Abstract
Objective
Epidemiological associations suggest that vitamin D status may play a role in inflammation and progression of atherosclerosis. Using frozen serum, carotid intima medial thickness (CIMT) measurements and other existing data from the Atherosclerosis Prevention in Pediatric Lupus Erythematosus (APPLE) trial, we assessed interactions between serum 25-hydroxyvitamin D (25(OH)D), atorvastatin randomisation and CIMT progression rate.
Methods
Participants in the 3-year APPLE trial were randomised to placebo or atorvastatin and CIMT progression rate was measured. Baseline frozen serum was used to measure 25(OH)D concentrations. Mixed effect longitudinal models for CIMT progression at 3 years were used to evaluate interaction between vitamin D deficiency (serum 25(OH)D <20 ng/mL) at baseline and atorvastatin or placebo treatment, adjusting for key systemic lupus erythematosus disease variables and cardiovascular risk factors.
Results
201/221 APPLE participants had available samples and were included in this analysis; 61/201 (30%) had vitamin D deficiency at baseline. In adjusted longitudinal modelling, there was significant interaction between baseline vitamin D deficiency and atorvastatin randomisation in 3-year progression of mean-max CIMT. In four out of six carotid segments, there was a greater decrease in mean-max CIMT progression rate in subjects who were treated with atorvastatin compared with placebo if they had baseline serum 25(OH)D levels ≥20 ng/mL.
Conclusions
Subjects with serum 25(OH)D ≥20 ng/mL had less mean-max CIMT progression following 3 years of atorvastatin treatment. Results from secondary analyses must be interpreted cautiously, but findings suggest that underlying vitamin D deficiency may be involved in response to atorvastatin in atherosclerosis prevention.
Trial registration number
Keywords: Systemic Lupus Erythematosus, Childhood/paediatric lupus, Inflammation, Cardiovascular Disease
Key messages.
Vitamin D deficiency at baseline was associated with increased baseline hsCRP levels in children and adolescents with SLE.
No change in longitudinal disease activity measures was seen based on baseline vitamin D status.
Findings suggest that underlying vitamin D deficiency may be involved in response to atorvastatin in atherosclerosis prevention in children and adolescents with SLE.
Introduction
Over the last three decades, systemic lupus erythematosus (SLE)-related mortality has decreased in all areas except cardiovascular disease (CVD).1 Women with SLE who are less than 40 years of age are at a 50-fold increased risk of myocardial infarction compared with control populations.2 This increase in risk cannot be attributed solely to traditional cardiovascular risk factors, and immune and vascular pathology in SLE are postulated to contribute to the increased CVD risk.3 4
Vitamin D deficiency has emerged as a potential risk factor for CVD.5 In epidemiological studies of the general population, lower vitamin D levels have been associated with CVD, hypertension, diabetes, high-density lipoprotein cholesterol and low-density lipoprotein (LDL) cholesterol, and surrogate measurements of cardiovascular risk such as coronary artery calcification and carotid intima medial thickness (CIMT).5 One prospective study found that a serum 25(OH)D <15 ng/mL had a multivariable-adjusted HR of 1.62 (95% CI 1.11 to 2.36, p=0.01) for incident CVD events.6
Vitamin D status is mainly determined by the level of circulating 25-hydroxyvitamin D (25(OH)D), which is converted into an active secosteroid hormone, 1,25-dihydroxyvitamin D (1,25(OH)2D) by the kidney and cells of the immune system. The activated hormone then regulates transcription of several inflammatory cytokines.7 Studies have shown that 25(OH)D deficiency (defined as a serum level <20 ng/mL) is common in SLE and has been associated with increased photosensitivity, fatigue, renal disease, SLE disease activity and proteinuria.8–12 In vitro, 1,25(OH)2D blocks dendritic cell differentiation, lowers interleukin 12 secretion, and modulates T lymphocyte proliferation and function.13–16 Differentiation of dendritic cells and type I interferon are important in the pathogenesis of SLE.17
The Atherosclerosis Prevention in Pediatric Lupus Erythematosus (APPLE) trial was originally designed to prospectively assess the effect of atorvastatin on progression of CIMT in 221 children and young adults (aged 10–21 years) with SLE.18 Subjects were randomised to 36 months of atorvastatin (10–20 mg/day based on weight) versus placebo treatment. Primary results showed overall, no significant difference in mean-mean CIMT progression between treatment and placebo groups.18 Atorvastatin is a hydroxymethyl glutaryl coenzyme A (HMG-coA) reductase inhibitor, which decreases the synthesis of cholesterol and is used in adults with hyperlipidaemia to reduce CVD progression. Cholesterol is one of the precursors of vitamin D. 1,25(OH)2D activates an enzyme of the cytochrome P450 system which metabolises atorvastatin. One study showed differential hypolipaemic response to atorvastatin based on serum 25(OH)D levels.19 Another study found that in dyslipidaemic subjects, the addition of vitamin D to atorvastatin synergistically lowered total cholesterol and LDL cholesterol levels.20 Two large studies in SLE (APPLE and Lupus Atherosclerosis Prevention Study (LAPS)) showed no significant change in CIMT between subjects taking atorvastatin and those taking placebo,18 21 but given the high proportion of patients with SLE with vitamin D deficiency, it is possible that these results may be confounded by vitamin D deficiency. There are no studies that have evaluated the relationship between vitamin D status, inflammation and subclinical vascular disease in paediatric subjects with lupus.
The objective of this subanalysis was to use samples prospectively obtained during participation in the APPLE trial to evaluate the relationship between vitamin D status and atorvastatin treatment on CIMT progression.
Methods
Subjects
Participants in the 3-year APPLE trial were randomised to placebo or atorvastatin in addition to routine care and CIMT progression was measured. The design and methods of the APPLE trial have been reported previously.18 SLE was classified by American College of Rheumatology criteria from 21 North American centres. Subjects were excluded from the study if they had baseline fasting total cholesterol >350 mg/dL, familial hypercholesterolaemia, nephrotic syndrome, renal insufficiency, liver disease, or were pregnant or nursing. Subjects were randomised to daily atorvastatin (>50 kg: 10 mg/day, increasing to 20 mg/day at day 30; ≤50 kg: 10 mg/day). Hydroxychloroquine, low-dose aspirin, multivitamins containing folate and American Heart Association Therapeutic Lifestyle Changes diet were recommended.
Two baseline CIMT examinations were performed using an ultrasound protocol described previously.18 CIMT measurements have been used in paediatric populations as a surrogate marker of cardiovascular risk in multiple diseases including chronic renal failure, chronic hypertension, obesity and familial hypercholesterolaemia.22 Ultrasound scans were read by a single experienced reader at Ward A. Riley Ultrasound Center, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA using Image Pro software (Media Cybernetics, Bethesda, Maryland, USA). Standardised longitudinal B-mode images were collected for three arterial segments defined relative to the tip of the flow divider (TFD) as the common carotid artery (10–20 mm proximal to the TFD), the carotid bifurcation (from the TFD to 10 mm proximal to the TFD) and the proximal 10 mm of the internal carotid artery. Near and far walls were imaged simultaneously in the common carotid artery, but separately in the carotid bifurcation and internal carotid artery to improve the ability to align each wall horizontally in these segments. For each arterial segment, Meijer's Arc was used to collect images at 90°, 120°, 150° and 180° on the right side and at 270°, 240°, 210° and 180° on the left side. For a set of 68 studies reread to evaluate intrareader reliability, the intraclass correlation coefficient was 0.74 (95% CI 0.61 to 0.83) for mean-mean common and 0.71 (95% CI 0.56 to 0.81) for mean-max CIMT measurements. The combination of 3 arterial segments, 2 walls, and 2 sides of the neck provided a set of 12 CIMT measurement sites, each imaged from 4 angles. For each measurement site, a maximum CIMT value was defined as the largest of the four angle-specific maximum CIMT values. The 12 maximum CIMT values were then averaged to determine the mean-max CIMT over near and far walls of the right and left common carotid artery, carotid bifurcation and internal carotid artery. For each of the four measurement sites in the common carotid artery, a mean CIMT value defined as the average of the four angle-specific mean CIMT values was also calculated. The four mean CIMT values were then averaged to determine the mean-mean common CIMT. Overall mean-mean and other segment/wall-specific mean-max or mean-mean CIMT measures were computed accordingly.
Other assessments including fasting lipid levels, disease activity scores (Safety of Estrogens in Lupus Erythematosus, National Assessment (SELENA), SLE Disease Activity Index (SLEDAI)), and a disease-related damage score (Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI)) were obtained as previously described.18 High sensitivity C reactive protein (hsCRP) was obtained along with lipid profiles after 12-h or 4-h fasts at randomisation and analysed at a central commercial laboratory (purified protein derivative Global Central Laboratories, Highland Heights, Kentucky, USA). Institutional Review Board (IRB) approval was obtained for the original APPLE trial and additionally for this secondary analysis.
Serum 25(OH)D determinations
Frozen serum collected at baseline and at 1-year follow-up was used to measure 25(OH)D levels after IRB approval was secured. Frozen serum samples stored in −80° freezers were shipped to the laboratory of Dr Vin Tangpricha at Emory University, Atlanta, Georgia, USA. Serum 25(OH)D was measured by chemiluminescent assay using the Immuno Diagnostic Systems immunoassay System (IDS-iSYS) automated system (Fountain Hills, Arizona, USA). Laboratory technicians were blinded to the study assignment of the samples. Intra-assay and interassay coefficients of variation for serum 25(OH)D were 1.8–4.0% and 10.1–13.0%, respectively. The laboratory participates in a vitamin D external quality control assessment schema (http://www.deqas.org) and the NIH standard quality control programme for vitamin D and tested proficient in the measurement of 25(OH)D during the study period.
Statistical analysis
All statistical analysis was performed using SAS V.9.2 statistical software (SAS, Cary, North Carolina, USA). All statistical tests were two-sided with p values less than 0.05 considered statistically significant for this analysis. Baseline characteristics were summarised using descriptive statistics with categorical data presented as percentages and continuous data presented as means, SDs and medians. The primary efficacy analysis for APPLE compared rates of mean-mean common CIMT progression between treatment groups based on a test of two-way interaction between treatment group and time in a longitudinal linear mixed effects model under data missing at random assumptions. From that model, it was assumed that the effect of treatment could be estimated as the difference in mean progression rates between participants assigned to atorvastatin versus placebo, with negative differences indicating progression for those on atorvastatin was slower than for those on placebo. Similar mixed-effects models were used for analysing continuous secondary longitudinal end points or changes from baseline over time for lipid data. Log transformation was used for hsCRP to achieve normality. Generalised estimating equations were used for binary longitudinal outcomes.
To examine heterogeneity of treatment effects by vitamin D status (25(OH)D levels <20 ng/mL), the efficacy model used in the primary APPLE analysis was extended to include an indicator variable for subgroup as well as two-way and three-way interactions between subgroup, treatment group and time. From these models, we provide estimated mean progression rates with 95% CIs for each combination of subgroup and treatment group. Finally, the three-way interaction between subgroup, treatment group and time provides a test of whether treatment effects in terms of progression rate differ significantly between subgroups. Initially, models were fit examining one subgroup variable at a time, then adjusted for lupus duration, sex, systolic blood pressure, pubertal status, LDL and natural log of hsCRP. These results should be interpreted cautiously as hypothesis generating and not hypothesis testing.
Results
Baseline characteristics
A total of 201/221 (91%) of APPLE subjects had available baseline samples and were included in the analysis; 98 were randomised to atorvastatin and 103 to placebo. At 1-year follow-up, 79 subjects in each group had available serum for analysis. In the original APPLE trial, 81.6% of each arm completed the 3-year study, 70% of them still on study drug. Among the 201 subjects included in the current subanalysis, 180 remained on study drugs at the 1-year follow-up. As shown in table 1, subjects were 83% female, 51% Caucasian, 27% African American and had a mean age of 16 years at entry into the study.
Table 1.
Baseline characteristics of APPLE substudy subjects
| Variable | All patients, n=201 |
|---|---|
| Female | 167 (83.1%) |
| Age, years (SD) | 15.7 (2.7) |
| Latitude (SD) | 39.3 (3.3) |
| Season | |
| 1st quarter | 36 (17.9%) |
| 2nd quarter | 54 (26.9%) |
| 3rd quarter | 52 (25.9%) |
| 4th quarter | 59 (29.4%) |
| Race | |
| White | 102 (50.7%) |
| Black | 54 (26.9%) |
| Asian | 19 (9.5%) |
| Native American | 3 (1.5%) |
| Native Hawaiian | 5 (2.5%) |
| Hispanic or Latino | 47 (23.4%) |
| History of smoking | 6 (3.0%) |
| Postmenarchal | 137/167 (82.0%) |
| Annual household income | |
| <$25 000 | 57/187 (30.5%) |
| $25 000–49 999 | 51/187 (27.3%) |
| $50 000–74 999 | 31/187 (16.6%) |
| $75 000–99 999 | 24/187 (12.8%) |
| $100 000–149 999 | 16/187 (8.6%) |
| >$150 000 | 8/187 (4.3%) |
| Body mass index percentile (SD) | 72.1 (25.2) |
| Duration of lupus, months (SD) | 30.4 (28.9) |
| SLEDAI (SD) | 4.5 (4.0) |
| SDI=0 | 151 (75.1%) |
| Hypertension | 65/195 (33.3%) |
| Glomerulonephritis | 81/200 (40.5%) |
| Creatinine clearance (SD) | 138.7 (31.8) |
| Timed urine protein, mg/24 h (SD) | 214.6 (491.5) |
| Serologies | |
| Lupus anticoagulant | 68/190 (35.8%) |
| Anticardiolipin antibody | 86/196 (43.9%) |
| AntidsDNA antibody | 163/201 (81.1%) |
| Corticosteroid usage | 163/200 (81.5%) |
| Multivitamin usage | 147 (73.1%) |
| Hydroxychloroquine usage | 196 (97.5%) |
| Baseline hsCRP, mg/L (SD) | 2.9 (8.4) |
| Homocysteine, μmol/L (SD) | 7.4 (3.0) |
| Lipids, mg/dL (SD) | |
| Total cholesterol | 154.7 (38.5) |
| HDL cholesterol | 46.0 (13.0) |
| LDL cholesterol | 86.0 (31.4) |
| Triglycerides | 115.2 (68.6) |
| Lipoprotein A, mg/dL (SD) | 22.6 (25.3) |
| Baseline mean-mean mm (SD) common CIMT mm (SD) | 0.468 (0.042) |
| Baseline mean-max CIMT mm (SD) | 0.583 (0.055) |
APPLE, Atherosclerosis Prevention in Pediatric Lupus Erythematosus; CIMT, carotid intima media thickening; dsDNA, double-stranded DNA; HDL, high-density lipoprotein; hsCRP, high-sensitivity C reactive protein; LDL, low-density lipoprotein; SDI, Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; SLICC, Systemic Lupus International Collaborating Clinics.
Vitamin D status
Overall, 61/201 (30%) had vitamin D deficiency at baseline and 139 (69%) had vitamin D insufficiency (25(OH)D <30 ng/mL); 12 subjects (6%) had levels less than 10 ng/mL indicating severe deficiency. Mean baseline 25(OH)D levels were 25.9 ng/mL (SD 11.0). At 1 year follow-up, mean 25(OH)D levels were 27.7 ng/mL (SD 14.0), with no statistically significant difference between atorvastatin and placebo groups in mean vitamin D levels after 1 year (p=0.97). There was no statistically significant difference between baseline and 1 year follow-up levels within or between arms. Sixty-six per cent of subjects stayed in their original vitamin D status category at follow-up (sufficient, insufficient or deficient). Percentage taking corticosteroids, and mean prednisone dose adjusted for weight did not differ between deficient and insufficient/sufficient groups (0.19 mg/kg for both groups, p=0.80).
Vitamin D status and CIMT progression
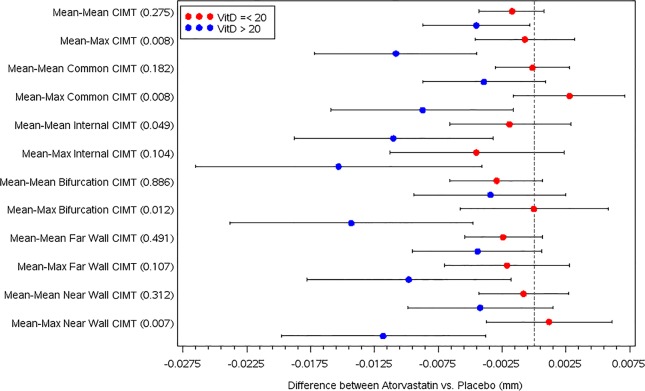
In unadjusted longitudinal modelling, baseline vitamin D deficiency was associated with increased baseline mean-max CIMT (p=0.01). Other baseline associations between vitamin D deficiency and cardiovascular risk factors were detailed in a previous paper.23 In adjusted longitudinal modelling, there was a significant interaction effect between baseline vitamin D deficiency and atorvastatin treatment in 3-year progression of mean-max CIMT (see table 2 and figure 1). In four out of six carotid segments, there was a greater decrease in mean-max CIMT progression rate in subjects treated with atorvastatin compared with placebo if they had baseline 25(OH)D levels ≥20 ng/mL. In only one of six carotid segments, there was a greater decrease in mean-mean CIMT progression rate in those treated with atorvastatin with sufficient vitamin D levels. Of the subjects who changed in vitamin D status from deficient to either insufficient or sufficient at 1 year into the trial, there was a trend towards response to atorvastatin treatment in 3-year CIMT progression, but this did not reach statistical significance.
Table 2.
CIMT progression in participants treated with atorvastatin or placebo for 3 years by baseline serum 25(OH)D levels (mg/dL)*
| Segment | CIMT progression with atorvastatin (mm) | CIMT progression with placebo (mm) | Interaction effect | p Value |
|---|---|---|---|---|
| Mean-mean CIMT | ||||
| 25(OH)D ≤20 | 0.0014 (−0.0005, 0.0032) | 0.0031 (0.0013, 0.0049) | −0.0027 (−0.0077, 0.0022) | 0.275 |
| 25(OH)D >20 | 0.0039 (0.0013, 0.0066) | 0.0084 (0.0051, 0.0117) | ||
| Mean-max CIMT | ||||
| 25(OH)D ≤20 | 0.0024 (−0.0004, 0.0052) | 0.0031 (0.0004, 0.0057) | −0.0101 (−0.0175, −0.0027) | 0.008 |
| 25(OH)D >20 | 0.0021 (−0.0019, 0.0061) | 0.0129 (0.0080, 0.0178) | ||
| Mean-mean common CIMT | ||||
| 25(OH)D ≤20 | 0.0006 (−0.0015, 0.0027) | 0.0007 (−0.0013, 0.0027) | −0.0038 (−0.0095, 0.0018) | 0.182 |
| 25(OH)D >20 | 0.0002 (−0.0029, 0.0032) | 0.0041 (0.0004, 0.0078) | ||
| Mean-max common CIMT | ||||
| 25(OH)D ≤20 | 0.0000 (−0.0032, 0.0032) | −0.0028 (−0.0058, 0.0003) | −0.0115 (−0.0199, −0.0031) | 0.008 |
| 25(OH)D >20 | −0.0015 (−0.0061, 0.0031) | 0.0073 (0.0017, 0.0129) | ||
| Mean-mean internal CIMT | ||||
| 25(OH)D ≤20 | 0.0047 (0.0013, 0.0082) | 0.0066 (0.0033, 0.0099) | −0.0092 (−0.0183, −0.0000) | 0.049 |
| 25(OH)D >20 | 0.0057 (0.0007, 0.0106) | 0.0167 (0.0107, 0.0227) | ||
| Mean-max internal CIMT | ||||
| 25(OH)D ≤20 | 0.0073 (0.0024, 0.0122) | 0.0118 (0.0071, 0.0165) | −0.0108 (−0.0239, 0.0023) | 0.104 |
| 25(OH)D >20 | 0.0074 (0.0003, 0.0145) | 0.0227 (0.0141, 0.0314) | ||
| Mean-mean bifurcation CIMT | ||||
| 25(OH)D ≤20 | 0.0007 (−0.0020, 0.0033) | 0.0036 (0.0011, 0.0061) | −0.0005 (−0.0075, 0.0065) | 0.886 |
| 25(OH)D >20 | 0.0045 (0.0008, 0.0083) | 0.0080 (0.0034, 0.0126) | ||
| Mean-max bifurcation CIMT | ||||
| 25(OH)D ≤20 | 0.0034 (−0.0008, 0.0075) | 0.0034 (−0.0006, 0.0073) | −0.0143 (−0.0254, −0.0032) | 0.012 |
| 25(OH)D >20 | −0.0007 (−0.0067, 0.0052) | 0.0135 (0.0062, 0.0209) | ||
| Mean-mean far wall CIMT | ||||
| 25(OH)D ≤20 | 0.0026 (0.0004, 0.0048) | 0.0049 (0.0028, 0.0071) | −0.0021 (−0.0080, 0.0038) | 0.491 |
| 25(OH)D > 20 | 0.0051 (0.0019, 0.0083) | 0.0095 (0.0056, 0.0134) | ||
| Mean-max far wall CIMT | ||||
| 25(OH)D ≤20 | 0.0033 (−0.0002, 0.0068) | 0.0054 (0.0020, 0.0088) | −0.0077 (−0.0171, 0.0017) | 0.107 |
| 25(OH)D > 20 | 0.0036 (−0.0014, 0.0087) | 0.0135 (0.0073, 0.0197) | ||
| Mean-mean near wall CIMT | ||||
| 25(OH)D ≤20 | −0.0001 (−0.0026, 0.0024) | 0.0007 (−0.0017, 0.0031) | −0.0034 (−0.0101, 0.0032) | 0.312 |
| 25(OH)D > 20 | 0.0026 (−0.0010, 0.0062) | 0.0069 (0.0025, 0.0113) | ||
| Mean-max near wall CIMT | ||||
| 25(OH)D ≤20 | 0.0012 (−0.0024, 0.0047) | −0.0001 (−0.0035, 0.0033) | −0.0130 (−0.0224, −0.0036) | 0.007 |
| 25(OH)D > 20 | 0.0003 (−0.0048, 0.0054) | 0.0121 (0.0059, 0.0183) | ||
Bold represents p<0.05.
*Multivariable mixed effects longitudinal modelling adjusted for lupus duration, female gender, systolic blood pressure, pubertal level, LDL cholesterol and hsCRP.
CIMT, carotid intima medial thickness; hsCRP, high-sensitivity C reactive protein; LDL, low-density lipoprotein; 25(OH)D, 25-hydroxyvitamin D.
Figure 1.
Forest plot of CIMT progression rate for atorvastatin treatment versus placebo for 3 years by baseline serum 25-hydroxyvitamin D status. Multivariable mixed effects longitudinal modelling adjusted for lupus duration, female gender, systolic blood pressure, pubertal level, LDL cholesterol and hsCRP. VitD, serum 25-hydroxyvitamin D status, ng/mL; CIMT, carotid intima medial thickness, in mm; hsCRP, high-sensitivity C reactive protein; LDL, low-density lipoprotein. p Values for the interaction effect are listed in parentheses on the y-axis.
When we repeated this analysis with 30 ng/mL as our new 25(OH)D cut point, we found similar results with interaction in overall mean-max CIMT progression rate with 2/6 carotid mean-mean segments and 2/6 carotid mean-max segments showing evidence of interaction between vitamin D insufficiency and sufficiency with atorvastatin usage (p<0.05). All interaction effects were in the same direction with greater decrease in CIMT progression rate in those treated with atorvastatin with higher serum 25(OH)D levels. Using race and ethnicity as an additional adjustment variable, we noted no changes in our conclusions, and the numbers of subjects in each subgroup were too small to evaluate changes in CIMT progression with any accuracy.
Vitamin D status and secondary outcomes
In the original APPLE trial, the atorvastatin group displayed reductions from baseline in total cholesterol, LDL and hsCRP, which were maintained over time. Changes from baseline in SLEDAI and SDI did not differ between groups. We found no evidence of interaction between vitamin D deficiency and response to atorvastatin in change in LDL, SLEDAI or SDI over 2 years. When looking at SLEDAI and SDI by vitamin D status alone, there was a trend towards higher SLEDAI area under the curve over 3 years (65.8, 95% CI −14.9 to 146.5) and higher proportion of subjects with damage index greater than 0 (3.4%, 95% CI −1.3% to 8.0%) in those with baseline vitamin D deficiency, but this was not statistically significant. Baseline vitamin D deficiency was not a predictor of change in hsCRP over 3 years and change in vitamin D over 1 year was not associated with change in hsCRP at 1 year.
Discussion
For the first time in SLE, we find that vitamin D status may affect response to atorvastatin in CVD risk and CIMT progression over time. The associations we found between vitamin D deficiency and increased age, body mass index, winter season and minority status in the APPLE paediatric cohort are consistent with those seen in larger epidemiological studies of the general population.24 A higher, although not statistically significant, proportion of subjects with vitamin D deficiency were also on multivitamins, but multivitamin use in this population was high overall, and it is possible that subjects with a history of vitamin D deficiency may have been encouraged by their providers to take multivitamins. In addition, in this cohort we found notable differences at baseline between vitamin D deficient subjects and those not deficient, notably in some CVD risk measures (mean-max CIMT, LDL cholesterol hsCRP), and in SLE disease related variables (duration of SLE, SDI and proteinuria).25
The relationship between vitamin D status and the inflammation marker hsCRP has been previously described in adults with SLE, specifically in the LAPS study, a randomised study of atorvastatin in adults with lupus.26 In LAPS, baseline 25(OH)D levels ≥21 ng/mL were associated with lower baseline hsCRP levels. HsCRP is associated with higher SLE disease activity measured by physician's global assessment or SLEDAI, and has been associated with increased serositis and arthritis.27–29 In newly diagnosed patients with SLE, hsCRP levels have correlated with disease activity.30 Inflammation is important in the pathogenesis of atherosclerosis,31 and is an independent predictor of future stroke and myocardial infarction in the general population.32 However, the relationship between hsCRP and CVD events in SLE is less clear. For instance, cross-sectional studies of patients with SLE evaluating hsCRP and CIMT have shown inconsistent results. Secondary analysis of the APPLE cohort suggested that atorvastatin may reduce atherosclerosis prevention in pubertal patients with lupus with higher hsCRP.33
Studies suggest that vitamin D has potent effects on innate and acquired immunity, including modulation of T lymphocyte proliferation and function, and inhibition of Th1 cytokine expression while augmenting the antiatherogenic Th2 cytokines.34 35 In addition, vitamin D inhibits tumor necrosis factor (TNF)-α-induced adhesion molecule expression in endothelial cells.36 Thus, through modulation of inflammatory cells and inflammatory cytokine secretion, low vitamin D may adversely affect the cardiovascular system in several chronic inflammatory conditions. Indeed, vitamin D receptors have broad tissue distribution that includes vascular smooth muscle, endothelium and cardiomyocytes. In vitro, activated 1,25(OH)2D directly suppresses renin gene expression37 and regulates the growth and proliferation of vascular smooth muscle cells and cardiomyocytes.38 Vitamin D suppresses foam cell formation by reducing oxidised LDL-cholesterol uptake, suppresses CD36 expression, and improves insulin signalling.39 Clinical studies have reported associations between lower vitamin D levels and hypertension,23 40 low high-density lipoprotein cholesterol, coronary artery calcification,41 increased CIMT42 43 and prevalent CVD.44 45 Thus, putative vascular effects of vitamin D include modulation of smooth muscle cell proliferation, inflammation, thrombosis, insulin sensitivity and blood pressure.
The findings in this study suggest that there may be interaction between vitamin D levels and response to atorvastatin in CIMT progression, especially in mean-max CIMT measurements. In general, we note that the lowest CIMT progression rates were seen in the atorvastatin-treated subgroup with serum 25(OH)D >20 ng/mL, although these numbers should be interpreted cautiously in segments where the p value of the interaction effect was greater than 0.05. In the original APPLE trial, mean-max CIMT progression was not found to be significantly different between atorvastatin and placebo groups (0.0037 mm/year in the atorvastatin group vs 0.0064 mm/year in the placebo group, p=0.083). Further analysis of the LAPS trial concluded that 25(OH)D levels were not associated with progression of coronary artery calcium or CIMT over 2 years, although the mean hsCRP decreased over the study period.25 Difference in results between the LAPS trial secondary analyses and the present study could be due to the fact that in LAPS, no adjustments were made for atorvastatin or placebo usage, there was a shorter duration of follow-up, and only single measurements of the common carotid arteries were performed. Baseline CIMT was not controlled for in this analysis, which looked strictly at progression alone, nor was it controlled for in the original APPLE study. However, vitamin D levels and CIMT levels were not statistically different between atorvastatin and placebo groups at baseline.
Mean-mean and mean-max CIMT commonly have been used in trials of statins to moderate cardiovascular risk. In the original APPLE trial, due to slow recruitment, the primary outcome was changed during the trial to mean-mean common CIMT from mean-max CIMT. The initial analysis showed a difference in mean-max CIMT progression over 3 years after controlling for covariates related to baseline CIMT.18 Mean-max CIMT is thought to be more predictive of clinical cardiovascular events than mean-mean CIMT, and more strongly associated with the presence of symptomatic CVD in adults.46 The mean-max IMT is a measure of plaque taken at the carotid bifurcation and proximal internal carotid artery (ICA), where complex oscillatory low shear stress promotes the primary deposition of LDL cholesterol in the wall. However, carotid plaque is a later effect of atherosclerosis and is not present in children and adolescents. Further research is needed to better define the biological relevance of different CIMT measures in patients with SLE compared with the general population.
In our study of APPLE trial data, the relationship between vitamin D status and mean-max CIMT progression persisted despite adjustment for multiple confounders. Evaluation of the interaction effect between atorvastatin and vitamin D levels is a strength of this analysis, especially after adjustment for factors such as duration of lupus, gender, baseline blood pressure, baseline pubertal status, baseline LDL cholesterol and baseline hsCRP.
The trend towards higher SLEDAI over 3 years, and higher proportion of participants with SDI>0 in subjects with baseline vitamin D deficiency found in this analysis was interesting, and matches with prior studies finding associations between vitamin D deficiency and increased disease activity,8–12 although the difference did not reach statistical significance. This may be related to the fact that subjects with severe disease such as proteinuria were excluded from entry into the trial, and the cohort reported overall low SLEDAI and SDI scores.
Conclusions
APPLE participants with higher serum 25(OH)D (≥20 ng/mL) had less mean-max CIMT progression in multiple carotid segments following 3 years of atorvastatin treatment than participants receiving placebo. Results from secondary analyses must be interpreted cautiously, but these findings suggest that underlying vitamin D deficiency may negatively impact the efficacy of atorvastatin in atherosclerosis prevention. More studies are needed to determine if vitamin D replacement therapy can boost response to atorvastatin in prevention of CVD in SLE and the general population.
Footnotes
Collaborators: The following investigators participated in this study by enrolling patients at sites or by performing study procedures at sites: Stacy Ardoin, Esi Morgan Dewitt, C Egla Rabinovich, Janet Ellis, Kelly Mieszkalski, Janet Wootton (Duke University Medical Center, Durham, North Carolina, USA), Peter Chira, Joyce Hsu, Tzielan Lee, Christy Sandborg, Jan Perea (Stanford University School of Medicine, Palo Alto, California, USA), Beth Gottlieb, Patricia Irigoyen, Jennifer Luftig, Shaz Siddiqi, Zhen Ni, Marilynn Orlando, Eileen Pagano (Cohen Children's Medical Center, New Hyde Park, New York, USA), Andrew Eichenfield, Lisa Imundo, Deborah Levy, Philip Kahn, Candido Batres, Digna Cabral (Morgan Stanley Children's Hospital of New York Presbyterian, New York, New York, USA), Kathleen A Haines, Yukiko Kimura, Suzanne C Li, Jennifer Weiss, Mary Ellen Riordan, Beena Vaidya (Hackensack University Medical Center, Hackensack, New Jersey, USA), Emily von Scheven, Michelle Mietus-Snyder (University of California at San Francisco Medical Center, San Francisco, California, USA), Earl Silverman, Lawrence Ng (Hospital for Sick Children, Toronto, Ontario, Canada), Suzanne Bowyer, Susan Ballinger, Thomas Klausmeier, Debra Hinchman, Andrea Hudgins (Indiana University School of Medicine, Indianapolis, Indiana, USA), Marilynn Punaro, Shirley Henry, Shuzen Zhang (Texas Scottish Rite Hospital for Children, Dallas, Texas, USA), Nora G Singer, Elizabeth B Brooks, Stacy Miner, Nancy Szabo, Lisabeth Scalzi (University Hospitals/Case Medical Center, Cleveland, Ohio, USA), David Sherry, Libby Dorfeld, Sarajane Wilson, Jenna Tress (Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, USA), Deborah McCurdy, Tatiana Hernandez, Jyotsna Vitale (University of California Los Angeles Medical Center, Los Angeles, California, USA), Marisa Klein-Gitelman, Angela Kress, Nicole Lowe, Falguni Patel (Children's Memorial Hospital, Chicago, Illinois, USA), Carol Wallace, Stephanie Hamilton (Seattle Children's Hospital and Regional Medical Center, Seattle, Washington, USA), Richard Silver, Katie Caldwell, Diane Kamen (Medical University of South Carolina, Charleston, South Carolina, USA), Linda Wagner-Weiner, Becky Puplava, Atanas Lonchev (University of Chicago, Chicago, Illinois, USA), Gloria Higgins, Monica Bacani (Nationwide Children's Hospital, Columbus, Ohio, USA), Hermine Brunner, Cynthia Rutherford, Jamie Meyers-Eaton, Shannen Nelson, Alexei Grom (Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio, USA), Larry Jung, Teresa Conway, Lacey Frank, Lori Kuss (Creighton University Medical Center, Omaha, Nebraska, USA), Jenny Soep, Hazel Senz (University of Colorado, Aurora, Colorado, USA), Ann Reed, Thomas Mason, Jane Jaquith, Diana E Paepke-Tollefsrud (Mayo Clinic, Rochester, Minnesota, USA).
Contributors: All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. ABR and EY had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: ABR, VT, EY, RG, LES, GAMcC. Acquisition of data: ABR, VT, LES. Analysis and interpretation of data: ABR, VT, EY, RG, LES, GAMcC.
Funding: APPLE is supported by the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases contract N01-AR-2-2265), the Edna and Fred L. Mandel Jr. Center for Hypertension and Atherosclerosis and Pfizer, which provided atorvastatin and matching placebo. Secondary analysis was supported by the Rainbow Babies and Children's Hospital Pediatrics Pilot Award and the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases contract 5P30-AR-047363-12). The investigators have no conflicts of interest to disclose. This publication was made possible by the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All vitamin D data have been added to the original APPLE database and is open to investigators who apply through APPLE data sharing policies.
Contributor Information
APPLE investigators:
Stacy Ardoin, Esi Morgan Dewitt, C Egla Rabinovich, Janet Ellis, Kelly Mieszkalski, Janet Wootton, Peter Chira, Joyce Hsu, Tzielan Lee, Christy Sandborg, Jan Perea, Beth Gottlieb, Patricia Irigoyen, Jennifer Luftig, Shaz Siddiqi, Zhen Ni, Marilynn Orlando, Eileen Pagano, Andrew Eichenfield, Lisa Imundo, Deborah Levy, Philip Kahn, Candido Batres, Digna Cabral, Kathleen A Haines, Yukiko Kimura, Suzanne C Li, Jennifer Weiss, Mary Ellen Riordan, Beena Vaidya, Emily von Scheven, Michelle Mietus-Snyder, Earl Silverman, Lawrence Ng, Suzanne Bowyer, Susan Ballinger, Thomas Klausmeier, Debra Hinchman, Andrea Hudgins, Marilynn Punaro, Shirley Henry, Shuzen Zhang, Nora G Singer, Elizabeth B Brooks, Stacy Miner, Nancy Szabo, Lisabeth Scalzi, David Sherry, Libby Dorfeld, Sarajane Wilson, Jenna Tress, Deborah McCurdy, Tatiana Hernandez, Jyotsna Vitale, Marisa Klein-Gitelman, Angela Kress, Nicole Lowe, Falguni Patel, Carol Wallace, Stephanie Hamilton, Richard Silver, Katie Caldwell, Diane Kamen, Linda Wagner-Weiner, Becky Puplava, Atanas Lonchev, Gloria Higgins, Monica Bacani, Hermine Brunner, Cynthia Rutherford, Jamie Meyers-Eaton, Shannen Nelson, Alexei Grom, Larry Jung, Teresa Conway, Lacey Frank, Lori Kuss, Jenny Soep, Hazel Senz, Ann Reed, Thomas Mason, Jane Jaquith, and Diana E Paepke-Tollefsrud
Collaborators: APPLE investigators
References
- 1.Nossent J, Cikes N, Kiss E, et al. Current causes of death in systemic lupus erythematosus in Europe, 2000–2004: relation to disease activity and damage accrual. Lupus 2007;16:309–17. [DOI] [PubMed] [Google Scholar]
- 2.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol 1997;145:408–15. [DOI] [PubMed] [Google Scholar]
- 3.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum 2001;44:2331–7. [DOI] [PubMed] [Google Scholar]
- 4.Westerweel PE, Luyten RK, Hoomans HA, et al. Premature atherosclerotic cardiovascular disease in systemic lupus erythemarosus. Arthritis Rheum 2007;56:1384–96. [DOI] [PubMed] [Google Scholar]
- 5.Kienrich K, Tomaschitz A, Verheyen N, et al. Vitamin D and cardiovascular disease. Nutrients 2013;5:3005–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008;117:503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiRosa M, Malaguarnera G, DeGregorio C, et al. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol 2012;280:36–43. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Irastorza G, Egurbide MV, Olivares N, et al. Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatology 2008;47:920–3. [DOI] [PubMed] [Google Scholar]
- 9.Kamen DL, Cooper GS, Bouali H, et al. Vitamin D deficiency in systemic lupus erythematosus. Autoimmunity Rev 2006;5:114–17. [DOI] [PubMed] [Google Scholar]
- 10.Wright TB, Shults J, Leonard MB, et al. Hypovitaminosis D is associated with greater body mass index and disease activity in pediatric systemic lupus erythematosus. J Pediatr 2009;155:260–5. [DOI] [PubMed] [Google Scholar]
- 11.Borba VZC, Vieira JGH, Kasamatsu T, et al. Vitamin D deficiency in patients with active systemic lupus erythematosus. Osteoporosis Int 2009;20:427–33. [DOI] [PubMed] [Google Scholar]
- 12.Robinson AB, Thierry-Palmer M, Gibson KL, et al. Disease activity, proteinuria, and vitamin D status in children with systemic lupus erythematosus and juvenile dermatomyositis. J Pediatr 2012;160:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab 2008;4:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piemonti L, Monti P, Sironi M, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol 2000;164:4443–51. [DOI] [PubMed] [Google Scholar]
- 15.D'Ambrosio D, Cippitelli M, Cocciolo MG, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest 1998;101:252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Sims GP, Chen XX, et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 2007;179:1634–47. [DOI] [PubMed] [Google Scholar]
- 17.Ronnblom L, Pascual V. The innate immune system in SLE: type I interferons and dendritic cells. Lupus 2008;17:394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schanberg LE, Sandborg C, Barnhart HX, et al. Use of atorvastatin in systemic lupus erythematosus in children and adolescents. Arthritis Rheum 2012;64:285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Castrillon JL, Abad Manteca L, Vega G, et al. Vitamin D levels and lipid response to atorvastatin. Int J Endocrinol 2010;2010:320721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz JB. Effects of vitamin D supplementation in atorvastatin-treated patients: a new drug interaction with an unexpected consequence. Clin Pharmacol Ther 2009;85:198–203. [DOI] [PubMed] [Google Scholar]
- 21.Petri MA, Kiani AN, Post W, et al. Lupus Atherosclerosis Prevention Study (LAPS). Ann Rheum Dis 2011;70:760–5. [DOI] [PubMed] [Google Scholar]
- 22.Lamotte C, Iliescu C, Libersa C, et al. Increased intima-media thickness of the carotid artery in childhood: a systematic review of observational studies. Eur J Pediatr 2011;170:719–29. [DOI] [PubMed] [Google Scholar]
- 23.Robinson AB, Tangpricha V, Yow E, et al. Vitamin D deficiency is common and associated with increased C-reactive protein in children and young adults with lupus: An Atherosclerosis Prevention in Pediatric Lupus Erythematosus substudy. Lupus Sci Med 2014;1:e000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar J, Muntner P, Kaskel FJ, et al. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics 2009;124:e362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson AB, Tangpricha V, Yow E, et al. for the APPLE investigators. Vitamin D deficiency is common and associated with increased C-reactive protein in children and young adults with lupus: an Atherosclerosis Prevention in Pediatric Lupus Erythematosus substudy. Lupus Sci Med 2014;1:e000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiani AN, Fang H, Magder LS, et al. Vitamin D deficiency does not predict progression of coronary artery calcium, carotid intima-media thickness or high-sensitivity C-reactive protein in systemic lupus erythematosus. Rheumatology 2013;52:2071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SS, Singh S, Magder LS, et al. Predictors of high sensitivity C-reactive protein levels in patients with systemic lupus erythematosus. Lupus 2008;17:114–23. [DOI] [PubMed] [Google Scholar]
- 28.Mochizuki T, Aotsuka S, Satoh T. Clinical and laboratory features of lupus patients with complicating pulmonary disease. Respir Med 1999;93:95–101. [DOI] [PubMed] [Google Scholar]
- 29.Moutsopoulos HM, Mavridis AK, Acritidis NC, et al. High C-reactive protein response in lupus polyarthritis. Clin Exp Rheumatol 1983;1:53–5. [PubMed] [Google Scholar]
- 30.Liou LB. Serum and in vitro production of IL-1 receptor antagonist correlate with C-reactive protein levels in newly diagnosed, untreated lupus patients. Clin Exp Rheumatol 2001;19:515–23. [PubMed] [Google Scholar]
- 31.Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009;54:2129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. NEJM 2000;342:836–43. [DOI] [PubMed] [Google Scholar]
- 33.Ardoin SA, Schanberg LE, Sandborg CI, et al. Secondary analysis of APPLE study suggests atorvastatin may reduce atherosclerosis progression in pubertal lupus patients with higher C reactive protein. Ann Rheum Dis 2014;73:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemire JM, Archer DC, Beck L, et al. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr 1995;125(6 Suppl):1704S–8S. [DOI] [PubMed] [Google Scholar]
- 35.Boonstra A, Barrat FJ, Crain C, et al. 1alpha,25-Dihydroxyvitamin D3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol 2001;167:4974–80. [DOI] [PubMed] [Google Scholar]
- 36.Martinesi M, Bruni S, Stio M, et al. 1,25-Dihydroxyvitamin D3 inhibits tumor necrosis factor-alpha-induced adhesion molecule expression in endothelial cells. Cell Biol Int 2006;30:365–75. [DOI] [PubMed] [Google Scholar]
- 37.Sigmund CD, Okuyama K, Ingelfinger J, et al. Isolation and characterization of renin-expressing cell lines from transgenic mice containing a renin-promoter viral oncogene fusion construct. J Biol Chem 1990;265:19916–22. [PubMed] [Google Scholar]
- 38.O'Connell TD, Berry JE, Jarvis AK, et al. 1,25-Dihydroxyvitamin D3 regulation of cardiac myocyte proliferation and hypertrophy. Am J Physiol 1997;272(4 Pt 2):H1751–8. [DOI] [PubMed] [Google Scholar]
- 39.Oh J, Weng S, Felton SK, et al. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation 2009;120:687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lind L, Hanni A, Lithell H, et al. Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men. Am J Hypertens 1995;8:894–901. [DOI] [PubMed] [Google Scholar]
- 41.Watson KE, Abrolat ML, Malone LL, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation 1997;96:1755–60. [DOI] [PubMed] [Google Scholar]
- 42.Targher G, Bertolini L, Padovani R, et al. Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol (Oxf) 2006;65:593–7. [DOI] [PubMed] [Google Scholar]
- 43.Ross AC, Tangpricha V, Judd S, et al. Vitamin D is Linked to Carotid Intima-Media Thickness, Inflammation, and Immune Reconstitution in HIV-Infected Individuals. Antivir Ther 2011;16:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scragg R, Jackson R, Holdaway IM, et al. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study. Int J Epidemiol 1990;19:559–63. [DOI] [PubMed] [Google Scholar]
- 45.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med 2008;168:1340–9. [DOI] [PubMed] [Google Scholar]
- 46.Baldassarre D, Hamsten A, Veglia F, et al. Measurements of carotid intima-media thickness and of interadventitia common carotid diameter improve prediction of cardiovascular events: results of the IMPROVE (Carotid Intima Media Thickness [IMT] and IMT-Progression as Predictors of Vascular Events in a High Risk European Population) study. J Am Coll Cardiol 2012;60:1489–99. [DOI] [PubMed] [Google Scholar]