Abstract
Despite anti-dsDNA antibodies constitute a wide range of specificities, they are considered as the hallmark for systemic lupus erythematosus (SLE).
Objective
To identify clinical phenotypes associated with anti-dsDNA antibodies, independently of any clinical diagnoses.
Methods
Patients with recent onset of any rheumatic symptoms were screened for antinuclear antibodies (ANA). All ANA-positive and matching ANA-negative patients were examined, and their clinical phenotypes were registered, using a systematic chart formulated after consensus between the participating centres. All patients were tested for different anti-dsDNA antibody specificities with assays habitually used in each participating laboratory. Crithidia Luciliae Immuno Fluorescence Test (CLIFT) was performed three times (with two different commercial kits); solid and solution phase ELISA were performed four times. Associations between clinical phenotypes and results of anti-dsDNA assays were evaluated by linear regression analysis (LRA) and principal component analysis (PCA).
Results
Totally, 292 ANA-positive and 292 matching ANA-negative patients were included in the study. A full dataset for statistical analysis was obtained in 547 patients. Anti-dsDNA antibodies were most frequently detected by ELISA. LRA showed that overall positivity of anti-dsDNA antibodies was associated with proteinuria and pleuritis. Alopecia was significantly associated only with CLIFT-positivity. Besides confirming the same findings, PCA showed that combined positivity of CLIFT and ELISA was also associated with lymphopenia.
Conclusions
Our results show that different anti-dsDNA antibody specificities are associated with nephropathy, pleuritis, alopecia and lymphopenia, regardless of the diagnosis. It may challenge the importance of anti-dsDNA antibodies as a diagnostic hallmark for SLE.
Keywords: Autoantibodies, Autoimmunity, Systemic Lupus Erythematosus, Lupus Nephritis, Autoimmune Diseases
Key messages.
In patients with recent onset of rheumatic symptoms, the assessment of anti-dsDNA antibodies with different techniques results in a considerable discrepancy of outcomes and of correlations to various clinical and biochemical manifestations.
Anti-dsDNA antibodies are associated with presence of proteinuria, regardless of clinical diagnosis, outcome of ANA screening and laboratory method used for the assessment of anti-dsDNA antibodies.
In distinct subgroups of patients, anti-dsDNA antibodies are also variously associated with presence of other clinical manifestations, such as haematuria, leukopenia, pleuritis and alopecia.
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease with unknown aetiology. Whether SLE represents one disease entity or is a continuous overlap of aetiologically unrelated organ manifestations is not established.
This is particularly challenging when attempting to determine biomarkers for SLE. Anti-double-stranded DNA (dsDNA) antibodies are regarded as fairly specific for SLE.1–3 B-cell-mediated and T-cell-mediated autoimmunity to the individual components of nucleosomes are considered important in establishing a diagnosis,4–7 but the pathogenic and diagnostic roles played by anti-dsDNA and other antibodies are still debated.7 8
Antibodies to dsDNA may have a direct pathogenic effect in lupus nephritis,9 lupus dermatitis10 11 and possibly also in certain aspects of cerebral lupus.12 How anti-dsDNA antibodies relate to the rest of the clinical components of current classification criteria5 8 remains to be determined.
When emphasising anti-dsDNA antibodies as a central biomarker in SLE, it is important to perceive that these antibodies basically are not representing a homogenous antibody population.13–16 Growing insight into the different possible mechanisms of production of antibodies specifically binding to dsDNA17–24 challenges the notion of a specific relationship between all anti-dsDNA antibodies per se with SLE. Which and how anti-dsDNA antibodies are pathogenic has also been questioned.9 25–38
We aim to explore in the present investigation whether the positivity of anti-dsDNA antibodies is a biomarker indicating presence of defined clinical phenotypes, such as, for example, arthropathy or nephropathy or serositis, rather than a defined diagnosis, such as SLE.
The current literature is mainly composed of investigations of patients with established SLE and other defined and classified diagnoses. Studies concerning the association of anti-dsDNA antibodies with clinical manifestations in unselected patients with early onset of rheumatic symptoms are scarce.
The main purpose of this study is to correlate the presence in serum of anti-dsDNA antibodies with individual clinical manifestations and laboratory variables. We intend to use an unbiased approach that mirrors an ordinary clinical setting, where the physician is challenged to make the right diagnosis in patients with newly developed rheumatic manifestations, based upon clinical signs and symptoms, with the support of various diagnostic procedures, including laboratory tests. This approach also allows us to perform a comparison between serum levels of anti-dsDNA antibodies obtained in different laboratories with different methods.
Methods and patients
Patients
Consecutive patients with recent onset of suspected rheumatic disorder, referred for the first time to the participating rheumatologic units (Rigshospitalet in Copenhagen, Denmark; University Hospital in Tromsø, Norway; University Hospital in Lund, Sweden) were recruited to the study between February 2003 and December 2007.
Exclusion criteria were: established autoimmune disease, treatment with any biological drug, corticosteroids (equivalent Prednisolon >20 mg/day), immune-modulating, immunosuppressive or cytostatic drugs. Patients who previously had been examined by a rheumatologist, and patients unable to fully collaborate in the study (not confident with the language, actual cognitive, speech, hearing or memory impairment) were also excluded.
The patients were examined by a rheumatologist who made an initial working clinical diagnosis, based on anamnesis, symptoms, physical examination and laboratory test results.
All the patients were screened for antinuclear antibodies (ANA) by local testing. All the ANA-positive patients and the same amount (1:1 ratio) of randomly selected sex-matched and age-matched ANA-negative patients built together a nested cohort, which underwent further clinical and laboratory assessments. A systematic chart including clinical data and routine laboratory variables (see online supplementary table S1) was completed, ensuring a common trunk of data on which the clinical phenotypes of the patients could be characterised. Aliquots of serum samples from all patients were collected and sent to each centre for simultaneous parallel analysis of anti-dsDNA antibodies by several different assays, as described below. All clinical data were collected without knowledge of the results of the anti-dsDNA testing.
Ethics
All patients entered the study after giving informed written consent. The participating centres performed the study according to the approval from the local ethics committees.
Clinical phenotype description
Consensus as to the content of the clinical and laboratory dataset in the systematic chart (see online supplementary table S1) was obtained through a Delphi-like process, spanning over four meetings. A uniform definition of the final clinical dataset was assured by agreeing on the definitions of the various manifestations prior to the study (see online supplementary table S2). Manifestations were recorded as being absent ever, ongoing/active, previous/inactive or unknown and the date any manifestations first appeared was noted. In the present study, calculations were based on the presence ever of a manifestation.
Detection of ANA
In the various centres, respective current routine methodology was used for detection of ANA. In Copenhagen and Lund, screening for ANA was performed by indirect immunofluorescence (IIF) technique. In Copenhagen, HEp-2 cells (ImmunoConcepts, Sacramento, California, USA) and patient sera in dilution 1/160 were used together with an FITC-labelled antihuman IgG conjugate (DAKO Denmark A/S, Glostrup, Denmark). The diagnostic ANA titre was 1:160 as established by determination of the 95th percentile for negativity in healthy blood donors. In Lund, Hep-2 or Hep-20-10 cells (Euroimmun, Lübeck, Germany) and patient sera in dilution 1/400 were used which corresponded to detection of ANA with homogeneous pattern at 14 IU/mL (WHO reference serum 66/233). The diagnostic ANA titre was 1:400 as established by determination of the 96.5th percentile for negativity in healthy blood donors. In Tromsø, the detection of ANA was performed with the ELISA Varelisa ReCombi ANA Screen (Pharmacia Diagnostics, Freiburg, Germany). This is an indirect non-competitive enzyme immunoassay for the qualitative and semiquantitative determination of eight preselected ANA (dsDNA, Sm (B,B′,D), ribo-nucleo protein (68 kDa, A, C), SS-A/Ro(52 and 60 kDa), SS-B/La, Scl-70, centromere and Jo-1) in serum or plasma. The assay was performed as recommended by the manufacturer, while validation and determination of cut-off values were performed in accordance with an internal and external quality assessment programme, with validation against both controls and other disease categories.
Detection of anti-dsDNA antibodies
Without knowledge of any clinical information, all the patients included in the nested cohort had IgG anti-dsDNA antibodies determined by different indirect immunofluorescence (IIF) tests and immunoassays available at the participating centres (table 1).
Table 1.
Overview of anti-dsDNA tests used in the participating laboratories for analysis of serum aliquots from all patients
| Name | Methodology | Antigen | Reference interval | Manufacturer | Centre |
|---|---|---|---|---|---|
| CLIFT 1 | IIF | Crithidia luciliae kinetoplast | <titre 10 | ImmunoConcept | CPH |
| CLIFT 2 | IIF | Crithidia luciliae kinetoplast | <titre 10 | ImmunoConcept | Tromsø |
| CLIFT 3 | IIF | Crithidia luciliae kinetoplast | <titre 10 | Euroimmune | Lund |
| EliA | Solid phase ELISA | Recombinant plasmid dsDNA | <10 IU/mL | Phadia | Tromsø |
| SPADE | Solution phase ELISA | Biotinylated plasmid dsDNA and biotinylated, S1 nucleased human dsDNA | <1 AU/mL | Inhouse | Tromsø |
| Varelisa 1 | Solid phase ELISA | Recombinant plasmid dsDNA | <55 IU/mL | Phadia | Tromsø |
| Varelisa 2 | Solid phase ELISA | Recombinant plasmid dsDNA | <35 IU/mL | Phadia | CPH |
AU, arbitrary units; CLIFT, Crithidia Lucillia Immunofluorescence Test; CPH, Copenhagen; IIF: indirect immunofluorescence technique; IU, international units; SPADE, solution phase anti-dsDNA ELISA.
Immunofluorescent tests
Presence of anti-dsDNA antibodies was assessed in all the three participating laboratories by Crithidia Luciliae Immunofluorescence Test (CLIFT) with two different commercial kits, according to the manufacturers’ instructions. By using a fluorescence microscope, the results were based on the fluorescence intensity and categorised as negative or positive.
Immunoassays
The determination of anti-dsDNA antibodies by the EliA test and the Varelisa were performed according to the manufacturer's instructions.
The solution phase anti-dsDNA ELISA (SPADE) is previously described in detail.13 It measures antibody binding to dsDNA in solution using biotinylated dsDNA.39 Biotinylation of the pUC18 DNA (1 μg/μL H2O) was carried out as recommended by the manufacturer (Pierce Chemical Company, Rockford, Illinois, USA). SPADE was performed by mixing 0.5 μg of pUC18 DNA (1 μg/μL H2O) with serially diluted serum samples (solution phase step). After incubation for 30 min, the mixtures were added to microtitre plates (Nunc MaxiSorp, Nunc, Denmark) coated overnight at 37°C with 50 μL streptavidin (5 μg/mL PBS). After incubation for 30 min, the plates were washed and incubated with horseradish peroxidase conjugated anti-human Fcγ antibodies (Sigma–Aldrich, St Louis, Missouri, USA) in phosphate buffered saline with tween 20. The reaction was developed by adding o-phenylenediamine dihydrochloride, and stopped by 1 M HCl. The reaction was read at 490 nm.
Statistical analysis
After finalising data retrieval, all data were registered in a common database accessible for all centres. Statistical analyses using SPSS statistics V.20.0 software (IBM) included contingency table analysis and binary logistic regression analysis. OR and corresponding 95% CIs were initially calculated as crude values using dichotomised anti-dsDNA results as the dependent variable and each dichotomised clinical manifestation registered in our database as explanatory variables. OR and corresponding 95% CI were then adjusted, using all the crude significant associations as covariates.
Principal component analysis (PCA) using R (http://www.R-project.org) included clinical phenotype data as well as positivity in the ANA test, any of the CLIFTs and any of the ELISAs. The PCA results were presented as biplots with variable vectors (arrows) indicating by direction which variables had the highest degree of covariation and influence, positive or negative, in discriminating the patients.
Results
Altogether, 1073 patients were recruited from February 2003 to December 2007. Among these patients, 292 were found to be ANA positive by local testing. From the remaining ANA-negative patients, each centre in a 1:1 ratio randomly selected sex-matched and age-matched patients, reaching a total of 292 ANA-negative control patients. The resulting nested cohort of 584 patients (138 from Copenhagen, 144 from Tromsø and 302 from Lund) was investigated concerning clinical phenotype at inclusion and anti-dsDNA antibodies assessed by different assays.
Clinical and biochemical manifestations in ANA positive and ANA-negative patients
A full serological dataset was obtained for 547 patients, 288 ANA positive (78.5% females, median age 51.8 years, range 15.4–83.7 years) and 259 ANA negative (83.4% females, median age 51.1 years, range 15.9–84.2 years). The remaining 37 patients, 4 ANA positive (75% females, median age 64 years, range 49–77 years) and 33 ANA negative (76% females, median age 59 years, range 22–81 years) withdrew from the study because of technical and logistic problems that hampered the collection of a full serological dataset.
The initial clinical diagnoses of the patients based on the conclusion of the examining rheumatologist are listed in table 2.
Table 2.
Clinical diagnoses formulated by the examining rheumatologists in the participating centre
| Nested cohort—547 patients |
37 dropout patients |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Diagnoses | ANA pos (N=288) |
ANA neg (N=259) |
Rate ratio* | Total |
Total |
||||
| N | Per cent | n | Per cent | n | Per cent | n | Per cent | ||
| Systemic lupus erythematosus | 57 | 19.8 | 6 | 2.3 | 8.61 | 63 | 11.5 | 2 | 5.4 |
| Inflammatory connective tissue disease | 44 | 15.3 | 12 | 4.6 | 3.33 | 56 | 10.2 | 0 | 0 |
| Inflammatory joint disease | 49 | 17 | 95 | 36.7 | 0.46 | 144 | 26.3 | 13 | 35.1 |
| Systemic inflammatory disease | 10 | 3.5 | 17 | 6.6 | 0.53 | 27 | 4.9 | 2 | 5.4 |
| Arthralgia | 45 | 15.6 | 32 | 12.35 | 1.26 | 77 | 14.1 | 6 | 16.2 |
| Osteoarthritis | 25 | 8.7 | 29 | 11.2 | 0.78 | 54 | 10 | 5 | 13.5 |
| Soft-tissue rheumatism | 8 | 2.8 | 25 | 9.65 | 0.29 | 33 | 6.0 | 3 | 8.1 |
| Non-rheumatic disease | 34 | 11.8 | 26 | 10 | 1.18 | 60 | 11 | 3 | 8.1 |
| Dermatological disorder | 10 | 3.5 | 7 | 2.7 | 1.30 | 16 | 2.9 | 2 | 5.4 |
| Unspecified | 7 | 2.4 | 10 | 3.9 | 0.61 | 17 | 3.1 | 1 | 2.7 |
*Ratio between prevalence of manifestation in ANA positive and ANA negative patients.
ANA, antinuclear antibodies.
The most prevalent American College of Rheumatology (ACR) classification criteria for SLE,5 40 and the most relevant remaining clinical and biochemical manifestations are listed in table 3. Arthritis, photosensitivity, oral/nasal ulcers, haematuria, and proteinuria were the five most prevalent SLE specific manifestations. Malar rash was the only clinical variable significantly associated with the presence of ANA.
Table 3.
Clinical and biochemical manifestations recorded during the initial work-up of the included 547 patients. Listed are manifestations included in systemic lupus erythematosus (SLE) classification criteria and other most prevalent clinical manifestations
| All patients |
ANA pos (N=288) |
ANA neg (N=259) |
Rate ratio* | p Value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Per cent | N | Per cent | n | Per cent | |||
| Manifestations among SLE classification criteria | ||||||||
| Peripheral arthritis | 157 | 28.7 | 82 | 28.5 | 75 | 29 | 1.0 | 0.90 |
| Photosensitivity | 57 | 10.7 | 35 | 12.2 | 22 | 8.5 | 1.4 | 0.16 |
| Oral/nasal ulcers | 32 | 5.9 | 21 | 7.3 | 11 | 4.2 | 1.7 | 0.13 |
| Haematuria | 20 | 3.7 | 11 | 3.8 | 9 | 3.5 | 1.1 | 0.82 |
| Proteinuria | 17 | 3.1 | 9 | 3.1 | 8 | 3.1 | 1 | 0.98 |
| Malar rash | 15 | 2.7 | 12 | 4.2 | 3 | 1.2 | 3.5 | 0.03 |
| Leukopenia | 14 | 2.6 | 10 | 3.5 | 4 | 1.5 | 2.3 | 0.15 |
| Alopecia | 12 | 2.2 | 6 | 2.1 | 6 | 2.3 | 0.9 | 0.85 |
| Lymphopenia | 11 | 2 | 8 | 2.8 | 3 | 1.2 | 2.3 | 0.17 |
| Discoid LE | 8 | 1.5 | 2 | 0.7 | 6 | 2.3 | 0.3 | 0.13 |
| Thrombocytopenia | 8 | 1.5 | 6 | 2.1 | 2 | 0.8 | 2.6 | 0.19 |
| Pleuritis | 5 | 0.9 | 4 | 1.5 | 1 | 0.3 | 5 | 0.16 |
| Other manifestations | ||||||||
| Arthralgia | 308 | 56.3 | 168 | 58.3 | 140 | 54.1 | 1.1 | 0.29 |
| Morning joint stiffness | 128 | 23.4 | 69 | 24.0 | 59 | 22.8 | 1.1 | 0.74 |
| Raynaud's phenomenon | 73 | 13.3 | 47 | 16.3 | 26 | 10.0 | 1.6 | 0.03 |
| Headache | 72 | 13.2 | 49 | 17.0 | 23 | 8.9 | 1.9 | 0.004 |
| Xerostomia | 70 | 12.8 | 43 | 14.9 | 27 | 10.4 | 1.4 | 0.11 |
| Arterial hypertension | 67 | 12.2 | 35 | 12.2 | 32 | 12.4 | 1 | 0.94 |
| Tendinitis | 49 | 9 | 27 | 9.4 | 22 | 8.5 | 1.1 | 0.72 |
| Psoriasis | 40 | 7.4 | 14 | 4.9 | 26 | 10.2 | 0.5 | 0.02 |
| Affective disorder | 40 | 7.3 | 27 | 9.4 | 13 | 5.0 | 1.9 | 0.05 |
| Keratoconjunctivitis sicca | 39 | 7.2 | 25 | 8.7 | 14 | 5.5 | 1.6 | 0.14 |
| Asthma bronchiale | 38 | 7.0 | 25 | 8.7 | 13 | 5.0 | 1.7 | 0.09 |
| Puffy fingers | 36 | 6.6 | 25 | 8.7 | 11 | 4.2 | 2.1 | 0.35 |
| Thyreoiditis | 33 | 6.1 | 18 | 6.3 | 15 | 5.8 | 1.1 | 0.81 |
| Peripheral neuropathy | 32 | 5.9 | 14 | 4.9 | 18 | 6.9 | 0.7 | 0.30 |
| Weight loss | 28 | 5.2 | 15 | 5.2 | 13 | 5.0 | 1 | 0.92 |
| Axial arthritis | 25 | 4.6 | 15 | 5.2 | 10 | 3.9 | 1.3 | 0.45 |
| Fibromyalgia | 21 | 3.9 | 12 | 4.2 | 9 | 3.5 | 1.2 | 0.67 |
| Anaemia, non-haemolytic | 20 | 3.7 | 13 | 4.5 | 7 | 2.7 | 1.7 | 0.26 |
| Non-infectious fever | 19 | 3.5 | 9 | 3.1 | 10 | 3.9 | 1.3 | 0.65 |
| Arrhythmia | 16 | 2.9 | 9 | 3.1 | 7 | 2.7 | 0.8 | 0.77 |
| Cutaneous vasculitis | 14 | 2.6 | 7 | 2.5 | 7 | 2.7 | 0.9 | 0.85 |
| Miscarriage/abortion | 14 | 2.6 | 9 | 3.1 | 5 | 1.9 | 1.6 | 0.32 |
| Chronic urticaria | 12 | 2.2 | 7 | 2.4 | 5 | 1.9 | 1.3 | 0.69 |
| Livedo reticularis | 12 | 2.2 | 9 | 3.1 | 3 | 1.2 | 2.6 | 0.11 |
| Palindromic arthritis | 12 | 2.2 | 6 | 2.1 | 6 | 2.3 | 0.9 | 0.85 |
*Ratio between prevalence of manifestation in ANA positive and ANA-negative patients.
ANA, antinuclear antibodies.
Arthralgia, morning joint stiffness, Raynaud's phenomenon, headache, and subjective xerostomia were the five most prevalent manifestations among those not included in ACR classification criteria. Raynaud’s phenomenon, headache, puffy fingers and affective disorders were significantly more prevalent in the presence of ANA, whereas psoriasis was significantly more prevalent in ANA-negative patients.
ANA and anti-dsDNA antibody assays
The number of patients positive in the different anti-dsDNA assays performed ranged from 33 (6.1%) to 61 (11.1%). Among all 547 sera, a substantial difference between the centres was seen, being the number of CLIFT-positive results 33 (6%), 36 (6.6%) and 45 (8.2%) for Copenhagen, Tromsø and Lund, respectively. A total of 59 patients were positive by at least one CLIFT and 99 by any ELISA assessment, with low agreement of results (k=0.34).
Of the 288 ANA-positive sera, 39 (13.5%) were positive in any CLIFT, while 50 sera (17.4%) were positive in any of the immunoassays (Varelisa, SPADE or EliA). Of the 259 ANA-negative sera, 20 (7.7%) were positive by any CLIFT, and 49 (18.9%) were positive by any immunoassay. One hundred and twenty-four patients (68 ANA positive and 56 ANA negative) were positive at least by one assay, whereof 34 patients (21 ANA positive and 13 ANA negative) were positive by at least one CLIFT and one ELISA assessment.
In general, the CLIFT tests were least often positive. None of the single anti-dsDNA tests was associated with the presence of ANA, with the only exception of the CLIFT performed in Lund. Nonetheless, the positivity for any CLIFT was strongly associated with ANA positivity (table 4). According to our results, having a negative ANA did not rule out having a positive anti-dsDNA test.
Table 4.
Prevalence of positivity in the anti-dsDNA tests performed
| All patients |
ANA pos (N=288) |
ANA neg (N=259) |
Rate ratio* | p Value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Per cent | n | Per cent | n | Per cent | |||
| CLIFT 1 | 33 | 6.1 | 18 | 6.3 | 15 | 5.8 | 1.1 | 0.82 |
| CLIFT 2 | 36 | 6.6 | 22 | 7.6 | 14 | 5.4 | 1.4 | 0.29 |
| CLIFT 3 | 45 | 8.2 | 30 | 10.4 | 15 | 5.8 | 1.8 | 0.05 |
| Any CLIFT | 59 | 10.8 | 39 | 13.5 | 20 | 7.7 | 1.75 | 0.03 |
| EliA | 61 | 11.1 | 27 | 9.4 | 34 | 13.1 | 0.7 | 0.16 |
| SPADE | 57 | 10.4 | 28 | 9.7 | 29 | 11.2 | 0.9 | 0.57 |
| Varelisa 1 | 50 | 9.1 | 26 | 9.0 | 24 | 9.3 | 1 | 0.92 |
| Varelisa 2 | 59 | 10.8 | 30 | 10.4 | 29 | 11.2 | 0.9 | 0.77 |
| Any ELISA | 99 | 18.1 | 50 | 17.4 | 49 | 18.9 | 0.9 | 0.64 |
| Any test | 124 | 22.7 | 68 | 23.6 | 56 | 21.6 | 1.1 | 0.58 |
| CLIFT+ELISA | 34 | 6.2 | 21 | 7.3 | 13 | 5.0 | 1.6 | 0.27 |
Test details are presented in table 1.
*Ratio between prevalence of positive anti-dsDNA test in ANA positive and ANA-negative patients.
ANA, antinuclear antibodies; CLIFT, Crithidia Lucillia Immunofluorescence Test; SPADE, solution phase anti-dsDNA ELISA.
Anti-dsDNA antibodies and clinical manifestations
The prevalence of clinical and laboratory manifestations in anti-dsDNA-positive patients are reported in the online supplementary table S3. Malar rash, cutaneous vasculitis, alopecia, leukopenia, lymphopenia, non-haemolytic anaemia, pleuritis, proteinuria and haematuria are more prevalent in patients with combined CLIFT and ELISA positivity (CLIFT+ ELISA+). Sixty-five patients resulted negative by CLIFT and positive by ELISA (CLIFT− ELISA+), with higher prevalence of oral or nasal ulcers, tendinitis, xeroftalmia, xerostomia and fibromyalgia. The combination of CLIFT positivity with negative outcome of ELISA (CLIFT+ ELISA−) was more frequent in patients with arthralgia, peripheral arthritis, morning joint stiffness, photosensitivity, Raynaud’s phenomenon, puffy fingers, thrombocytopenia, livedo reticularis, chronic urticaria, discoid LE, asthma, peripheral neuropathy and headache.
Associations between clinical and biochemical manifestations and positivity of anti-dsDNA antibodies are listed in table 5. ORs with 95% CIs are given as crude values (univariate) and as adjusted values (multivariate) for all the individual manifestations significantly associated with presence of anti-dsDNA antibodies, assessed by any CLIFT and any ELISA, namely proteinuria, haematuria, alopecia, leukopenia, thrombocytopenia, cutaneous vasculitis and pleuritis.
Table 5.
Association between most relevant clinical manifestations and positive outcome of anti-dsDNA tests (any CLIFT and any ELISA)
| Any CLIFT positive (39 ANA positive+20 ANA negative) |
Any ELISA positive (50 ANA positive+49 ANA negative) |
|||
|---|---|---|---|---|
| CRUDE OR (95% CI) | ADJUSTED OR (95% CI) | CRUDE OR (95% CI) | ADJUSTED OR (95% CI) | |
| Peripheral arthritis | 1.1 (0.6–2) | 0.6 (0.4–1.04) | ||
| Photosensitivity | 0.6 (0.2–1.7) | 0.5 (0.2–1.2) | ||
| Oral ulcers | 0.8 (0.25–2.9) | 1.8 (0.8–4.1) | ||
| Haematuria | 3.8 (1.4–10.4) | 0.6 (0.1–3.4) | 3.2 (1.3–8) | 0.5 (0.1–2.8) |
| Proteinuria | 14 (5.1–38.4) | 13 (2.9–57.7) | 16.7 (5.3–52.6) | 18.8 (3.7–95.2) |
| Malar rash | 3.1 (0.97–10.2) | 1.7 (0.5–5.4) | ||
| Anaemia | 3.8 (1.4–10.4) | 1.1 (0.4–3.5) | ||
| Leukopenia | 6.8 (2.3–20.3) | 2.3 (0.5–10.7) | 6.5 (2.2–19.1) | 3.5 (0.8–14.2) |
| Alopecia | 4.4 (1.3–15) | 4.3 (1.1–16) | 3.35 (1–10.8) | 3.1 (0.9–10.8) |
| Lymphopenia | 10.9 (3.2–37) | 2.7 (0.8–9.2) | ||
| Discoid LE | 1.2 (0.1–9.8) | 0.6 (0.8–5.3) | ||
| Thrombocytopenia | 8.8 (2.1–36.2) | 3.1 (0.5–20.2) | 4.7 (1.1–19) | 1.1 (0.2–7.6) |
| Pleuritis | 13 (2.1–79.6) | 11.1 (1.5–83.8) | 18.8 (2.1–170.3) | 14.5 (1.4–148.2) |
| Arthralgia | 0.6 (0.3–1.02) | 0.5 (0.3–0.8) | ||
| Morning joint stiffness | 0.5 (0.2–1.04) | 0.4 (0.2–0.7) | ||
| Raynaud's phenomenon | 1.6 (0.8–3.2) | 1.2 (0.6–2.2) | ||
| Headache | 0.7 (0.3–1.75) | 0.8 (0.4–1.6) | ||
| Xerostomia | 0.75 (0.3–1.8) | 1 (0.5–2) | ||
| Arterial hypertension | 0.96 (0.4–2.2) | 1 (0.5–1.9) | ||
| Tendinitis | 0.2 (0.02–1.2) | 1.3 (0.7–2.7) | ||
| Psoriasis | 0.6 (0.2–2.2) | 0.5 (0.2–1.4) | ||
| Affective disorder | 0.7 (0.2–2.2) | 0.8 (0.3–1.9) | ||
| Keratoconjunctivitis sicca | 0.4 (0.1–1.8) | 0.8 (0.3–2) | ||
| Cutaneous vasculitis | 6.7 (2.25–20.2) | 2.1 (0.5–9.4) | 6.4 (2.2–18.9) | 2.4 (0.6–9.5) |
| Asthma or COL | 2.4 (1.04–5.5) | 1.4 (0.7–3.15) | ||
| Lymphadenopaty | 6.5 (1.4–29.7) | 1.8 (0.3–9.6) | ||
Crude OR with corresponding 95% CIs in brackets is reported for all the variables. Adjusted OR is reported only for variables significantly associated with positivity of any CLIFT and any ELISA.
ANA, antinuclear antibodies; CLIFT, Crithidia Lucillia Immunofluorescence Test.
Lymphopenia and non-haemolytic anaemia were significantly associated with positive CLIFT, as well as morning stiffness and arthralgia were inversely associated only with a positive ELISA result.
The clinical manifestations most strongly associated with positive result of CLIFT had higher OR in ANA-positive patients, except for pleuritis. In patients with any positive ELISA, only OR for cutaneous vasculitis, thrombocytopenia and leukopenia were higher in ANA-positive patients. Having a positive anti-dsDNA test was associated with pleuritis and proteinuria even in ANA-negative patients.
The overall association of anti-dsDNA with typical manifestations of SLE
A PCA of the ACR classification criteria5 40 items included in table 4, alopecia (included in the Systemic Lupus International Collaborating Clinics (SLICC) criteria8) and outcomes of anti-dsDNA tests by ELISA (any test positive) and CLIFT (any test positive) is shown as a three-dimensional plot for the three major principal components explaining 16.1%, 10.7% and 8.6%, respectively, of the variance in the dataset (figure 1). From this plot it is seen that having positive CLIFT and ELISA tests for anti-dsDNA (n=34) was closely associated with nephropathy (proteinuria and haematuria), hematological abnormalities and pleuritis; patients with a positive CLIFT and negative ELISA (n=25) or negative CLIFT and positive ELISA (n=65) did not cluster with any particular subset of the clinical manifestations.
Figure 1.
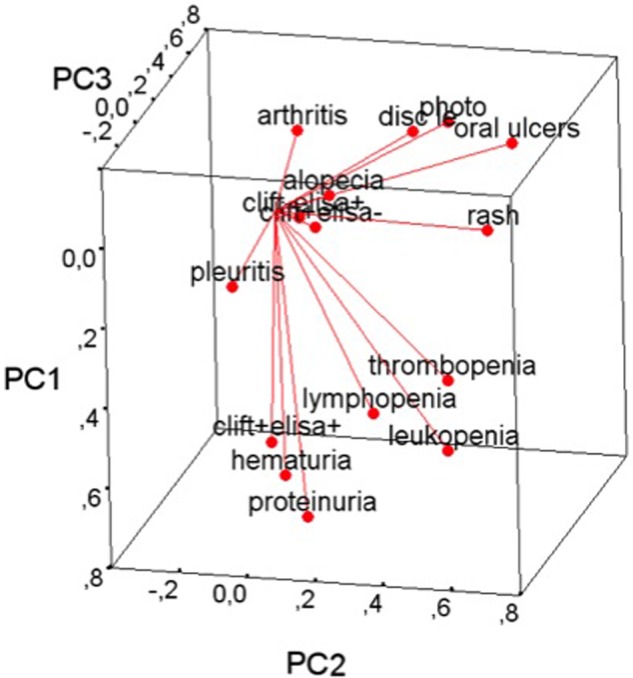
Principal component (PC) analysis of typical manifestations of systemic lupus erythematosus, positivity of any Chritidia Luciliae Immunofluorescence Test (CLIFT) and positivity of any ELISA included in this study. PC 1, 2 and 3 explained 16.1%, 10.7% and 8.6%, respectively. This PC plot aims to optimally display variances and not correlations; however, the angles between the various plot vectors serve as good indicators of the correlations among the variables, and the length of the vectors provide good indications as to which variables have had the largest effect on the variation in the dataset. The plot shows how having a positive CLIFT and ELISA(clift+elisa+) is associated with signs of nephropathy, haematological abnormalities and pleuritis; having a single positive test (CLIFT or ELISA) did not cluster with any of the manifestations included in the PC analysis.
Discussion
The uniqueness of the present study is that the association between clinical phenotypes and presence in serum of anti-dsDNA antibodies has been investigated in consecutive patients with recent onset of rheumatic symptoms, regardless of the diagnosis. By this approach, we aimed to reproduce the usual clinical setting, where the physician, at an early stage, is challenged to formulate a diagnosis and predict the outcome, based on clinical manifestations and suitable diagnostic tools available locally.
This study demonstrates that different techniques for the detection of anti-dsDNA antibodies result in a considerable variation in anti-dsDNA antibody status and relation hereof to various clinical and biochemical manifestations. Shared findings for the regression analyses and the PCA are the relationships between nephropathy and pleuritis with a positive CLIFT. The results obtained for immunoassays were, instead, highly variable and less selective with respect to several typical SLE manifestations. Furthermore, anti-dsDNA antibodies in general were only associated with a few manifestations typically used to classify SLE, and notably with lupus nephritis, where anti-dsDNA antibodies are demonstrated to be involved in the pathogenesis.
These observations raise the discussion about the performance of the various anti-dsDNA detection techniques, the general pathogenic role of the various anti-dsDNA antibodies identified, their clinical associations, and how the syndrome of SLE is currently delineated. Anti-dsDNA antibodies represent a heterogeneous population of antibodies with respect to origin, intrinsic affinity, fine structural DNA specificity and potential to cross-react with non-DNA structures. From this consideration, diverse anti-dsDNA antibody assays may have been designed without implementing this knowledge, but rather with a general view that anti-dsDNA is a homogeneous antibody population. Therefore, in order to analyse this, diverse assay principles are included in this study (table 1). These assays are partly claimed to detect antibodies of low versus high avidity, and those recognised in the CLIFT assays are reported to recognise highly bent structures on DNA, that are disclosed from the general B helical DNA used in some of the ELISA assays. Consistent with this, the kinetoplast DNA has one of the greatest known degrees of stable curvature.41 42 Thus, the assays may disclose antibody binding to DNA structures that are only formed by strong deformations from the more common linear B helical DNA structure. Antibodies recognising the kinetoplast DNA of the haemoflagellate Crithidia luciliae may specifically bind unique structures shared by nucleosomes.41 42 This stringent antibody specificity may well reflect structures on eukaryotic nucleosomal DNA that is believed to induce such immune responses in vivo.
We analysed anti-dsDNA antibodies by a variety of anti-dsDNA tests commonly used in clinical practice. CLIFT was performed on all sera at all three laboratories. Each laboratory also performed additional anti-dsDNA antibody assays on all patient sera, depending on their interests and expertise. This approach uncovered a high degree of variation in the reporting of presence of anti-dsDNA between the individual assays. Even when using a common antigen source (CLIFT) in all laboratories, a high degree of variation in the assay results was demonstrated. It cannot be regarded as acceptable, and it supports the need for further international efforts in assay standardisation, and a broad discussion of what we want to analyse. Do we want to detect as many antibodies as possible, or only those with stringent characteristics, like high avidity and strong correlation with organ manifestations?
The present study shows a high degree of variation in the associations of anti-dsDNA antibodies with clinical and biochemical manifestations, which is in line with previous observations.1 43 44 We do confirm also that immunoassays had higher rates of positivity than CLIFT, as previously reported.13 45
Anti-dsDNA antibodies do not represent a well-defined autoantibody entity. Given the multiple and diverse mechanisms for their production, it is less obvious that anti-dsDNA antibodies per se are associated with all aspects of the broad syndrome constituting SLE.
As others and we have observed, individuals may produce anti-dsDNA antibodies without having organ manifestations, like, for example, nephritis or dermatitis. It has been known for decades that not all anti-dsDNA antibodies are pathogenic.
A renal target for potentially nephritogenic anti-dsDNA antibodies has been demonstrated to be extracellular, poorly degraded chromatin fragments in both murine30 46 47 and human48 lupus nephritis. Antibodies that bind exclusively in ELISA recognise B helical DNA, but not the highly bent structure in the kinetoplast or in the chromatin fragments.41 42 Thus, what makes anti-dsDNA antibodies pathogenic is their potential to bind chromatin structures, as reflected in, for example, the CLIFT assay, and not just because they bind any DNA structure. This basic information should encourage us to consider this as a problem, and to develop a new consensus with respect to how we should test for anti-dsDNA antibodies.
In an effort to clinically identify the pathogenic potential of the various anti-dsDNA antibodies measured in this study, we have correlated these findings to clinical and biochemical features of patients referred to our clinics for further evaluation of rheumatic disease. The data support the notion that various anti-dsDNA antibodies impact differently on the classification of SLE.
We focused on whether any of the anti-dsDNA tests were discriminatory towards manifestations included in the current ACR classification criteria.5 Anti-dsDNA antibodies detected by CLIFT are associated with a clinical picture characterised by proteinuria, haematuria, pleuritis and leukopenia. When the presence of anti-dsDNA antibodies is confirmed by any immunoassay, then the prevalence of malar rash, cutaneous vasculitis, alopecia, lymphopenia and non-haemolytic anaemia seems to increase.
Arthralgia, cutaneous vasculitis, morning stiffness and alopecia are variously associated with the anti-dsDNA tests. Except for alopecia, however, these associations are not strong, and these findings may be spurious. Our results may indicate the existence of a cluster of patients with anti-dsDNA antibodies and diffuse non-scarring alopecia. It supports the recent reinstatement of non-scarring alopecia as a clinical criterion in the SLICC classification criteria of SLE.8
With regard to the immunological ACR classification criterion of SLE ‘antibody to native DNA in an abnormal titre’,5 this study has demonstrated an up to twofold difference in prevalence of positivity, and considerable variation in the clinical association profile between the anti-dsDNA tests performed. This may relate to varying test properties with regard to avidity and structural antibody specificity.13 Immunoassays are often used to make a preliminary screening of anti-dsDNA antibodies. Positive results are then verified by other more specific assays, such as CLIFT. In our investigation, the positivity of ELISA combined with CLIFT negativity was found in 65 patients, whereof 13 were diagnosed with SLE. A moderately increased prevalence of tendinitis, fibromyalgia, xeroftalmia, xerostomia, oral and nasal ulcers was observed, a clinical picture often found in patients affected by Sjögren’s Syndrome. However, these and other parallel findings49–54 indicate that currently the anti-dsDNA antibodies cannot be considered precise enough as a classification criterion. Low specificity of ELISA testing for anti-dsDNA antibodies has in newly proposed classification criteria been offset by raising the cut-off to twice above lower laboratory reference range.8 Whether this is sufficient to generate a valid, clinically relevant anti-dsDNA antibody test situation has not yet been validated. To this end, it is noteworthy that even the CLIFT test, which according to the present results is the anti-dsDNA assay best correlated with a typical nephritic SLE phenotype, had only low diagnostic value in the diagnosis of SLE in this population of patients with recent onset of rheumatic symptoms.55
When analysing the ANA-positive subset of the patients, the CLIFT association with clinical phenotypes consisting of proteinuria, haematuria, leukopenia, thrombocytopenia, cutaneous vasculitis and alopecia persisted. On the other hand, pleuritis and proteinuria defined the clinical phenotypes associated with any of the anti-dsDNA tests in ANA-negative patients. These observations suggest that the presence of anti-dsDNA antibodies in ANA-negative patients may be of less clinical significance, supporting the view that testing for antibodies to dsDNA is not indicated in ANA-negative sera.56 Detection in ANA-negative patients of anti-dsDNA antibodies with low avidity may, in a worst case scenario, lead to incorrect diagnosis and classification.
The purpose of this study was to reach more insight into the linkage between anti-dsDNA antibody detection and clinical and laboratory manifestations without the restriction posed by existing SLE classification criteria. Our findings provide clinical support for a diagnostic role of CLIFT-determined anti-dsDNA antibodies, however, only in a limited number of key SLE manifestations, proteinuria in particular. These findings challenge the broad role of ELISA-based anti-dsDNA antibody testing in diagnosis and classification of SLE as presently defined.
Supplementary Material
Footnotes
Contributors: All the authors contributed to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work. All the authors contributed to drafting the work or revising it critically for important intellectual content. All the authors approved the final version to be published. All the authors achieved agreement for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: Supported by academic research funding from the participating centres, the Danish Rheumatism Association (SJ), the Novo Nordisk Research Foundation (SJ), Foundation for Health and Rehabilitation through the Norwegian Rheumatology Organisation (project 2004/2/0250), Northern Norway Regional Health Authority Medical Research Program (Grants # SFP-100-04, SFP-101-04), University of Tromsø as a Milieu support (OPR), Swedish Rheumatic Patients’ Association (AAB), The Foundation of King Gustaf V 80-year fund (AAB).
Competing interests: None.
Ethics approval: Tromsø, Norway: Regional Committees for Medical and Health Research Ethics - REC North, project no. P Rek Nord 03/2004; Lund, Sweden: Research Ethics Committee, Medical Faculty, Lund University, project no. LU 30-03 (LU-P12-03); Copenhagen, Denmark: Research Ethics Committees for Copenhagen and Frederiksberg, project no. (KF) 01-024/03.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Isenberg DA, Ehrenstein MR, Longhurst C, et al. The origin, sequence, structure, and consequences of developing anti-DNA antibodies. A human perspective . Arthritis Rheum 1994;37:169–80. [DOI] [PubMed] [Google Scholar]
- 2.Khalil M, Spatz L, Diamond B. Anti-DNA antibodies. In: Lahita R. ed. Systemic lupus erythematosus. 3rd edn. Academic Press, 1999:197–217. [Google Scholar]
- 3.van Bruggen MC, Walgreen B, Rijke TP, et al. Antigen specificity of anti-nuclear antibodies complexed to nucleosomes determines glomerular basement membrane binding in vivo. Eur J Immunol. 1997;27:1564–9. [DOI] [PubMed] [Google Scholar]
- 4.van Bavel CC, Fenton KA, Rekvig OP, et al. Glomerular targets of nephritogenic autoantibodies in systemic lupus erythematosus. Arthritis Rheum 2008;58:1892–9. [DOI] [PubMed] [Google Scholar]
- 5.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 6.Hahn BH. Antibodies to DNA. N Engl J Med 1998;338:1359–68. [DOI] [PubMed] [Google Scholar]
- 7.Foster MH. T cells and B cells in lupus nephritis. Semin Nephrol 2007;27:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenton K, Fismen S, Hedberg A, et al. Anti-dsDNA antibodies promote initiation, and acquired loss of renal Dnase1 promotes progression of lupus nephritis in autoimmune (NZBxNZW)F1 mice. PLoS One 2009;4:e8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fismen S, Hedberg A, Fenton KA, et al. Circulating chromatin-anti-chromatin antibody complexes bind with high affinity to dermo-epidermal structures in murine and human lupus nephritis. Lupus 2009;18:597–607. [DOI] [PubMed] [Google Scholar]
- 11.Grootscholten C, van Bruggen MC, van der Pijl JW, et al. Deposition of nucleosomal antigens (histones and DNA) in the epidermal basement membrane in human lupus nephritis. Arthritis Rheum 2003;48:1355–62. [DOI] [PubMed] [Google Scholar]
- 12.Huerta PT, Kowal C, DeGiorgio LA, et al. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci USA 2006;103:678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haugbro K, Nossent JC, Winkler T, et al. Anti-dsDNA antibodies and disease classification in antinuclear antibody positive patients: the role of analytical diversity. Ann Rheum Dis 2004;63:386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang YJ, Stollar BD. Anti-DNA antibodies: aspects of structure and pathogenicity. Cell Mol Life Sci 2003;60:309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalsi JK, Martin AC, Hirabayashi Y, et al. Functional and modelling studies of the binding of human monoclonal anti-DNA antibodies to DNA. Mol Immunol 1996;33:471–83. [DOI] [PubMed] [Google Scholar]
- 16.Stollar BD. Immunochemistry of DNA. Int Rev Immunol 1989;5:1–22. [DOI] [PubMed] [Google Scholar]
- 17.Berden JH. Lupus nephritis: consequence of disturbed removal of apoptotic cells? Neth J Med 2003;61:233–8. [PubMed] [Google Scholar]
- 18.Fismen S, Mortensen ES, Rekvig OP. Nuclease deficiencies promote end-stage lupus nephritis but not nephritogenic autoimmunity in (NZB x NZW) F1 mice. Immunol Cell Biol 2011;89:90–9. [DOI] [PubMed] [Google Scholar]
- 19.Kruse K, Janko C, Urbonaviciute V, et al. Inefficient clearance of dying cells in patients with SLE: anti-dsDNA autoantibodies, MFG-E8, HMGB-1 and other players. Apoptosis 2010;15:1098–113. [DOI] [PubMed] [Google Scholar]
- 20.Munoz LE, Janko C, Schulze C, et al. Autoimmunity and chronic inflammation—two clearance-related steps in the etiopathogenesis of SLE. Autoimmun Rev 2010;10:38–42. [DOI] [PubMed] [Google Scholar]
- 21.Napirei M, Karsunky H, Zevnik B, et al. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet 2000;25:177–81. [DOI] [PubMed] [Google Scholar]
- 22.Urbonaviciute V, Furnrohr BG, Meister S, et al. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med 2008;205:3007–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Ghelue M, Moens U, Bendiksen S, et al. Autoimmunity to nucleosomes related to viral infection: a focus on hapten-carrier complex formation. J Autoimmun 2003;20:171–82. [DOI] [PubMed] [Google Scholar]
- 24.Rekvig OP. Anti-dsDNA antibodies as a classification criterion and a diagnostic marker for SLE: critical remarks. Clin Exp Immunol 2014. doi:10.1111/cei.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mostoslavsky G, Fischel R, Yachimovich N, et al. Lupus anti-DNA autoantibodies cross-react with a glomerular structural protein: a case for tissue injury by molecular mimicry. Eur J Immunol 2001;31:1221–7. [DOI] [PubMed] [Google Scholar]
- 26.Amital H, Heilweil M, Ulmansky R, et al. Treatment with a laminin-derived peptide suppresses lupus nephritis. J Immunol 2005;175:5516–23. [DOI] [PubMed] [Google Scholar]
- 27.Deocharan B, Qing X, Lichauco J, et al. Alpha-actinin is a cross-reactive renal target for pathogenic anti-DNA antibodies. J Immunol 2002;168:3072–8. [DOI] [PubMed] [Google Scholar]
- 28.Mageed RA, Zack DJ. Cross-reactivity and pathogenicity of anti-DNA autoantibodies in systemic lupus erythematosus. Lupus 2002;11:783–6. [DOI] [PubMed] [Google Scholar]
- 29.Sabbaga J, Line SR, Potocnjak P, et al. A murine nephritogenic monoclonal anti-DNA autoantibody binds directly to mouse laminin, the major non-collagenous protein component of the glomerular basement membrane. Eur J Immunol 1989;19:137–43. [DOI] [PubMed] [Google Scholar]
- 30.Kalaaji M, Mortensen E, Jorgensen L, et al. Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells. Am J Pathol 2006;168:1779–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lefkowith JB, Gilkeson GS. Nephritogenic autoantibodies in lupus: current concepts and continuing controversies. Arthritis Rheum 1996;39:894–903. [DOI] [PubMed] [Google Scholar]
- 32.Mjelle JE, Rekvig OP, Fenton KA. Nucleosomes possess a high affinity for glomerular laminin and collagen IV and bind nephritogenic antibodies in murine lupus-like nephritis. Ann Rheum Dis 2007;66:1661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortensen ES, Fenton KA, Rekvig OP. Lupus nephritis: the central role of nucleosomes revealed. Am J Pathol 2008;172:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mortensen ES, Rekvig OP. Nephritogenic potential of anti-DNA antibodies against necrotic nucleosomes. J Am Soc Nephrol 2009;20:696–704. [DOI] [PubMed] [Google Scholar]
- 35.Seredkina N, Rekvig OP. Acquired loss of renal nuclease activity is restricted to DNaseI and is an organ-selective feature in murine lupus nephritis. Am J Pathol 2011;179:1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seredkina N, Zykova SN, Rekvig OP. Progression of murine lupus nephritis is linked to acquired renal Dnase1 deficiency and not to up-regulated apoptosis. Am J Pathol 2009;175:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Bruggen MC, Kramers C, Berden JH. Autoimmunity against nucleosomes and lupus nephritis. Ann Med Interne (Paris) 1996;147:485–9. [PubMed] [Google Scholar]
- 38.van Bruggen MC, Kramers C, Hylkema MN, et al. Pathophysiology of lupus nephritis: the role of nucleosomes. Neth J Med 1994;45:273–9. [PubMed] [Google Scholar]
- 39.Radic MZ, Seal SN. Selection of recurrent V genes and somatic mutations in autoantibodies to DNA. Methods 1997;11:20–6. [DOI] [PubMed] [Google Scholar]
- 40.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 41.Griffith J, Bleyman M, Rauch CA, et al. Visualization of the bent helix in kinetoplast DNA by electron microscopy. Cell 1986;46:717–24. [DOI] [PubMed] [Google Scholar]
- 42.Hirota Y, Ohyama T. Adjacent upstream superhelical writhe influences an Escherichia coli promoter as measured by in vivo strength and in vitro open complex formation. J Mol Biol 1995;254:566–78. [DOI] [PubMed] [Google Scholar]
- 43.Isenberg DA, Dudeney C, Williams W, et al. Measurement of anti-DNA antibodies: a reappraisal using five different methods. Ann Rheum Dis 1987;46:448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan EM, Smolen JS, McDougal JS, et al. A critical evaluation of enzyme immunoassays for detection of antinuclear autoantibodies of defined specificities. I. Precision, sensitivity, and specificity. Arthritis Rheum 1999;42:455–64. [DOI] [PubMed] [Google Scholar]
- 45.Werle E, Blazek M, Fiehn W. The clinical significance of measuring different anti-dsDNA antibodies by using the Farr assay, an enzyme immunoassay and a Crithidia luciliae immunofluorescence test. Lupus 1992;1:369–77. [DOI] [PubMed] [Google Scholar]
- 46.Kalaaji M, Sturfelt G, Mjelle JE, et al. Critical comparative analyses of anti-alpha-actinin and glomerulus-bound antibodies in human and murine lupus nephritis. Arthritis Rheum 2006;54:914–26. [DOI] [PubMed] [Google Scholar]
- 47.Zykova SN, Seredkina N, Benjaminsen J, et al. Reduced fragmentation of apoptotic chromatin is associated with nephritis in lupus-prone (NZB x NZW)F(1) mice. Arthritis Rheum 2008;58:813–25. [DOI] [PubMed] [Google Scholar]
- 48.Kalaaji M, Fenton KA, Mortensen ES, et al. Glomerular apoptotic nucleosomes are central target structures for nephritogenic antibodies in human SLE nephritis. Kidney Int 2007;71:664–72. [DOI] [PubMed] [Google Scholar]
- 49.Antico A, Platzgummer S, Bassetti D, et al. Diagnosing systemic lupus erythematosus: new-generation immunoassays for measurement of anti-dsDNA antibodies are an effective alternative to the Farr technique and the Crithidia luciliae immunofluorescence test. Lupus 2010;19:906–12. [DOI] [PubMed] [Google Scholar]
- 50.Conrad K, Ittenson A, Reinhold D, et al. High sensitive detection of double-stranded DNA autoantibodies by a modified Crithidia luciliae immunofluorescence test. Ann N Y Acad Sci 2009;1173: 180–5. [DOI] [PubMed] [Google Scholar]
- 51.Kim KH, Han JY, Kim JM, et al. Clinical significance of ELISA positive and immunofluorescence negative anti-dsDNA antibody. Clin Chim Acta 2007;380:182–5. [DOI] [PubMed] [Google Scholar]
- 52.Neogi T, Gladman DD, Ibanez D, et al. Anti-dsDNA antibody testing by Farr and ELISA techniques is not equivalent. J Rheumatol 2006;33:1785–8. [PubMed] [Google Scholar]
- 53.Roggenbuck D, Conrad K, Reinhold D. High sensitive detection of double-stranded DNA antibodies by a modified Crithidia luciliae immunofluorescence test may improve diagnosis of systemic lupus erythematosus. Clin Chim Acta 2010;411:1837–8. [DOI] [PubMed] [Google Scholar]
- 54.Tzioufas AG, Terzoglou C, Stavropoulos ED, et al. Determination of anti-ds-DNA antibodies by three different methods: comparison of sensitivity, specificity and correlation with lupus activity index (LAI). Clin Rheumatol 1990;9:186–92. [DOI] [PubMed] [Google Scholar]
- 55.Compagno M, Jacobsen S, Rekvig OP, et al. Low diagnostic and predictive value of anti-dsDNA antibodies in unselected patients with recent onset of rheumatic symptoms: results from a long-term follow-up Scandinavian multicentre study. Scand J Rheumatol 2013;42:311–16. [DOI] [PubMed] [Google Scholar]
- 56.Manoussakis MN, Garalea KL, Tzioufas AG, et al. Testing for antibodies to ENA and to dsDNA is not indicated in FANA-negative sera. Clin Rheumatol 1988;7:465–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


