A, Wagner S, Gaidzik N, Kowalczyk D, Westerlind U, Gerlitzki B, Schmitt E, Kunz H. Angew Chem, Int Ed. 2010;49:8498. doi: 10.1002/anie.201003810. [DOI] [PubMed] [Google Scholar]
- 19.Rich JR, Wakarchuk WW, Bundle DR. Chem– Eur J. 2006;12:845. doi: 10.1002/chem.200500518. [DOI] [PubMed] [Google Scholar]
- 20.Livingston POW, Wong GYC, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Calves MJ, Helling F, Ritter G, Oettgen HF, Old LJ. J Clin Oncol. 1994;12:1036. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 21.Heegaard PMH, Boas U, Sorensen NS. Bioconjugate Chem. 2010;21:405. doi: 10.1021/bc900290d. [DOI] [PubMed] [Google Scholar]
- 22.Lo-Man R, Vichier-Guerre S, Perraut R, Deriaud E, Huteau V, BenMohamed L, Diop OM, Livingston PO, Bay S, Leclerc C. Cancer Res. 2004;64:4987. doi: 10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- 23.Grigalevicius S, Chierici S, Renaudet O, Lo-Man R, Deriaud E, Leclerc C, Dumy P. Bioconjugate Chem. 2005;16:1149. doi: 10.1021/bc050010v. [DOI] [PubMed] [Google Scholar]
- 24.Brinas RP, Sundgren A, Sahoo P, Morey S, Rittenhouse-Olson K, Wilding GE, Deng W, Barchi JJ. Bioconjugate Chem. 2012;23:1513. doi: 10.1021/bc200606s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ojeda R, de Paz JL, Barrientos AG, Martin-Lomas M, Penades S. Carbohydr Res. 2007;342:448. doi: 10.1016/j.carres.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Lakshminarayanan V, Thompson P, Wolfert MA, Buskas T, Bradley JM, Pathangey LB, Madsen CS, Cohen PA, Gendler SJ, Boons GJ. Proc Natl Acad Sci U S A. 2012;109:261. doi: 10.1073/pnas.1115166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez LE, Alonso DF, Gomez DE, Vazquez AM. Expert Rev Vaccines. 2003;2:817. doi: 10.1586/14760584.2.6.817. [DOI] [PubMed] [Google Scholar]
- 28.De Silva RA, Wang Q, Chidley T, Appulage DK, Andreana PR. J Am Chem Soc. 2009;131:9622. doi: 10.1021/ja902607a. [DOI] [PubMed] [Google Scholar]
- 29.Yin Z, Nguyen HG, Chowdhury S, Bentley P, Bruckman MA, Miermont A, Gildersleeve JC, Wang Q, Huang X. Bioconjugate Chem. 2012;23:1694. doi: 10.1021/bc300244a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miermont A, Barnhill H, Strable E, Lu XW, Wall KA, Wang Q, Finn MG, Huang X. Chem– Eur J. 2008;14:4939. doi: 10.1002/chem.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narla SN, Nie H, Li Y, Sun XL. J Carbohydr Chem. 2012;31:67. [Google Scholar]
- 32.Narain R. Engineered Carbohydrate-Based Materials for Biomedical Applications: Polymers, Surfaces, Dendrimers, Nanoparticles, and Hydrogels. John Wiley & Sons, Inc; 2011. [Google Scholar]
- 33.Sun XL, Faucher KM, Houston M, Grande D, Chaikof EL. J Am Chem Soc. 2002;124:7258. doi: 10.1021/ja025788v. [DOI] [PubMed] [Google Scholar]
- 34.Suriano F, Pratt R, Tan JPK, Wiradharma N, Nelson A, Yang YY, Dubois P, Hedrick JL. Biomaterials. 2010;31:2637. doi: 10.1016/j.biomaterials.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Kim B, Peppas NA. J Biomater Sci Polymer Ed. 2002;13:1271. doi: 10.1163/156856202320893000. [DOI] [PubMed] [Google Scholar]
- 36.Hudak JE, Canham SM, Bertozzi CR. Nat Chem Biol. 2013;10:69. doi: 10.1038/nchembio.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu L, Sampson NS. ACS Chem Biol. 2014;9:468. doi: 10.1021/cb400550j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parry AL, Clemson NA, Ellis J, Bernhard SSR, Davis BG, Cameron NR. J Am Chem Soc. 2013;135:9362. doi: 10.1021/ja4046857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nuhn L, Hartmann S, Palitzsch B, Gerlitzki B, Schmitt E, Zentel R, Kunz H. Angew Chem, Int Ed. 2013;52:10652. doi: 10.1002/anie.201304212. [DOI] [PubMed] [Google Scholar]
- 40.Sun XL, Grande D, Baskaran C, Hanson SR, Chaikof EL. Biomacromolecules. 2002;3:1065. doi: 10.1021/bm025561s. [DOI] [PubMed] [Google Scholar]
- 41.Baskaran S, Grande D, Sun XL, Yayon A, Chaikof EL. Bioconjugate Chem. 2002;13:1309. doi: 10.1021/bc0255485. [DOI] [PubMed] [Google Scholar]
- 42.Grande D, Baskaran S, Chaikof EL. Macromolecules. 2001;34:1640. [Google Scholar]
- 43.Grande D, Baskaran S, Baskaran C, Gnanou Y, Chaikof EL. Macromolecules. 2000;33:1123. [Google Scholar]
- 44.Cazet A, Julien S, Bobowski M, Burchell J, Delannoy P. Breast Canc Res. 2010;12:204. doi: 10.1186/bcr2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Q, Anver MR, Butcher DO, Gildersleeve JC. Mol Cancer Ther. 2009;8:971. doi: 10.1158/1535-7163.MCT-08-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Springer GF. Science. 1984;224:1198. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- 47.Buskas T, Li Y, Boons GJ. Chem– Eur J. 2004;10:3517. doi: 10.1002/chem.200400074. [DOI] [PubMed] [Google Scholar]
- 48.Yin Z, Comellas-Aragones M, Chowdhury S, Bentley P, Kaczanowska K, BenMohamed L, Gildersleeve JC, Finn MG, Huang X. ACS Chem Biol. 2013;8:1253. doi: 10.1021/cb400060x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leclerc C, Deriaud E, Mimic V, van der Werf S. J Virol. 1991;65:711. doi: 10.1128/jvi.65.2.711-718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng K, Adams MM, Damani P, Livingston PO, Ragupathi G, Gin DY. Angew Chem, Int Ed. 2008;47:6395. doi: 10.1002/anie.200801885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sad S, Gupta HM, Talwar GP, Raghupathy R. Immunology. 1991;74:223. [PMC free article] [PubMed] [Google Scholar]
- 52.Di John D, Wasserman SS, Torres JR, Cortesia MJ, Murillo J, Losonsky GA, Herrington DA, Stürcher D, Levine MM. Lancet. 1989;334:1415. doi: 10.1016/s0140-6736(89)92033-3. [DOI] [PubMed] [Google Scholar]
- 53.Herzenberg LA, Tokuhisa T. J Exp Med. 1982;155:1730. doi: 10.1084/jem.155.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakada H, Inoue M, Tanaka N, Numata Y, Kitagawa H, Fukui S, Yamashina I. Biochem Biophys Res Commun. 1991;179:762. doi: 10.1016/0006-291x(91)91882-d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.