Abstract
Objective
To compare the performance characteristics of cell-bound complement (C4d) activation products (CBCAPS) on erythrocyte (EC4d) and B cells (BC4d) with antibodies to double-stranded DNA (anti-dsDNA) and complement C3 and C4 in systemic lupus erythematosus (SLE).
Methods
The study enrolled 794 subjects consisting of 304 SLE and a control group consisting of 285 patients with other rheumatic diseases and 205 normal individuals. Anti-dsDNA and other autoantibodies were measured using solid-phase immunoassays while EC4d and BC4d were determined using flow cytometry. Complement proteins were determined using immunoturbidimetry. Disease activity in SLE was determined using a non-serological Systemic Lupus Erythematosus Disease Activity Index SELENA Modification. A two-tiered methodology combining CBCAPS with autoantibodies to cellular and citrullinated antigens was also developed. Statistical analyses used area under receiver operating characteristic curves and calculations of area under the curve (AUC), sensitivity and specificity.
Results
AUC for EC4d (0.82±0.02) and BC4d (0.84±0.02) was higher than those yielded by C3 (0.73±0.02) and C4 (0.72±0.02) (p<0.01). AUC for CBCAPS was also higher than the AUC yielded by anti-dsDNA (0.79±0.02), but significance was only achieved for BC4d (p<0.01). The combination of EC4d and BC4d in multivariate testing methodology with anti-dsDNA and autoantibodies to cellular and citrullinated antigens yielded 80% sensitivity for SLE and specificity ranging from 70% (Sjogren's syndrome) to 92% (rheumatoid arthritis) (98% vs. normal). A higher proportion of patients with SLE with higher levels of disease activity tested positive for elevated CBCAPS, reduced complement and anti-dsDNA (p<0.03).
Conclusions
CBCAPS have higher sensitivity than standard complement and anti-dsDNA measurements, and may help with the differential diagnosis of SLE in combination with other autoantibodies.
Keywords: Autoimmune Diseases, Systemic Lupus Erythematosus, Autoantibodies
Key messages.
CBCAPS have higher sensitivity for SLE than standard complement measures.
CBCAPS in multivariate assay panel yield high sensitivity and specificity for SLE.
Introduction
The diagnosis of systemic lupus erythematosus (SLE) remains challenging partly because of the heterogeneity of the disease, its evolutive nature and also because there are significant limitations with currently available diagnostic immunology tests.1 2 In addition, SLE can be associated with irreversible and unpredictable organ damage, and the disease results in substantial economic burden to the patient and healthcare system.3 4 Therefore, an accurate diagnosis is critical. Currently, the diagnosis of SLE rests on a combination of clinical features (history and physical examination), other tests and immunological testing along with classification criteria.5 6
Among the immunological laboratory measurements performed routinely, the detection of antinuclear antibodies (ANA) is pivotal for screening purposes, owing to its high sensitivity. However, the ANA test is imperfect with a 10%–25% false positive rate among healthy individuals.7 8 More specific immunological tests such as antibodies to double-stranded DNA (anti-dsDNA) and/or anti-Smith (anti-Sm) are also useful in diagnosing SLE, but these markers lack sensitivity.9 Other autoantibodies to cellular antigens including antibodies to extractable nuclear antigens (ENA: Sjogren's syndrome (SS)-A/Ro, SS-B/La, Centromere (CENP), Jo-1 U1RNP, scleroderma (Scl)-70) are also routinely measured in the clinical immunology laboratory, and while of value, none of these markers have sufficient predictive value on their own to differentiate SLE from other connective tissue diseases.9 Since patients with SLE often share many clinical features with other connective tissue diseases, the potential for misdiagnosis is not insignificant. It follows that the availability of more sensitive and specific diagnostic tests is highly desirable.
Many patients with SLE experience activation of the classical complement pathway, resulting in reduced complement levels and formation of complement C4 activation products (CBCAPS) that are stably deposited on various cell membranes including erythrocytes (EC4d) and B-lymphocytes (BC4d).10–12 In particular, the deposition of C4d on erythrocytes has a significant impact on erythrocyte membrane deformability,13 thereby potentially impairing the ability of red blood cells to deliver oxygen to tissues. These CBCAPS were initially reported as valuable in SLE diagnostics,11 and their performance characteristics were recently validated in a prospective multicentre study.10 In the present study, we sought to compare the sensitivity of CBCAPS with reduced complement C3/C4 levels as these markers are often used to support SLE diagnosis and are now part of the new Systemic Lupus International Collaborating Clinics (SLICC) criteria for classifying SLE.14 We also compared the performances of CBCAPS with anti-dsDNA antibody levels and developed a multivariate diagnostic methodology combining these markers with other autoantibodies to cellular and citrullinated antigens. Finally, in this large cross-sectional study, the relationship between CBCAPS, standard complement measurements and anti-dsDNA antibody levels and disease activity was evaluated.
Methods
This study was multicentred and enrolled adult patients with SLE who fulfilled the 1982 American College of Rheumatology (ACR) classification criteria for SLE,5 6 patients with other rheumatic diseases and normal healthy volunteers. Methods have been described in detail elsewhere.10 Two cohorts were defined. The first cohort (cohort 1) consisted of 593 subjects previously characterised and enrolled from April to August 2010 (210 SLE, 120 rheumatoid arthritis (RA), 21 Scl, 9 SS, 16 polymyositis/dermatomyositis (PM/DM), 12 other rheumatic diseases and 205 normal healthy volunteers).10 The second cohort (cohort 2) consisted of 201 subjects (94 SLE, 41 RA, 14 Scl, 24 SS, 11 PM/DM and 17 other diseases) enrolled from June 2011 to September 2013. The study was approved by internal review boards at each participating site, and all subjects provided informed consent. All autoantibodies, complement C3/C4 and CBCAPS determinations were performed in our centralised clinical laboratory. ANA, anti-dsDNA and anti-mutated citrullinated vimentin (anti-MCV) were determined using validated ELISA, while CBCAPS (EC4d and BC4d expressed as net mean fluorescence intensity (MFI)) were determined using flow cytometry.10 All analytes measured were stable during transportation at least 48 h post-phlebotomy. All anti-dsDNA-positive results (as per manufacturer cut-off) detected by ELISA were confirmed using the Crithidia Luciliae immunofluorescence assay (Inova Diagnostics, San Diego, California, USA).15 Antibodies to ENA (SS-A/Ro, SS-B/La, CENP, Scl-70, Jo-1, anti-Sm, U1RNP) were measured using fluorescent-enzyme immunoassays (ThermoFisher, Uppsala, Sweden). Cut-offs recommended by the manufacturer were used for all autoantibodies. Complement C3 and C4 levels were determined using immunoturbidimetry (The Binding Site, San Diego, California, USA), and low complement cut-offs for C3 or C4 were set at a concentration lower than the 95th centile of normal healthy subjects. Among patients with SLE, disease activity was determined using the Systemic Lupus Erythematosus Disease Activity Index SELENA Modification (SELENA-SLEDAI)16 subscore (without the immunology components (i.e. low complement and anti-dsDNA reactivity—modified SELENA-SLEDAI)).
Statistical analysis was conducted using the R software V.2.15. Statistical analyses used area under receiver operating characteristic (ROC) curves and calculations of area under the curve (AUC), sensitivity and specificity (SLE vs. rheumatic disease controls and normal healthy volunteers). A two consecutive tier methodology was used to develop the multivariate assay panel10 in which AUC calculation included both tiers, with tier 1-positive samples given an index value consistent with a logistic function of 0.99. The reported performance statistics (sensitivity, specificity) were calculated using leave-one-out cross-validation. Correlation between variables was evaluated using non-parametric Spearman's rank tests while differences between groups were evaluated using Kruskal–Wallis test. Differences in sensitivity and specificity between the two cohorts of subjects were evaluated using χ2 test.
Results
Patient characteristics
Characteristics for the 794 subjects enrolled are presented in table 1 and consisted of 304 SLE, 161 RA, 33 SS, 35 Scl, 27 PM/DM, 29 patients with other rheumatic diseases and 205 normal healthy volunteers. As expected, the ANA test (≥20 units) was a sensitive (89%) yet non-specific marker in distinguishing SLE from other rheumatic diseases (53%). Conversely, anti-dsDNA and anti-Sm were less sensitive (33% and 14%, respectively) but highly specific for SLE (>95%; at manufacturer cut-offs). Increased titres for anti-MCV (>70 units), SS-B/La (>10 units), Scl-70 (>10 units), CENP (>10 units) and Jo-1 (>10 units) were specific in distinguishing SLE from RA, SS, Scl and PM/DM subjects, respectively (table 1). Among subjects with rheumatic diseases other than SLE, 40% tested positive for one or more of these 5 specific antibodies (anti-MCV, SS-B/La, CENP, Scl-70 and Jo-1). EC4d and BC4d levels were several fold higher in SLE than other rheumatic diseases.
Table 1.
Characteristics of 794 subjects and single marker assay results
| SLE | RA | SS | Scl | PM/DM | Others | NHV | |
|---|---|---|---|---|---|---|---|
| Age (years) | 41±1 | 59±1 | 54±2 | 51±2 | 56±2 | 53±3 | 41±1 |
| Gender (% female) | 91 | 85 | 91 | 80 | 81 | 83 | 65 |
| Ethnicity Caucasians (%) | 41 | 61 | 73 | 74 | 63 | 48 | 56 |
| African–Americans (%) | 30 | 15 | 18 | 14 | 26 | 10 | 35 |
| Asians (%) | 8 | 4 | 0 | 0 | 0 | 10 | 2 |
| Hispanics (%) | 20 | 19 | 9 | 9 | 11 | 31 | 6 |
| Other (%) | 2 | 2 | 0 | 3 | 0 | 0 | 1 |
| Duration of disease (years) | 11±1 | 12±1 | 10±3 | 8±1 | 3±1 | 6±1 | NA |
| ANA ≥20 units (%) | 89 | 32 | 89 | 66 | 74 | 31 | 10 |
| dsDNA >301 units (%) | 33 | 4 | 0 | 6 | 0 | 7 | 0 |
| Anti-Sm >10 units (%) | 14 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anti-MCV >70 units (%) | 3 | 47 | 9 | 6 | 4 | 3 | 0 |
| Jo1 >10 units (%) | 0 | 0 | 0 | 0 | 15 | 0 | 0 |
| Scl-70 >10 units (%) | 0 | 0 | 3 | 20 | 0 | 0 | 0 |
| SS-A/Ro >10 units (%) | 39 | 10 | 79 | 11 | 26 | 3 | 1 |
| SS-B/La >10 units (%) | 9 | 1 | 39 | 3 | 0 | 0 | 0 |
| CENP >10 units (%) | 2 | 3 | 3 | 17 | 0 | 0 | 1 |
| Reduced C3 (%) | 33 | 4 | 6 | 12 | 0 | 0 | 6 |
| Reduced C4 (%) | 32 | 7 | 3 | 0 | 4 | 4 | 4 |
| Reduced C3 or C4 (%) | 45 | 10 | 9 | 12 | 4 | 4 | 8 |
| U1 RNP >10 units (%) | 27 | 0 | 3 | 6 | 0 | 10 | 0 |
| EC4d net MFI | 21±3 | 7±1 | 10±2 | 7±1 | 8±1 | 8±1 | 5±1 |
| >14 units (%) | 46 | 5 | 12 | 11 | 10 | 1 | |
| >75 units (%) | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| BC4d net MFI | 106±6 | 32±2 | 26±4 | 32±5 | 27±4 | 46±19 | 23±1 |
| >60 units (%) | 53 | 9 | 9 | 6 | 4 | 10 | 1 |
| >200 units (%) | 14 | 0 | 0 | 0 | 0 | 3 | 0 |
| Antibody specificity comp. (%) | 12 | 48 | 52 | 46 | 15 | 3 | 1 |
Age, EC4d, BC4d and disease duration are expressed as average±SEM. The group of subjects with other rheumatic diseases included granulomatosis with polyangiitis (5 patients), fibromyalgia (13 patients), vasculitis (10 patients) and antiphospholipid syndrome (1 patient)). The antibody specificity component corresponds to positivity to either anti-MCV (>70 units), SS-B/La (>10 units), Jo-1 (>10 units), Scl-70 (>10 units) or CENP (>10 units), and was used to calculate the index score (tier 2).
ANA, antinuclear antibodies; anti-Sm, anti-Smith; dsDNA, double-stranded DNA; MFI, mean fluorescence intensity; NA, not applicable; NHV, normal healthy volunteers; PM/DM, polymyositis/dermatomyositis; RA, rheumatoid arthritis; Scl, scleroderma; SLE, systemic lupus erythematosus; SS, Sjogren's syndrome; anti-MCV, anti-mutated citrullinated vimentin; EC4d, complement C4d levels on erythrocytes; BC4d, complement C4d levels on cells; CENP, Centromere extractable nuclear antigen.
CBCAPS have higher sensitivity than low complement C3/C4 and anti-dsDNA
The performance characteristics of EC4d and BC4d were compared with those of reduced complement (C3/C4) and anti-dsDNA in a total of 764 subjects (288 SLE and 476 non-SLE (274 other rheumatic diseases and 202 healthy subjects); serum was not available 30 subjects). Lower complement C4 levels were associated with higher EC4d levels (R=−0.249; p<0.001) and BC4d levels (R=−0.343; p<0.001). Similarly, lower complement C3 levels correlated with higher CBCAPS (EC4d: −0.248; BC4d: −0.314; p<0.001). Area under the ROC curves for EC4d (0.82±0.02 (SEM)) and BC4d (0.84±0.02) was higher than those yielded by C3 (0.73±0.02) and C4 (0.72±0.02) (p<0.001) (figure 1). Area under the ROC curves for CBCAPS was also higher than the AUC yielded by anti-dsDNA (0.79±0.02), but significance was achieved for BC4d (p=0.009) and not EC4d (p=0.108). The combination of reduced complement proteins (C3 or reduced C4, each below their respective cut-offs yielding 95% specificity) was 91% specific against the control group, and sensitivity was 44%. At that specificity, anti-dsDNA was 42% sensitive while elevated CBCAPS (EC4d or BC4d each above their respective cut-offs yielding 95% specificity) was 66% sensitive.
Figure 1.
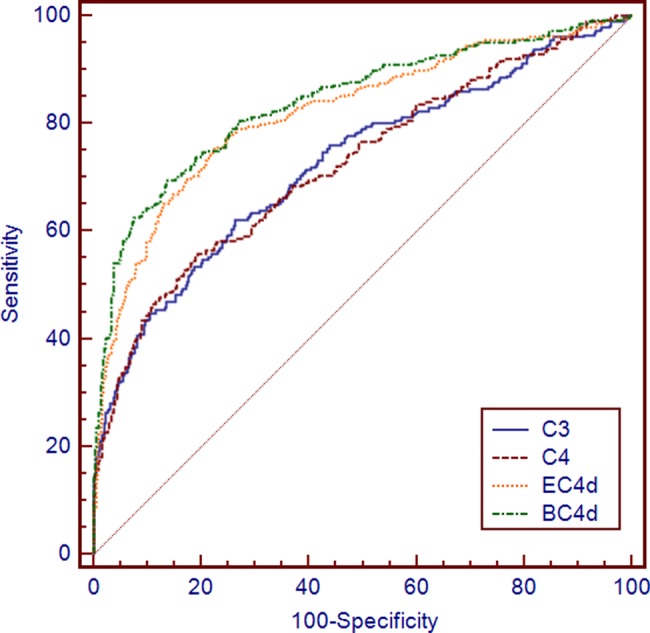
Receiver operating curve of soluble C3, C4, antibodies to double-stranded DNA (anti-dsDNA) compared with complement C4d levels on erythrocytes (EC4d) and on B cells (BC4d) in systemic lupus erythematosus (SLE) (n=288) vs. non-SLE (n=476). Area under the receiver operating characteristic curves for EC4d was 0.82±0.02 (SEM)), 0.84±0.02 for BC4d, 0.73±0.02 for C3, 0.72±0.02 for C4d and 0.79±0.02 for anti-dsDNA.
Performance characteristics of multivariate assay panel using CBCAPS
The multivariate diagnostic methodology involved two consecutive tiers of analysis (figure 2). Tier 1 relied on positivity for anti-dsDNA, anti-Sm or elevated EC4d (>75 net MFI) and BC4d (>200 net MFI). A total of 140 patients with SLE (46%), 9 patients with other diseases (3%) and 1 normal healthy subject (<1%) were positive for any one of the tier 1 markers. Altogether, the specificity (vs. other rheumatic diseases) for tier 1 markers was 96% for anti-dsDNA and >99% for EC4d (>75 net NFI), BC4d (>200 net MFI) and anti-Sm. Tier 2 and the index score were determined among subjects negative in tier 1 (644 subjects including 164 SLE, 276 other diseases and 204 normal healthy subjects). This index score (output of logistic regression) was calculated by combining an ANA component (using ANA cut-offs at 20 and 60 units), a CBCAPS component (sum of log normalised EC4d and BC4d net MFI) and an antibody specificity component (corresponding to positivity to either anti-MCV, SS-B/La, CENP, Scl-70 or Jo-1). As presented in figure 3, 102 of the 164 patients with SLE analysed in tier 2 (62%) presented with an index score >0 while 62 presented a score <0. Conversely, among the 276 subjects with rheumatic diseases other than SLE, a total of 245 subjects (148 RA, 23 SS, 32 Scl, 20 PM/DM and 22 others) presented with an index score <0, thus yielding a specificity of 89% (245/276). Altogether, when the two tiers were combined, the overall sensitivity for SLE was 80% (242/304) while the overall specificity in distinguishing SLE from other diseases ranged from 70% (23/33 SS) to 92% (148/161 RA) (overall specificity was 86% (245/285)). Specificity in distinguishing SLE from normal subjects was 98% (201/205).
Figure 2.
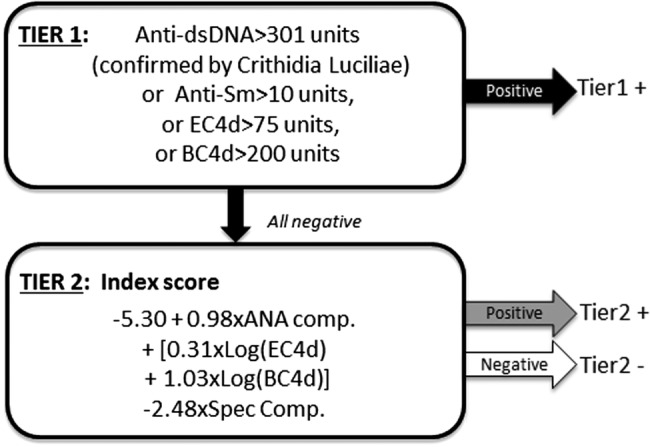
Multivariate assay panel for systemic lupus erythematosus (SLE) diagnosis. Two-tier diagnostic methodology for SLE diagnosis. Positivity in tier 1 consisted of reactivity to antibodies to double-stranded DNA (anti-dsDNA) (confirmed using Crithidia), anti-Smith (anti-Sm) or elevated complement C4d levels on erythrocytes (EC4d) and on B cells (BC4d). The index score (tier 2) was calculated among tier 1-negative subjects. Estimates with intercept from multivariate logistic regression are provided. The antinuclear antibodies component (ANA comp) used two thresholds and was affected with a value of 0 (ANA <20 units), a value of 1 (20≤ ANA <60 units) or a value of 2 (ANA ≥60 units). The complement C4 activation products (CBCAPS) component corresponds to log normalised EC4d plus BC4d values each affected by their coefficient. The antibody specificity component (Spec. Comp) was affected with a value of 0 (anti- mutated citrullinated vimentin (anti-MCV), Sjogren's syndrome (SS)-B, Centromere extractable nuclear antigen (CENP), Jo-1, scleroderma (Scl)-70 all negative) or 1 (either anti-MCV, SS-B, CENP, Jo-1, Scl-70 positive). For example, a tier 1-negative subject presenting with ANA=35 units, EC4d =15 net mean fluorescence intensity (MFI), BC4d=35 net MFI and Scl-70=50 units would present an index score of=−5.30+0.98×1+0.31×log (15)+1.03×log(35)−2.48°1=−2.3.
Figure 3.
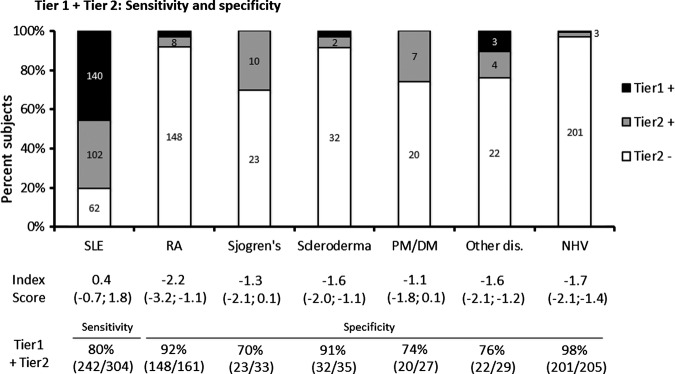
Performance characteristics for multivariate assay SLE panel. The number of subjects in tier 1 and tier 2 (positive and negative) is indicated. Median index score (IQR) in tier 2 is provided. Overall diagnostic sensitivities (sens.) are provided for SLE. Specificities for other rheumatology diseases (spec.) are also given. NHV, normal healthy volunteers; SLE, systemic lupus erythematosus; PM/DM, polymyositis/dermatomyositis; RA, rheumatoid arthritis.
The difference in sensitivity between the two cohorts (cohort 1=81% (170/210) vs. cohort 2=77% (72/94)) was not statistically significant (p=0.473). Also, the difference in specificity in distinguishing SLE from other diseases was not statistically significant between the two cohorts (cohort 1= 87% (155/178) vs. cohort 2=84% (90/107); p=0.602).
Finally, the performance characteristics of this two-tiered multivariate model combining CBCAPS with antibodies to cellular and citrullinated antigens (as presented in figure 2) were compared with those achieved without CBCAPS or without the antibody specificity component. As presented in table 2, the two-tiered model combining ANA, anti-dsDNA and anti-Sm (model #1) resulted in modest performances (AUC=0.781) with overall sensitivity of 89% and specificity of 53% (specificity in distinguishing normal subjects was 90%). However, the addition of CBCAPS (in both tier 1 and tier 2; model #3) significantly augmented the performances of the former model (AUC=0.894; p<0.001). Further enhancement in the specificity in distinguishing SLE from other connective tissue diseases (SS +18%; systemic sclerosis +34%; PM/DM +15% and RA +15%) was obtained with the addition of the antibody specificity component (AUC=0.913; p<0.01; model #4).
Table 2.
Stepwise addition of CBCAPS and antibody components to ANA, anti-dsDNA and anti-Sm serologies
| Model #1 | Model #2: model #1+specificity component | Model #3: model #1+CBCAPS | Model #4: model #1+CBCAPS+specificity component | |
|---|---|---|---|---|
| Tier1 | dsDNA; Sm | dsDNA; Sm | dsDNA; Sm; EC4d; BC4d | dsDNA; Sm; EC4d; BC4d |
| Tier 2 | ANA* | ANA*+Antibody specificity† | ANA*+CBCAPS‡ | ANA*+CBCAPS‡+Antibody specificity† |
| Total sensitivity (%) | 89 | 83 | 84 | 80 |
| Total specificity (others) (%) | 53 | 76 | 70 | 86 |
| Sjogren's syndrome (%) | 12 | 61 | 52 | 70 |
| Scleroderma (%) | 34 | 77 | 57 | 91 |
| PM/DM (%) | 26 | 41 | 59 | 74 |
| RA (%) | 67 | 86 | 77 | 92 |
| Other diseases (%) | 69 | 69 | 76 | 76 |
| Specificity (NHV) (%) | 90 | 91 | 95 | 98 |
| Two-tiered AUC | 0.781 | 0.804 | 0.894 | 0.913§ |
The diagnostic methodology involved two consecutive ‘tiers’ of analysis in which the aggregated diagnostic value of anti-dsDNA, anti-Sm, ANA combined with CBCAPS (EC4d and BC4d) and antibody specificity component (positivity to either anti-MCV, SS-B/La, Jo-1, Scl-70 or CENP) (model #4 as presented figure 1) was compared with model #1.
Model#1 relied on anti-dsDNA (>301 units confirmed by Crithidia) or anti-Sm (>10 units) reactivities in tier 1. Tier 2 was determined among subjects negative in tier 1 and consisted of an index score of the ANA component only (as defined in figure 1 legend); positivity for the index score (>0) was indicative of SLE, and the two-tier combination resulted in the overall performance characteristics (total sensitivity and total specificity). The stepwise addition of CBCAPS (model #3) and the antibody specificity component (model #4) to model #1 maximised the performances characteristics.
Total sensitivity and specificity are SLE vs. all others diseases. The individual sensitivities and specificities are SLE vs. the individual disease.
*ANA component.
†Antibody specificity component.
‡CBCAPS component.
§p<0.01 vs. model 1, 2 and 3.
ANA, antinuclear antibodies; anti-Sm, anti-Smith; AUC, area under the curve; CBCAPS, complement C4 activation products; anti-dsDNA, antibodies to double-stranded DNA; PM/DM, polymyositis/dermatomyositis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; NHV, normal healthy volunteers; anti-MCV, anti-mutated citrullinated vimentin; EC4d, complement C4d levels on erythrocytes; BC4d, complement C4d levels on cells; CENP, Centromere extractable nuclear antigen; Scl, scleroderma; SS, Sjogren's syndrome.
Contribution of disease activity to test sensitivity
The modified SELENA-SLEDAI subscores (non-serological SELENA-SLEDIA) together with anti-dsDNA, low complement C3/C4 and CBCAPS were available in 273 of the 304 SLE subjects (median 1.0; range 0–23). Among patients with a modified SELENA-SLEDAI subscore of 0 (131/273 patients), the sensitivity was 29% for anti-dsDNA, 36% for low complement, 62% for elevated CBCAPS and 77% for the two-tiered methodology (figure 4). Among SLE, those positive in tier 1 presented higher disease activity as assessed using the SELENA-SLEDAI subscore (3.3±0.4 points, average±SEM) than those positive (1.9±0.3 points) or negative in tier 2 (1.4±0.3) (Kruskal–Wallis test: p=0.002). Higher level of disease activity across three SELENA-SLEDAI subscore groups (0, 1–6 and >6) was associated with a higher proportion of patients testing positive for elevated CBCAPS (p=0.027) (χ2 test for homogeneity on a 3×2 contingency table). Similarly, higher level of disease activity was also associated with a higher proportion of patients testing positive for reduced complement (p=0.002) and positive anti-dsDNA (p=0.005). Among patients with SLE with less active disease (modified SELENA-SLEDAI subscore=0), the difference in sensitivity was 26% greater for elevated CBCAPS (62%) than for reduced complement C3 or C4 (36%) (p<0.001). Furthermore, the difference in sensitivity remained higher (17%) for CBCAPS compared with low complement among patients with SLE having a modified SELENA-SLEDAI subscore >6 points but without reaching statistical significance (p=0.21).
Figure 4.
Positivity rate for antibodies to double-stranded DNA (anti-dsDNA), low complement, high complement C4 activation products (CBCAPS) and two-tiered methodology stratified by disease activity score. Disease activity was determined by using the non-serological Systemic Lupus Erythematosus Disease Activity Index SELENA Modification (SELENA-SLEDAI) score (without anti-dsDNA and low complement). Anti-dsDNA positivity cut-off was at 301 units, low complement corresponds to reduced C3 or C4, high CBCAPS corresponds to EC4d>14 net mean fluorescence intensity (MFI) or BC4d>60 net MFI. The number in each of the non-serological SELENA-SLEDAI category is given.
Discussion
Because limitations to currently available immunological tests can result in underdiagnosis of SLE and inappropriate treatment,17 validation of new diagnostic biomarkers is needed. Although the SLE classification criteria established by the ACR and more recently SLICC are being used to diagnose SLE, these instruments were initially developed for clinical research and not as diagnostic criteria. Also, because of the evolution of organ involvement and laboratory abnormalities in SLE, it may take years for any given patient to meet the criteria.18 It follows that improvement in currently available laboratory tests and diagnostic immunology methods to assist clinicians with the diagnosis of the disease is warranted. This study builds upon our initial report that CBCAPs assays add significant value to accurate SLE diagnosis when combined with routinely determined ANA and anti-dsDNA,10 and we now report here that elevated EC4d or BC4d have a 22% higher sensitivity than reduced C3/C4, one of the components of the new SLICC criteria.14 It follows that the determination of CBCAPS could help address some of the limitations of reduced complement C3/C4 levels in the diagnosis of SLE. For example, complement activation and C3/C4 consumption in SLE may be masked by the production of these complement proteins as a function of inflammation while the detection of CBCAPS is de facto associated with previous complement activation.19
The results of this research suggest that the determination of CBCAPS may be another valuable diagnostic biomarker for SLE. However, while greater sensitivity was achieved with CBCAPS than for reduced complement proteins, the overall sensitivity was modest (i.e. 66%), thereby indicating that the addition of other markers would be warranted to achieve improved diagnostic performances in clinical practice. Here, we have integrated antibodies to ENA into our diagnostic methodology and included a second cohort of 201 subjects. Our original two-tiered diagnostic methodology relied on positivity for anti-dsDNA (tier 1) and a weighed score (tier 2) combining ANA titres, EC4d and BC4d with anti-MCV to maximise specificity in distinguishing patients with SLE from patients with RA. In this methodology, tier 1 relied on highly specific markers for SLE and included positivity for anti-dsDNA and anti-Sm (both part of the ACR classification criteria for SLE),5 6 14 with elevated EC4d and BC4d. As expected, the combination of tier 1 markers yielded high specificity (>96%). Tier 2 determination among tier 1-negative subjects consisted of a weighed score comprising an ANA component, a CBCAPS component (EC4d and BC4d densities) minus a specificity component composite of positivity for anti-MCV, SS-B/La, CENP, Scl-70 or Jo-1. The inclusion of SS-B/La maximised the specificity of the method in distinguishing SLE from patients with SS. Similarly, the inclusion of CENP and Scl-70 maximised the specificity in distinguishing SLE from those with Scl, while the inclusion of Jo-1 maximised the specificity for PM/DM subjects (table 2). Moreover, the specificity of the diagnostic methodology in distinguishing SLE from RA was maintained with the addition of anti-MCV. Altogether, the two-tier model achieved a balanced 80% sensitivity for SLE (34% improvement over tier 1 only) with a specificity of 86%. All serological tests that were part of our diagnostic immunology method used widely distributed platforms (e.g. ELISA or fluorescent-enzyme immunoassays) and it is important to keep in mind that the overall performance characteristics of our multivariate method could potentially differ if other platforms such as laser bead immunoassay, chemiluminescence immunoassay or line immunoassays20 were employed. However, there is usually a reasonable concordance between the various methods and reagents offered by various manufacturers.21
We also evaluated whether the combinations of these complementary markers in a multivariate assay panel collectively outperformed the performance characteristics of single markers. Our results indicated that the aggregate value of CBCAPS with serological markers outperformed the best performances achieved by combining the single serological markers together. These data not only illustrate the value of CBCAPS as complementary markers to commonly determined serologies but also the power of combining multianalytes in multivariate assay panels. The major strength of our study was the large number of patients enrolled and the fact that all laboratory analyses were centralised in only one clinical laboratory. However, we acknowledge that additional studies should establish the performance characteristics of our diagnostic methodology in distinguishing SLE from diseases such as antiphospholipid syndrome, primary fibromyalgia syndrome, autoimmune hepatitis, undifferentiated connective tissue diseases and autoimmune thyroiditis. It is also not known whether abnormal CBCAPS selectively indicates activity in a particular organ system (e.g. kidney) and additional studies will be essential to address this point.
The sensitivity of low complement, elevated CBCAPS, anti-dsDNA and our multivariate two-tiered method was compared between patients with various levels of disease activity as determined using the modified SELENA-SLEDAI subscore (without the low complement and anti-dsDNA components). Our analysis revealed that the sensitivity for elevated CBCAPS outperformed low complement among patients with active and inactive disease, and that higher sensitivity was observed using the multivariate panel. Thus, elevated CBCAPS is more likely among patients with active disease, and these data suggest that CBCAPS could help monitor SLE disease activity. Furthermore, the higher sensitivity of CBCAPS compared with reduced complement and anti-dsDNA was particularly significant in SLE having a modified SELENA-SLEDAI score of 0. Thus, CBCAPS may be particularly important for diagnosing SLE in patients having less active disease, such as outpatients with early or mild SLE. Whether the patients having inactive disease and complement activation will become clinically active is not known, and prospective study will help establish whether CBCAPS can predict disease flares.
In conclusion, CBCAPS have a higher diagnostic sensitivity than reduced complement and anti-dsDNA. The assay panel combining CBCAPS with antibodies to cellular and citrullinated antigens is sensitive and specific for SLE and may be clinically useful to help diagnose SLE.
Acknowledgments
We thank all subjects for participating in the study. We also thank Leilani Wolover, Joanne Ligayon, Lori Nacario, Deborah Stimson, Robert Apilado and the clinical coordinators at each of the sites for technical assistance and management of the clinical protocol.
Footnotes
Contributors: All contributors meet the criteria for authorship and approved the final version of the manuscript.
Funding: Exagen Diagnostics.
Competing interests: SM and JA have received research grants and consulting fees from Exagen Diagnostics. Both own patent related to cell bound complement activation products for the diagnosis of SLE. CP, RR-G, JB, RF, PC, AW, KK have received consulting fees and/or research grants from Exagen. TO'M, JC, CI, DB, and TD are employees of Exagen Diagnsotics. AW is a board member of Exagen diagnostics. TD and DB hold patent related to methods to diagnose systemic lupus Erythematosus.
Patient consent: Obtained.
Ethics approval: Institutional IRBs.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bertsias GK, Pamfil C, Fanouriakis A et al. . Diagnostic criteria for systemic lupus erythematosus: has the time come? Nat Rev Rheumatol 2013;9:687–94. [DOI] [PubMed] [Google Scholar]
- 2.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med 2008;358:929–39. [DOI] [PubMed] [Google Scholar]
- 3.Meacock R, Dale N, Harrison MJ. The humanistic and economic burden of systemic lupus erythematosus: a systematic review. Pharmacoeconomics 2013;31:49–61. [DOI] [PubMed] [Google Scholar]
- 4.Gordon C, Isenberg D, Lerstrom K et al. . The substantial burden of systemic lupus erythematosus on the productivity and careers of patients: a European patient-driven online survey. Rheumatology (Oxford) 2013;52:2292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan EM, Cohen AS, Fries JF et al. . The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 6.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 7.Satoh M, Chan EK, Ho LA et al. . Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum 2012;64:2319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meroni PL, Schur PH. ANA screening: an old test with new recommendations. Ann Rheum Dis 2010;69:1420–2. [DOI] [PubMed] [Google Scholar]
- 9.Hanly JG, Thompson K, McCurdy G et al. . Measurement of autoantibodies using multiplex methodology in patients with systemic lupus erythematosus. J Immunol Methods 2010;352:147–52. [DOI] [PubMed] [Google Scholar]
- 10.Kalunian KC, Chatham WW, Massarotti EM et al. . Measurements of cell bound complement activation products enhance diagnostic performance in systemic lupus erythematosus. Arthritis Rheum 2012;64:4040–7. [DOI] [PubMed] [Google Scholar]
- 11.Manzi S, Navratil JS, Ruffing MJ et al. . Measurement of erythrocyte C4d and complement receptor 1 in systemic lupus erythematosus. Arthritis Rheum 2004;50:3596–604. [DOI] [PubMed] [Google Scholar]
- 12.Yang DH, Chang DM, Lai JH et al. . Usefulness of erythrocyte-bound C4d as a biomarker to predict disease activity in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2009;48:1083–7. [DOI] [PubMed] [Google Scholar]
- 13.Ghiran IC, Zeidel ML, Shevkoplyas SS et al. . Systemic lupus erythematosus serum deposits C4d on red blood cells, decreases red blood cell membrane deformability, and promotes nitric oxide production. Arthritis Rheum 2011;63:503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petri M, Orbai AM, Alarcon GS et al. . Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agmon-Levin N, Damoiseaux J, Kallenberg C et al. . International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis 2014;73:17–23. [DOI] [PubMed] [Google Scholar]
- 16.Petri M, Kim MY, Kalunian KC et al. . Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med 2005;353:2550–8. [DOI] [PubMed] [Google Scholar]
- 17.Narain S, Richards HB, Satoh M, et al DIagnostic accuracy for lupus and other systemic autoimmune diseases in the community setting. Arch Intern Med 2004;164:2435–41. [DOI] [PubMed] [Google Scholar]
- 18.Alarcon GS, McGwin G Jr, Roseman JM et al. . Systemic lupus erythematosus in three ethnic groups. XIX. Natural history of the accrual of the American College of Rheumatology criteria prior to the occurrence of criteria diagnosis. Arthritis Rheum 2004;51:609–15. [DOI] [PubMed] [Google Scholar]
- 19.Liu CC, Ahearn JM, Manzi S. Complement as a source of biomarkers in systemic lupus erythematosus: past, present, and future. Curr Rheumatol Rep 2004;6:85–8. [DOI] [PubMed] [Google Scholar]
- 20.Fritzler MJ, Fritzler ML. The emergence of multiplexed technologies as diagnostic platforms in systemic autoimmune diseases. Curr Med Chem 2006;13:2503–12. [DOI] [PubMed] [Google Scholar]
- 21.Hanly JG, Su L, Farewell V et al. . Comparison between multiplex assays for autoantibody detection in systemic lupus erythematosus. J Immunol Methods 2010;358:75–80. [DOI] [PubMed] [Google Scholar]



