Abstract
Objective
Correlates of systemic lupus erythematosus (SLE) Responder Index (SRI) response with clinical trial end points were examined using pooled data from the Study of Belimumab in Subjects with SLE (BLISS) trials (N=1684).
Methods
Changes in clinical, laboratory and health-related quality of life measures from baseline at 52 weeks were compared between SRI responders (n=761) and non-responders (n=923).
Results
More SRI responders than non-responders had ≥4-point (100% vs 3.8%) and ≥7-point (40.3% vs 1.3%) Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index reductions, no new British Isles Lupus Assessment Group (BILAG) A and ≤1 new B scores (91.9% vs 35.9%), and a 25% reduction in corticosteroid dose decrease of 25% from >7.5 mg/d to ≤7.5 mg/d (25.5% vs 13.9%), and fewer had a corticosteroid increase from ≤7.5 mg/d to >7.5 mg/d (4.1% vs 21.3%; all p<0.001). More responders than non-responders had improved organ domains: Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index (mean 1.45 vs 0.40), BILAG (2.00 vs 0.39), and greater improvement in Physician's Global Assessment (all p<0.001). Risks for developing any SLE flare or severe flare were reduced in responders by 42% and 87%, respectively (p<0.001). Responders reported greater improvements in Medical Outcomes Survey Short Form version 2 Physical and Mental Components and all domain scores, and Functional Assessment of Chronic Illness Therapy-Fatigue score compared with non-responders (all p<0.001).
Conclusion
Overall, SRI response in patients with active, autoantibody-positive SLE was associated with improvements in clinical, laboratory and patient-reported outcome measures, indicating that SRI response was associated with a global benefit.
Trial registration number
Keywords: Belimumab, Bilag, Facit-Fatigue, Health-Related Quality of Life, PGA
Key messages.
SRI responders reported greater improvements from baseline in a range of clinical, laboratory and health-related quality of life measures, compared with non-responders.
SRI responses, irrespective of therapy, were associated with global benefits in patients with active, autoantibody-positive SLE.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease associated with considerable morbidity, increased mortality and poor health-related quality of life (HRQoL).1 2 Belimumab is a human immunoglobulin (Ig)-G1λ monoclonal antibody that inhibits the biological activity of soluble B lymphocyte stimulator, an immunomodulatory cytokine involved in B cell selection and survival that is overexpressed in SLE.3 In two placebo-controlled trials conducted in patients with active, autoantibody-positive SLE (Study of Belimumab in Subjects with SLE (BLISS)-52 and BLISS-76), belimumab plus standard SLE therapy resulted in significantly higher SLE Responder Index (SRI) response rates at 1 year compared with standard therapy (placebo), indicating greater reductions in SLE disease activity with treatment4 5 and improvements in HRQoL measures.6
The SRI is a novel composite end point that requires improvement in SLE disease activity without worsening in specific organ domains or global disease activity7 consistent with US Food and Drug Administration guidance for development of products for treatment of SLE.8 A SRI response requires clinically meaningful improvement in the Safety of Estrogens in Lupus Erythematosus National Assessment-SLE Disease Activity Index (SELENA-SLEDAI) and no worsening of disease, as measured by British Isles Lupus Assessment Group (BILAG) organ domain score and Physician's Global Assessment (PGA). The present post hoc analysis examined the association of SRI response at Week 52, irrespective of treatment assignment, with individual clinical and laboratory measures, and patient-reported HRQoL and fatigue among SRI responders and non-responders.
Materials and methods
Patients with SLE (n=1684) who were autoantibody-positive (antinuclear antibody titre ≥1:80 and/or antidouble-stranded DNA (anti-dsDNA) ≥30 IU/mL) with a SELENA-SLEDAI score ≥6 received placebo, belimumab 1 mg/kg or 10 mg/kg in addition to standard SLE therapy for 52 weeks (BLISS 52; NCT00424476) or 76 weeks (BLISS 76; NCT00410384).4 5 Doses of standard therapy were required to be stable for ≥30 days prior to enrolment. Patients could not have severe active lupus nephritis or severe active central nervous system SLE. Progressive restrictions on immunosuppressives and antimalarials began at treatment Week 16, and restrictions on corticosteroids began at treatment Week 24. Patients were stratified at screening by SELENA-SLEDAI score (6–9 vs ≥10), proteinuria (<2 g/24 h vs ≥2 g/24 h), and race (African descent or indigenous American vs other). SRI response rate at Week 52 was the primary end point, defined as a decrease of ≥4 points in SELENA-SLEDAI score, no new BILAG A score and ≤1 new B score, and no worsening (<0.3-point increase) in PGA score. Patients were considered non-responders if they did not meet SRI response criteria, withdrew before Week 52 or received protocol-prohibited medications.
The BLISS trials were conducted according to the principles of the Declaration of Helsinki and the appropriate ethical approvals were obtained.4 5
Fifty-two-week data from BLISS-52 and BLISS-76 were pooled.4 5 Of the 1684 patients enrolled, 761 were SRI responders and 923 were non-responders at Week 52. Clinical variables examined included the individual components of SRI response, the numbers of BILAG and SELENA-SLEDAI organ domains with improvement,9 the proportions of patients with flares and severe flares based on the modified SLE Flare Index (SFI),10–12 and changes in corticosteroid dose. Laboratory values consisted of changes in anti-dsDNA, complement (C3 and C4), and circulating B (CD20) cells. HRQoL, Medical Outcomes Survey Short Form version 2 (SF-36 v2), and fatigue (Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue questionnaire) were examined.
Comparisons between responders and non-responders for SELENA-SLEDAI and BILAG scores, changes in corticosteroid dose, and normalisation of anti-dsDNA, C3, and C4 biomarkers were performed using the likelihood ratio test. The two-sample t test was used to compare the improved SELENA-SLEDAI or BILAG organ domains and per cent changes in PGA. The Cox proportional hazards model was used to compare risk of SFI flares. Changes in FACIT-Fatigue score and SF-36 physical component summary (PCS) and mental component summary (MCS) and domain scores were analysed using an analysis of covariance model adjusted for baseline scores. Comparisons of per cent changes from baseline in anti-dsDNA, C3, C4 and CD20 B cells used the Wilcoxon test. No multiple test adjustments were made for the above analyses, as they were considered exploratory. The analyses were performed using SAS software V.9.2 or higher and R statistical software V.1.9.1.
To examine the robustness of the univariate analysis, baseline covariate adjusted analyses were performed to assess baseline differences and the association with responder status at Week 52. For clinical and serological measures, the independent variables included the baseline value and SRI response status at Week 52. The dependent variable was the change from baseline in the clinical or serological measure.
Results
SRI responses in patients receiving placebo, and belimumab 1 and 10 mg/kg plus standard therapy were 38.8%, 46.2% (p=0.006) and 50.6% (p<0.001), respectively, at Week 52. Baseline characteristics were balanced across treatment groups (table 1) and were generally similar between SRI responders and non-responders. Responders were more likely to have higher disease activity, less serological activity (based on anti-dsDNA titre (p<0.001) and percentage of patients with C3 or C4 levels less than the lower limits of normal (p<0.001 and p<0.0001, respectively)), and were more likely to have received a corticosteroid dose >7.5 mg/d (p<0.01), but not an immunosuppressant (p<0.0001). At baseline, there were no statistically significant differences in B cell subsets or plasma cell subsets (data not shown).13
Table 1.
Baseline characteristics of BLISS-52 and BLISS-76 SRI responders and non-responders
| Responders (n=761) | Non-responders (n=923) | All patients (n=1684) | |
|---|---|---|---|
| Demographics | |||
| Women, n (%) | 718 (94.3) | 867 (93.9) | 1585 (94.1) |
| Mean age±SD, y | 37.3±11.4 | 38.2±11.6 | 37.8±11.5 |
| Treatment assignment, n/N (%) | |||
| Placebo | 218/562 (38.8) | 344/562 (61.2) | NA |
| Belimumab 1 mg/kg | 258/559 (46.2) | 301/559 (53.8) | NA |
| Belimumab 10 mg/kg | 285/563 (50.6) | 278/563 (49.4) | NA |
| Baseline SLE characteristics | |||
| Mean SELENA-SLEDAI score±SD | 10.5±3.4§ | 9.1±3.9 | 9.7±3.8 |
| ≥10, n (%) | 483 (63.5)§ | 395 (42.8) | 878 (52.1) |
| BILAG scores, n (%) | |||
| ≥1 A or 2 B | 493 (64.8)+ | 531 (57.5) | 1024 (60.8) |
| ≥1 A | 125 (16.4) | 138 (15.0) | 263 (15.6) |
| ≥1 B | 714 (93.8)§ | 812 (88.0) | 1526 (90.6) |
| No A or B | 47 (6.2)§ | 111 (12.0) | 158 (9.4) |
| Mean PGA score±SD | 1.5±0.5+ | 1.4±0.5 | 1.4±0.5 |
| Mean SLICC damage index±SD | 0.7±1.1+ | 0.9±1.3 | 0.8±1.2 |
| Mean proteinuria±SD, g/24 h | 0.44±0.84 | 0.53±0.99 | 0.49±0.93 |
| Anti-dsDNA ≥30 IU/mL, n (%) | 517 (67.9)# | 651 (70.5) | 1168 (69.4) |
| ANA ≥1:80, n (%) | 696 (91.5) | 870 (94.2) | 1566 (93.0) |
| Mean IgG±SD, g/L | 16.6±6.0 | 16.4±6.2 | 16.5±6.1 |
| >16.2 g/L, n (%) | 358 (47.0) | 386 (41.8) | 744 (44.2) |
| Low C3 (<90 mg/dL), n (%) | 311 (40.9)# | 447 (48.4) | 758 (45.0) |
| Low C4 (<16 mg/dL), n (%) | 395 (51.9)§ | 549 (59.5) | 944 (56.1) |
| Corticosteroid use, n (%) | 668 (87.8) | 785 (85.0) | 1453 (86.3) |
| >7.5 mg/d, n (%) | 471 (61.9)+ | 505 (54.7) | 976 (58.0) |
| Immunosuppressant use, n (%) | 323 (42.4)§ | 497 (53.8) | 820 (48.7) |
| Baseline HRQoL | |||
| Mean SF-36 PCS±SD | 39.6±9.2* | 38.6±10.0 | 39.1±9.7 |
| Mean SF-36 MCS±SD | 40.6±10.9 | 41.0±11.6 | 40.8±11.3 |
| Mean FACIT-Fatigue score±SD | 30.9±11.5* | 29.4±12.1 | 30.1±11.9 |
*p<0.05; +p<0.01; #p<0.001; §p<0.0001 (note: p values represent comparison between responders and non-responders from the likelihood ratio test for categorical data and from the t-test for continuous variables).
ANA, antinuclear antibody; anti-dsDNA, antidouble-stranded DNA; BILAG, British Isles Lupus Assessment Group; C, complement; FACIT, Functional Assessment of Chronic Illness Therapy; HRQoL, health-related quality of life; IgG, immunoglobulin-G; MCS, Mental Component Summary; NA, not applicable; PCS, Physical Component Summary; PGA, Physician's Global Assessment; SELENA-SLEDAI, Safety of Estrogens in Lupus Erythematosus National Assessment-SLE Disease Activity Index; SF-36, Medical Outcomes Survey Short Form; SLE, Systemic Lupus Erythematosus; SLICC, Systemic Lupus International Collaborating Clinics; SRI, SLE Responder Index.
Clinical and serological measures of disease activity
Clinical and laboratory measures of disease activity at Week 52 are shown in table 2.
Table 2.
Changes in clinical and serological measures from baseline in SRI responders versus non-responders at Week 52
| SRI responders (n=761) | SRI non-responders (n=923) | p Value | Adjusted p value* | |
|---|---|---|---|---|
| Clinical measures | ||||
| SELENA-SLEDAI, n (%) | ||||
| ≥4-point reduction | 761 (100) | 35 (3.8) | <0.001† | <0.001‡ |
| ≥7-point reduction | 307 (40.3) | 12 (1.3) | <0.001† | <0.001‡ |
| Mean no. of organ domains with improvement, per patient (SE)§ | ||||
| BILAG | 1.45 (0.03) | 0.40 (0.02) | <0.001¶ | <0.001** |
| SELENA-SLEDAI | 2.00 (0.03) | 0.39 (0.02) | <0.001¶ | <0.001** |
| No new BILAG A score and ≤1 new B score, n (%) | 699 (91.9) | 331 (35.9) | <0.001† | <0.001** |
| Mean % change in PGA score from baseline in all patients (SE) | −58.3 (1.17) (n=761) | −13.7 (2.03) (n=923) | <0.001¶ | <0.001‡ |
| Mean % change in PGA score from baseline in patients with no worsening (SE) ≥0.3-point increase, n†† | −58.3 (1.17) 761 | −34.9 (1.75) 455 | <0.001¶ | <0.001‡ |
| Corticosteroid dose, n (%) | ||||
| Dose decrease to ≤7.5 mg/d from >7.5 mg/d at baseline†† | 120/471 (25.5) | 70/505 (13.9) | <0.001† | <0.001‡ |
| Dose increase to >7.5 mg/d from ≤7.5 mg/d at baseline‡‡ | 12/290 (4.1) | 89/418 (21.3) | <0.001† | <0.001‡ |
| SFI flare, n (%) | ||||
| Any | 532 (69.9) | 763 (82.7) | HR 0.58 95% CI 0.52 to 0.65 <0.001§§ | <0.001††† |
| Severe | 47 (6.2) | 269 (29.1) | HR 0.13 95% CI 0.09 to 0.17 <0.001§§ | <0.001††† |
| Serological measures | ||||
| Anti-dsDNA antibodies | ||||
| Median % change in patients positive (≥30 IU/L) at baseline (Q1, Q3)¶¶ | −34.2 (−57.04, −0.50) (n=434) | −26.1 (−50.81, 6.76) (n=479) | 0.01*** | 0.129** |
| Normalisation in patients positive at baseline, n (%)¶¶ | 69/479 (14.4) | 47/434 (10.8) | 0.10† | 0.243‡ |
| C3 | ||||
| Median % change in patients with low C3 (<90 mg/dL) at baseline (Q1, Q3)¶¶ | 14.5 (1.25, 35.46) (n=292) | 9.0 (−4.88, 26.51) (n=293) | 0.001*** | 0.009** |
| Normalisation in patients with low C3 at baseline, n (%)¶¶ | 89/292 (30.5) | 74/293 (25.3) | 0.16† | 0.044‡ |
| C4 | ||||
| Median % change (Q1,Q3) in patients with low C4 (<16 mg/dL) at baseline (Q1, Q3)¶¶ | 40.0 (13.33, 81.82) (n=361) | 28.6 (0.00, 63.64) (n=379) | 0.003*** | 0.049** |
| Normalisation in patients with low C4 at baseline, n (%)¶¶ | 134/361 (37.1) | 112/379 (29.6) | 0.03† | 0.013‡ |
*The analysis was adjusted for the baseline value for each listed parameter using the following methods of analysis.
†Likelihood ratio test.
‡logistic regression test.
§Improved from British Isles Lupus Assessment Group (BILAG) A to B score or better, or from B to C score or better; dropout=failure.
¶2-sample t test.
**Analysis of covariance test.
††Last observation carried forward.
‡‡Dropout=failure.
§§Log-rank test.
¶¶Based on modified Systemic Lupus Erythematosus (SLE) Responder Index (SRI) analysis that excluded anti-dsDNA and complement items from determination of 4-point decrease in Safety of Estrogens in Lupus Erythematosus National Assessment-SLE Disease Activity Index (SELENA-SLEDAI) component of SRI; includes patients with data available at Week 52/primary visit.
***Wilcoxon test.
†††Cox test.
anti-dsDNA, antidouble-stranded DNA; C, complement; PGA, Physician's Global Assessment; Q, quartile; SFI, SLE Flare Index.
SRI components: SELENA-SLEDAI, BILAG and PGA
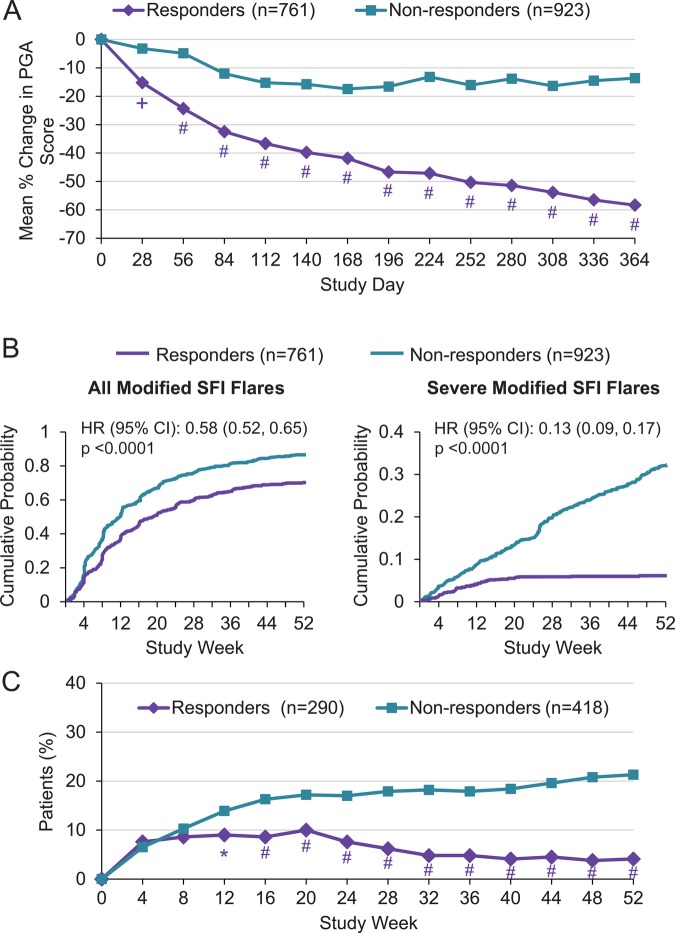
More responders than non-responders achieved a ≥4-point reduction in SELENA-SLEDAI score, with only 3.8% of non-responders meeting this SRI criterion versus 100% of responders (p<0.001) (table 2). A reduction of ≥7 in SELENA-SLEDAI score occurred in 40.3% of responders versus 1.3% of non-responders (p<0.001) at Week 52. Mean numbers of improved organ domains per patient were higher among responders as assessed by SELENA-SLEDAI and BILAG (all p<0.001). Mean improvements in PGA scores in all patients as well as those with no worsening of PGA scores at Week 52 were greater among responders versus non-responders (both p<0.001; 49.3% of non-responders had no worsening at Week 52). Responders had greater improvements in PGA than non-responders as early as Week 4 and this continued through Week 52 (figure 1A).
Figure 1.
Comparison of SRI responders and non-responders. (A) Mean % change in PGA score, (B) risk for flare by SFI, and (C) corticosteroid use over 52 weeks. *p<0.05; +p<0.01; #p<0.001. PGA, Physician's Global Assessment; SFI, SLE Flare Index; SRI, Systemic Lupus Erythematosus (SLE) Responder Index.
SRI response as a predictor of BILAG response
To evaluate whether a SRI response at Week 52 predicted improvement in BILAG items present at baseline, an analysis was performed that required a responder to have met SRI response criteria and to have had ≤1 BILAG B score present at Week 52. At baseline 64.8% of SRI responders and 57.5% of non-responders had >1 BILAG B or ≥1 A score. At Week 52 91.9% of SRI responders had ≤1 BILAG B score (table 2) compared with 35.9% of SRI non-responders (p<0.001).
SLE flare index
The risks of any flare and severe flare were lower in SRI responders (42% (HR 0.58; 95% CI 0.52 to 0.65; p<0.001) and 87% (0.13; 0.09 to 0.17; p<0.001), respectively: figure 1B).
Corticosteroid use
Approximately 62% of SRI responders and 55% of non-responders received a prednisone (or equivalent) dose >7.5 mg/d at baseline (table 1). Of these patients, more responders than non-responders had dose reductions ≥25% to <7.5 mg/d at Week 52 (25.5% vs 16.4%; p<0.001), and fewer responders who received prednisone ≤7.5 mg/d at baseline had dose increases to >7.5 mg/d at Week 52 (4.1% vs 21.3%). Over time, fewer SRI responders than non-responders had increases in prednisone dose >7.5 mg/d, with a difference beginning at Week 12 (figure 1C). The proportion of non-responders with increases in corticosteroid doses rose continually over the study period, whereas the proportion of responders did not rise after Week 4.
Serological measures
In all, 913 patients were anti-dsDNA-positive, 585 had low C3 levels (<90 mg/dL) and 740 had low C4 levels (<16 mg/dL) at baseline. Median anti-dsDNA antibody levels were lower in SRI responders than in non-responders at Week 52 (−34.2% vs −26.1%). Of patients with hypocomplementaemia at baseline, median per cent increases from baseline C3 and C4 levels were greater in responders than non-responders (C3: 14.5% vs 9.0%; C4: 40.0% vs 28.6%). More responders than non-responders exhibited normalisation of anti-dsDNA levels (14.4% vs 10.8%). Similarly, normalisation of low complement levels occurred more often in responders than in non-responders (C3: 30.5% vs 25.3%; C4: 37.1% vs 29.6%). Of 542 patients with measurements of circulating CD20 B cell subsets and plasma cell subsets at baseline and Week 52 in BLISS-76, the per cent reductions in these cell types at Week 52 were numerically greater in responders (data not shown). This finding was driven primarily by the SRI responders in the belimumab treatment groups who experienced greater reductions in B cell and plasma cell subsets than patients treated with standard therapy alone.13
Baseline covariate adjusted multivariate analysis of clinical and serological parameters
Overall results from the baseline adjusted analysis were similar to the univariate analysis. There was a greater response in SRI responders compared with non-responders, with similar p values for all the disease activity measures, including SELENA-SLEDAI, BILAG, PGA and SFI flare, as well as reduced corticosteroid use and improvement in complement levels. The only differences observed were in the serological parameters. The % median change and % normalisation of anti-dsDNA were not significantly different between SRI responders and non-responders; the normalisation of low C3 was significantly greater for SRI responders.
Patient-reported measures: HRQoL and fatigue
SRI responders were more likely to have higher baseline PCS scores than non-responders (p<0.05; table 1). Thresholds for minimum clinically important differences (MCIDs) from baseline are 2.5 points for the SF-36 PCS and MCS scores, and are generally considered 5 points for each of the eight domain scores.14 At Week 52, mean improvements in SF-36 PCS and MCS scores were greater in SRI responders versus non-responders (4.9 vs 2.6 and 4.4 vs 1.7, respectively; p<0.001) and exceeded MCID. A higher percentage of responders reported improvements ≥MCID than non-responders in PCS (59% vs 49%) and MCS (56% vs 44%). Similarly, improvements in individual domain scores were greater in SRI responders and exceeded MCID (all p<0.001). Improvements in non-responders exceeded MCID for PCS and role-physical, bodily pain and vitality domain scores. Mean improvements were ≥two-fold greater in responders versus non-responders in six of eight domains (figure 2 and see online supplementary figure S1); a consistently higher percentage of responders reported changes ≥MCID than non-responders in all domain scores (ranging from 54% vs 42%, respectively, for the role-emotional domain to 65% vs 53%, respectively, for the general health domain). At Week 52, more than twice as many responders versus non-responders reported feeling ‘somewhat better’ (76.1% vs 33.5%) and ‘much better’ (33.8% vs 14.6%) than 1 year ago.
Figure 2.
Mean change from baseline in SF-36 domain and summary scores. #p<0.001. MCID, minimum clinically important difference; MCS, Mental Component Summary; PCS, Physical Component Summary; SF-36, Medical Outcomes Survey Short Form.
Mean improvements in FACIT-Fatigue scores were higher in SRI responders than non-responders at Week 52 (5.2 vs 3.0). Improvement in the responder group exceeded MCIDs of 4 points as defined in patients with rheumatoid arthritis.15 Greater improvements in FACIT-Fatigue scores were observed by Week 8 in responders and were sustained through 52 weeks (figure 3). These findings are supported by improvements reported by responders in the SF-36 vitality domain (10.4 vs 6.5).
Figure 3.
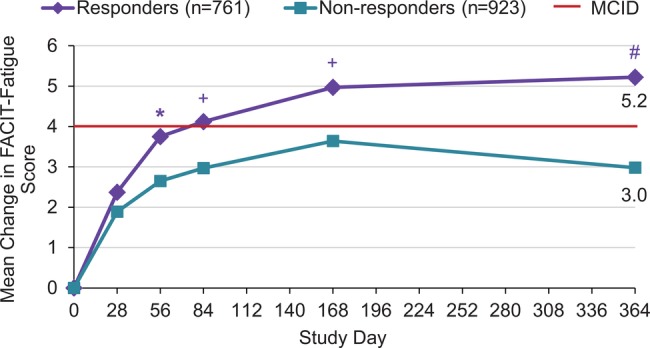
Comparison of SRI responders and non-responders for change in FACIT-Fatigue score over 52 weeks. *p<0.05; +p<0.01; #p<0.001. FACIT, Functional Assessment of Chronic Illness Therapy; MCID, minimum clinically important difference; SRI, Systemic Lupus Erythematosus (SLE) Responder Index.
Discussion
Although the lupus research community has become comfortable with SELENA-SLEDAI, BILAG and PGA as efficacy measures, the same level of understanding does not exist for the SRI. Therefore, we examined the clinical meaningfulness of SRI response in patients with active, autoantibody-positive SLE, irrespective of therapy. Improvements in a variety of clinical, serological and clinically meaningful changes in patient-reported outcome measures indicated that a SRI response was associated with global benefit beyond that measured by the components of the SRI. Overall, reductions in severe flares and corticosteroid use as well as clinically meaningful and statistically significant improvement in patient-reported outcomes correlated with SRI responder status.
While SRI responders would be expected to more frequently meet the SRI criteria (≥4-point improvement) for SELENA-SLEDAI than non-responders, 40% of responders in this analysis had improvement of ≥7 points on the SELENA-SLEDAI compared with 1% of non-responders. The improvement in PGA score in responders was greater than that achieved in non-responders, as well as in the subgroup of non-responders with no worsening in PGA scores, suggesting that SRI response is associated with a marked improvement in overall health. This finding is supported by clinically meaningful improvements in patient-reported HRQoL and fatigue, including PCS, MCS and all domain scores of SF-36, FACIT-Fatigue scores and the SF-36 transition question. In addition, SRI response was correlated with higher mean numbers of organ domains with improvement on SELENA-SLEDAI (2.00 vs 0.39) and BILAG (1.45 vs 0.40), as well as greater reductions in risk of any flare (42%) and severe flare (87%) over 52 weeks compared with non-responders. SRI response was also associated with lower overall corticosteroid use; nearly twice as many responders (25.5% vs 14%) with initial prednisone doses >7.5 mg/d were able to reduce corticosteroid doses, and 4% of responders versus 21% of non-responders with initial doses ≤7.5 mg/d had a dose increase at Week 52. These clinical benefits were observed in SRI responders as early as 8–12 weeks on study medications and this improvement generally increased over time. SRI response appeared to be predictive of BILAG response: 92% of patients, irrespective of SLE therapy, who achieved a SRI response also had ≤1 BILAG B organ score after 1 year of treatment.
Numerous studies have indicated that anti-dsDNA antibodies and low complement levels are associated with more severe disease and reduced HRQoL,16–22 and the American College of Rheumatology and European League Against Rheumatism recommend monitoring serum C3/C4 and anti-dsDNA.23 24 A SRI response was associated with a decrease in anti-dsDNA antibodies, and increases in C3 and C4 levels in patients with low complement levels at baseline compared with non-responders and more responders had normalisation of these markers. Improvements in these serological markers have been associated with reduced risk of severe flare and greater likelihood of achieving a SRI response, irrespective of therapy.13 Baseline values of B cell and plasma cell subsets were similar between SRI responders and non-responders. Although SRI responders generally had greater reductions in B cell and plasma cell subsets than non-responders, this was driven by a greater proportion of SRI responders receiving belimumab treatment, since belimumab treatment resulted in greater reductions in B cells and plasma cells than did standard therapy alone.
Other analyses of the BLISS trials have shown that the benefit of belimumab plus standard therapy over standard therapy was augmented in patients with higher disease activity, as defined by baseline SELENA-SLEDAI ≥10, low complement levels, anti-dsDNA-positivity, and corticosteroid treatment, and in patients with anti-dsDNA-positivity and low complement levels.13 25 Overall, SRI responders were more likely to have baseline high disease activity similar to the predictors of a belimumab SRI response. However, corticosteroid treatment was not predictive of a SRI response, whereas patients receiving prednisone >7.5 mg at baseline were more likely to have achieved a SRI response. Baseline serological activity was not associated with an overall greater likelihood of a SRI response, irrespective of therapy. This differential response can be partially explained by patients in the placebo and belimumab 1 mg/kg groups with high serological activity having lower rates of SRI response (31.7% and 41.5%, respectively) than the overall placebo and 1 mg/kg groups (38.8% and 46.2%, respectively), whereas the SRI responses in the 10 mg/kg group were similar in serologically active (51.5%) and all patients in that treatment group (50.6%).25
The HRQoL benefits in SRI responders support the association of a SRI response with broad improvements in SLE disease activity. The impact of SLE on HRQoL is comparable with or worse than other chronic diseases (eg, AIDS, rheumatoid arthritis, diabetes, congestive heart failure).1 21 22 26 Baseline SF-36 PCS and MCS scores in the BLISS trials reflected this high impact of SLE on HRQoL: compared with mean normative values of 50 in SF-36 summary scores, mean baseline scores were 39.1 for PCS and 40.8 for MCS. At Week 52, improvements from baseline in SF-36 PCS, MCS and all domain scores were greater in SRI responders and exceeded MCID for all scores.
Fatigue is one of the most common clinical manifestations of SLE and is associated with poor physical and mental functioning.27 Mean improvements in FACIT-Fatigue scores reported by SRI responders were greater than in non-responders, with changes from baseline exceeding MCID from Weeks 12 to 52 in those achieving a SRI response at Week 52. However, it should be noted that a MCID of 4, while valid in patients with rheumatoid arthritis, has not yet been validated in patients with SLE. However the MCIDs for SF-36 summary and domain scores were independently validated in SLE and correspond closely to those determined in rheumatoid arthritis. Reductions in fatigue were confirmed by a greater increase in SF-36 vitality domain score, consistent with other published data indicating a high correlation between these measurements.15 28 Finally, three-quarters of responders indicated that they felt ‘somewhat’ or ‘much better’ than 1 year before compared with a third of non-responders.
Interpretation of these study results is limited by the post hoc nature of the analyses. In addition, examining a clinical trial population based on achievement of the primary end point (SRI at Week 52) eliminates the randomised balance of baseline characteristics in the treatment groups. Baseline characteristics were, however, generally similar between SRI responders and non-responders. A baseline covariate adjusted analysis showed results similar to the unadjusted univariate analysis. Further, there was a greater magnitude of difference in clinical and patient reported outcomes between these groups than in the baseline characteristics. Although some individual variables examined were response criteria that, by definition, were met by responders, analysis of results for these criteria in non-responders remains instructive.
Conclusions
Our results indicate that SRI responses, irrespective of therapy, are associated with global, clinically meaningful benefits in patients with active, autoantibody-positive SLE.
Supplementary Material
Acknowledgments
Editorial support was provided by Matt Stenger and Eleanore Gross of BioScience Communications, New York, USA, and Louisa Pettinger, Fishawack Indicia Ltd, Knutsford, UK.
Footnotes
Contributors: RF, MAP, VS, DDG, ZJZ and WWF designed the analysis. RF, MAP, DDG, ZJZ and WWF acquired data. RF, MAP, VS, DDG, ZJZ and WWF analysed and interpreted the data. RF, MAP, VS, DDG, ZJZ and WWF prepared the manuscript. ZJZ performed statistical analysis. WWF was responsible for overall project management. RF had full access to the data in the studies and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This study was supported by Human Genome Sciences (HGS), Rockville, Maryland, USA, and GlaxoSmithKline (GSK), Uxbridge, Middlesex, UK. Editorial support was funded by HGS and GSK.
Competing interests: RF and MAP have received research or grant support, travel support and payment for review activities, board membership and consultancy from Human Genome Sciences and GlaxoSmithKline. VS has received consultancy fees from Human Genome Sciences and GlaxoSmithKline. DDG has received research or grant support from Human Genome Sciences and GlaxoSmithKline. ZJZ and WWF are employed and own stock or stock options in Human Genome Sciences.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: GSK facilitates annonymized data sharing through the www.ClinicalStudyDataRequest.com website.
References
- 1.Lau CS, Mak A. The socioeconomic burden of SLE. Nat Rev Rheumatol 2009;5:400–4. [DOI] [PubMed] [Google Scholar]
- 2.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med 2008;358:929–39. [DOI] [PubMed] [Google Scholar]
- 3.Cancro MP, D'Cruz DP, Khamashta MA. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J Clin Invest 2009;119:1066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:3918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarra SV, Guzmán RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomized, placebo-controlled phase 3 trial. Lancet 2011;377:721–31. [DOI] [PubMed] [Google Scholar]
- 6.Strand V, Levy R, Cervera R, et al. Improvements in health-related quality of life with belimumab, a B-lymphocyte stimulator-specific inhibitor, in patients with autoantibody-positive lupus erythematosus. Ann Rheum Dis 2014;73:838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furie R, Petri M, Wallace DJ, et al. Novel evidence based systemic lupus erythematosus responder index. Arthritis Rheum 2009;61:1143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United States (US) Food and Drug Administration (FDA). Guidance for Industry: Systemic lupus erythematosus—developing medical products for treatment. June 2010. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072063.pdf (accessed Jan 2014).
- 9.Manzi S, Sanchez-Guerrero J, Merrill JT, et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Ann Rheum Dis 2012;71:1833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buyon JP, Petri MA, Kim MY, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med 2005;142:953–62. [DOI] [PubMed] [Google Scholar]
- 11.Petri M, Buyon J, Kim M. Classification and definition of major flares in SLE clinical trials. Lupus 1999;8:685–91. [DOI] [PubMed] [Google Scholar]
- 12.Petri M, Kim MY, Kalunian KC, et al. OC-SELENA Trial: Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med 2005;353:2550–58. [DOI] [PubMed] [Google Scholar]
- 13.Stohl W, Hiepe F, Latinis KM, et al. Belimumab reduces autoantibodies, normalizes low complement, and reduces select B-cell populations in patients with systemic lupus erythematosus. Arthritis Rheum 2012;64:2328–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strand V, Crawford B. Improvement in health-related quality of life in patients with SLE following sustained reductions in anti-dsDNA antibodies. Expert Rev Pharmacoecon Outcomes Res 2005;5:317–26. [DOI] [PubMed] [Google Scholar]
- 15.Cella D, Yount S, Sorensen M, et al. Validation of the functional assessment of chronic illness therapy fatigue scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol 2005;32:811–19. [PubMed] [Google Scholar]
- 16.Biesen R, Dähnrich C, Rosemann A, et al. Anti-dsDNA-NcX ELISA: dsDNA-loaded nucleosomes improve diagnosis and monitoring of disease activity in systemic lupus erythematosus. Arthritis Res Ther 2011;13:R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merrill JT, Buyon JP. The role of biomarkers in the assessment of lupus. Best Pract Res Clin Rheumatol 2005;19:709–26. [DOI] [PubMed] [Google Scholar]
- 18.Petri M, Singh S, Tesfasyone H, et al. Prevalence of flare and influence of demographic and serologic factors on flare risk in systemic lupus erythematosus: a prospective study. J Rheumatol 2009;36:2476–80. [DOI] [PubMed] [Google Scholar]
- 19.Schur PH, Sandson J. Immunologic factors and clinical activity in systemic lupus erythematosus. N Engl J Med 1968;278:533–38. [DOI] [PubMed] [Google Scholar]
- 20.Strand V, Chu AD. Measuring outcomes in systemic lupus erythematosus clinical trials. Expert Rev Pharmacoecon Outcomes Res 2011;11:455–68. [DOI] [PubMed] [Google Scholar]
- 21.Strand V, Petri M, Buyon J, et al. Systemic lupus erythematosus (SLE) impacts all domains of health-related quality of life (HRQOL): baseline results from five randomized controlled trials (RCTs). Ann Rheum Dis 2007;66II):482. [Google Scholar]
- 22.Thumboo J, Strand V. Health-related quality of life in patients with systemic lupus erythematosus: an update. Ann Acad Med Singapore 2007;36:115–22. [PubMed] [Google Scholar]
- 23.ACR Guidelines for Referral and Management of SLE in Adults. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus guidelines. Guidelines for referral and management of systemic lupus erythematosus in adults. Arthritis Rheum 1999;42:1785–96. [DOI] [PubMed] [Google Scholar]
- 24.Bertsias G, Ioannidis JP, Boletis J, et al. Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics: EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis 2008;67:195–205. [DOI] [PubMed] [Google Scholar]
- 25.van Vollenhoven RF, Petri MA, Cervera R, et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis 2012;71:1343–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell R, Jr, Cooper GS, Gilkeson GS. Two aspects of the clinical and humanistic burden of systemic lupus erythematosus: mortality risk and quality of life early in the course of disease. Arthritis Rheum 2008;59:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zonana-Nacach A, Roseman JM, McGwin G, Jr, et al. Systemic lupus erythematosus in three ethnic groups. VI: factors associated with fatigue within 5 years of criteria diagnosis. LUMINA Study Group. LUpus in MInority populations: NAture vs Nurture. Lupus 2000;9:101–9. [DOI] [PubMed] [Google Scholar]
- 28.Strand V, Smolen JS, van Vollenhoven RF, et al. Certolizumab pegol plus methotrexate provides broad relief from the burden of rheumatoid arthritis: analysis of patient-reported outcomes from the RAPID 2 trial. Ann Rheum Dis 2011;70:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.