Abstract
Background
Urachal carcinomas occur mostly in the bladder dome, comprising 22 to 35% of vesical adenocarcinomas, and are generally treated by partial cystectomy with en bloc resection of the median umbilical ligament and umbilicus. Detailed pathologic studies with clinical outcome correlation are few.
Design
We reviewed histologic material and clinical data from 24 cases selected from a database of 67 dome-based tumors diagnosed and treated at our institution from 1984 to 2005. Follow-up information was available for all 24 patients.
Result
The mean age at diagnosis was 52 years (range 26-68). 15 patients were male and 9 were female. Location was the dome in 23, and dome and anterior wall in 1. Thirteen cases were pure adenocarcinoma, NOS, 9 were enteric type adenocarcinoma and 2 were adenocarcinoma with focal components of lymphoepithelioma-like carcinoma and urothelial carcinoma with cytoplasmic clearing. Signet ring cell features were focally seen in 2 cases. Cystitis cystica and cystitis glandularis were seen in 4 and 2 cases, respectively. In all instances but one, cystitis cystica/ glandularis was focal and predominantly in the bladder overlying the urachal neoplasm. Urachal remnants were identified in 15 cases: the urachal epithelium was benign urothelial-type in 6 cases and showed adenomatous changes in 9. The overlying bladder urothelium was colonized by adenocarcinoma in 3 cases. In all three, urachal remnants were identified and showed transition from benign to adenomatous epithelium. On immunohistochemistry, these tumors were positive for CK20 and variably positive for CK7 and 34BE12. The majority showed a cytoplasmic membranous staining pattern for beta-catenin, although in 1 case, focal nuclear immunoreactivity was identified.. The Sheldon pathologic stage was pT1 in 0, pT2 in 2, pT3a in 8, pT3b in 11, pT3c in 1, pT4a in 1 and pT4b in 1 patient. One patient had a positive soft tissue margin. The mean follow-up period was 40 months (range 0.3-157.6). Seven of 24 (29%) cases recurred locally. The incidence of local recurrence was higher in patients who underwent a partial cystectomy alone (37.5%) versus those who had a more radical surgery (27%). Distant metastases occurred in 9 (37.5%) patients, 4 of which had no prior local recurrence. Seven patients (29%) died of the disease. All cases with locally recurrent and metastatic disease belonged to stage pT3 or higher.
Conclusion
Pathologic stage is an important prognostic factor in urachal carcinoma. Surface urothelial involvement by carcinoma and presence of cystitis cystica/ glandularis do not necessarily exclude the diagnosis of urachal carcinoma. Immunostains do not unequivocally discriminate a urachal from a colorectal carcinoma, but diffuse positivity for 34BE12 would support, and diffuse nuclear immunoreactivity for beta catenin would militate against, a diagnosis of urachal carcinoma.. Local recurrence may be due to seeding within the distal urothelial tract, particularly in tumors with a configuration that is polypoid and which open into the bladder cavity. The type of surgery performed may have an effect on local recurrence despite negative margins of resection.
INTRODUCTION
Urachal carcinoma is rare and comprises 0.35 to 0.7 % of all bladder cancers and 22-35% of vesical adenocarcinomas (17, 19). The first extensive description of this entity was made by Begg in 1931 (2). Its rarity has translated into a very limited number of published clinicopathologic series, especially in the pathology literature (13, 15).
The urachus is a vestigial structure that connects the bladder to the allantois during early embryonic development. After birth it becomes a fibrous cord known as the median umbilical ligament. If remnants of the allantois remain within the ligament, they may develop into cysts and epithelial neoplasms. Urachal remnants have been identified in one third of cases in post mortem studies; in the dome and anterior wall commonly and rarely in the posterior wall of the bladder (2, 18). The urachus has intramucosal, intramuscular and supravesical segments. It contains three distinct tissue layers: an epithelial canal lined by urothelium, submucosal connective tissues and an outer layer of smooth muscle. Urachal neoplasms can arise in any of these layers, and can be epithelial or mesenchymal. Similar to urothelium at other sites, the epithelium often demonstrates focal glandular metaplasia, and this provides a morphologic basis for the development of intestinal-type tumors (2, 18).
The criteria for a diagnosis of urachal carcinoma are somewhat controversial but most investigators agree with those set forth by Sheldon et al (19) and Mostofi et al (14). These include (a) tumor in the dome of the bladder; (b) absence of cystitis cystica and cystitis glandularis; (c) predominant invasion of the muscularis or deeper tissues with a sharp demarcation between the tumor and surface bladder urothelium which is free of glandular or polypoid proliferation; (d) presence of urachal remnants within the tumor; (e) extension of tumor into the bladder wall with involvement of the space of Retzius, anterior abdominal wall or umbilicus, and (f) no evidence of a primary neoplasm elsewhere. A few staging systems have recently been proposed (13, 16), but commonly followed is that proposed by Sheldon et al (19): pT1 – no invasion beyond the urachal mucosa; pT2 – invasion confined to the urachus; pT3 – local extension to the (a) bladder, (b) abdominal wall, (c) viscera other than the bladder, and pT4 – metastasis to (a) regional lymph nodes, (b) distant sites.
The mainstay of treatment for these tumors is partial cystectomy with en bloc resection of the median umbilical ligament up to the umbilicus (9). There are conflicting results in the literature on whether prognosis and survival are worse for urachal as compared to nonurachal bladder adenocarcinoma (7, 14, 24). Because of the silent nature of early lesions, their propensity for local growth and the tendency to metastasize late in the clinical course, a large proportion of patients with carcinomas of urachal origin present with disease at stage pT3 or higher.
Very few studies have comprehensively described the clinicopathologic features with outcome correlation as a case series. The goal of this study was to carry out a detailed morphologic assessment of this entity, and correlate it with staging and outcome parameters.
MATERIALS AND METHODS
Approval to perform this study was obtained from the institutional review board as well as the human tissue utilization committee. A total of 67 tumors located in the dome of the urinary bladder diagnosed and treated at Memorial Sloan Kettering Cancer Center by radical cystectomy or partial cystectomy between 1984 to 2005 were identified. Hematoxylin and eosin stained slides were obtained from the pathology files of MSKCC. Of the 67 cases, 24 cases fulfilled the histologic criteria for urachal carcinoma. We used the following 4 histologic criteria, modified from the Sheldon criteria, for inclusion in our cohort:
location of the tumor in the dome/ anterior wall
epicenter of carcinoma in the bladder wall
Absence of widespread cystitis cystica/ glandularis beyond the dome/ anterior wall
Absence of a known primary elsewhere
This cohort was smaller that described in a recent study of urachal carcinoma from our institution by Herr et al (9), where the definition of ‘urachal’ included all dome-based tumors for which the therapeutic approach was similar.
Patient demographics, clinical history, treatment and follow-up details, including outcome information, were obtained from the patient charts. We reviewed all biopsies, transurethral resection specimens as well as the partial or radical cystectomy specimens with the corresponding pelvic lymph node dissections. The gross findings including the size and geographical location of the tumor were noted from the pathology reports. The morphologic characteristics of the carcinoma including location in the bladder wall, histologic subtype and invasion pattern, the presence and characteristics of the urachal remnants as well as the presence and location of associated cystitis cystica and glandularis were recorded. The presence of lymphovascular invasion, status of margins and peritoneal involvement or lack thereof were also noted. Pathologic staging was performed according to the staging system of Sheldon et al (19).
A panel of immunohistochemical stains was performed on a representative formalin fixed paraffin embedded tumor block from 15 of the 24 cases where adequate tumor was available for evaluation. These included CK7 (monoclonal; DAKO, Carpinteria, CA; CK20 (monoclonal, DAKO, Carpinteria, CA); 34BE12 (monoclonal; DAKO, Carpinteria, CA); CDX-2 (monoclonal; Biogenex, San Ramon, CA) and beta catenin (monoclonal, Ventana). For CK7, CK20 and 34BE12, any cytoplasmic staining was interpreted as positive (focal, when only patchy staining of the tumor cells was present or diffuse, when the majority of the tumor cells in most fields stained uniformly and strongly). For CDX-2 any nuclear staining was considered positive. For beta-catenin, both cytoplasmic membranous positivity as well as any nuclear immunoreactivity were evaluated. A control sample set of colonic adenocarcinoma cases available on a TMA was also stained for 34BE12 and beta-catenin.
RESULTS
The patient demographics and individual clinicopathologic features of the cases are tabulated in tables 1a and 1b. The mean age of the patients at diagnosis was 52 years (range 26-68). Fifteen patients were male and 9, female (male to female ratio 1.7:1). The breakdown of the initial surgical procedure performed is as follows: partial cystectomy with or without a bilateral pelvic lymph node dissection in 9 cases, an extended partial cystectomy with resection of the umbilicus with or without a pelvic lymph node dissection in 11 cases, a radical cystectomy in one case, radical cystoprostatectomy in 2 cases, and a resection of a presumptive urachal cyst in one case. In 6 cases (cases 5, 8, 11, 12, 17 and 24), surgery was followed by chemotherapy. In addition, cases 8, 10 and 24 also received adjuvant radiation therapy.
TABLE 1.
CLINICOPATHOLOGIC FEATURES OF INDIVIDUAL CASES OF URACHAL CARCINOMA
| Case | Age | Sex | Presenting symptoms | Location | Size(cm) | Histology | Mucin/ signet ring | CC/CG | Urachal remnants | Sheldon stage at diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | M | Gross hematuria | Dome | 1.7 | Adeno, E | >50%; No | CC + CCI | Yes, Adenomatous | pT3a |
| 2 | 60 | F | Gross hematuria | Dome | 3.5 | Adeno NOS + Urothelial (clear) | No, No | No | Yes, Adenomatous | pT3a |
| 3 | 62 | M | Gross hematuria | Dome | 0.7 | Adeno, E | Predominant; Yes | CC | Yes, Benign | pT3a |
| 4 | 46 | M | Urethral discharge with blood | Dome | 3 | Adeno, E | Predominant; Yes | No | Yes, Glandular intestinal + Benign transitional | pT3b |
| 5 | 43 | M | Gross hematuria | Dome | 4.8 | Adeno, NOS | No; No | No | Yes, Adenomatous | pT3b |
| 6 | 61 | F | Multiple recurrent UTI's | Dome | 4.5 | Adeno, E | >50%; No | No | None | pT3b |
| 7 | 29 | M | Pyuria followed by hematuria | Dome | NA | Adeno, NOS | >50%; No | CG | Yes; Villous adenoma + benign transitional | pT2/pT3a (TUR) |
| 8 | 61 | M | Gross hematuria | Dome + Ant wall | NA | Adeno, NOS | >50%; No | No | None | pT3b |
| 9 | 49 | M | Draining mucoid sinus to skin | Dome | NA | Adeno, NOS | >50%; No | CG | Yes; Benign transitional | pT3b |
| 10 | 65 | M | Obstructive | Dome | 1.9 | Adeno, E | >50%; No | CG | None | pT3b |
| 11 | 63 | M | N/A | Dome | NA | Adeno, E | >50%; Focal | No | None | pT4b |
| 12 | 37 | M | Mucus per urethra for years then gross hematuria | Dome | 0.5, 0.4 | Adeno, NOS | >50%; Focal | No | Suggestive; replaced by carcinoma | pT3b |
| 13 | 42 | M | Gross hematuria | Dome | 2.2 | Adeno, E | No; No | No | Yes; Benign transitional | pT3c |
| 14 | 26 | M | Gross hematuria | Dome | NA | Adeno, NOS | >50%; No | No | Yes; Benign transitional | pT3b |
| 15 | 44 | F | N/A | Dome | NA | Adeno, NOS | >50%; No | No | Suggestive; replaced by carcinoma | pT3b |
| 16 | 60 | F | Gross hematuria | Dome | NA | Adeno, NOS | >50%; No | CC | Yes; Adenomatous | pT2 |
| 17 | 35 | F | Abdominal mass | Dome | NA | Adeno, NOS | >50%; No | CC | Yes; Benign transitional | pT3a |
| 18 | 68 | F | N/A | Dome | NA | Adeno, NOS | >50%; No | No | None | pT3b |
| 19 | 59 | M | Gross hematuria | Dome | NA | Adeno, NOS | >50%; No | No | None | pT3a |
| 20 | 55 | F | Gross hematuria | Dome | 0.9 (micro) | Adeno, NOS | >50%; Yes | CC | Yes; Adenomatous with IM | pT2 |
| 21 | 60 | F | Bleeding (hematuria vs. vaginal) | Dome | 2.5 | Adeno, NOS + Lymphoepithelioma-like CA | No; No | No | Yes; Severe dysplasia + Benign transitional | pT3b |
| 22 | 50 | M | Gross hematuria | Dome | 8 | Adeno, E + Micropapillary CA | No; No | No | Yes; Adenomatous | pT4a |
| 23 | 53 | M | Gross hematuria | Dome | 6 | Adeno E | <50%; No | No | None | pT3a |
| 24 | 58 | M | Gross hematuria | Dome | NA | Adeno E + Villous adenoma | <50%; No | CG | Yes; Villous adenoma and benign transitional | pT3a |
The tumor was located in the dome of the bladder in 23 cases, and the dome and anterior wall in 1. The overlying urothelium was involved by adenocarcinoma in 3 cases. In all three, urachal remnants were identified, and showed a transition from benign to adenomatous epithelium (FIGURE 4 inset). Lymphovascular invasion was identified in two cases.
4.
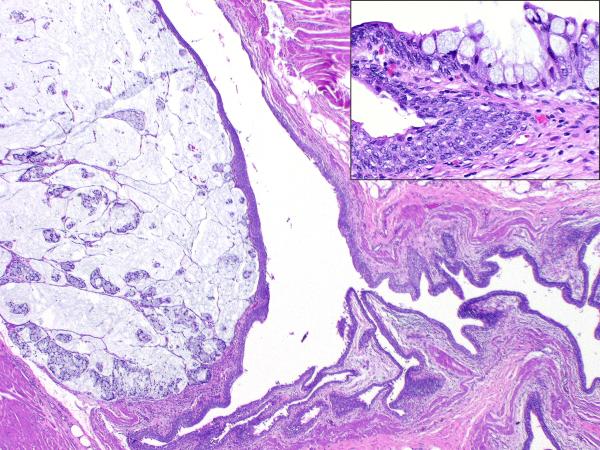
Low magnification image showing a colloid carcinoma arising in a urachal remnant; Inset – transition from benign urothelium lining the remnant to adenomatous epithelium containing goblet cells
The breakdown of disease stage at the time of definitive surgery was as follows: pT1 (0 cases); pT2 (2 cases); pT3a (8 cases); pT3b (11 cases); pT3c (1 case); pT4a (1 case); pT4b (1 case). 7 of 24 (29%) patients recurred locally. The histologic features are tabulated in Table 2 and demonstrated in figures 2A-D. On histology, 13 showed adenocarcinoma NOS, 9 were adenocarcinoma with enteric/ colonic differentiation, one of these also with signet ring cells. One case each showed adenocarcinoma mixed with urothelial carcinoma with clear cell features, and lymphoepithelioma-like carcinoma. There was no case of predominant urothelial carcinoma, NOS. The immunohistochemical staining results are summarized in table 3 and demonstrated in figures 3A-B. The mean follow-up period was 43 months (range 0.9-155.6). The clinical outcome data are summarized in table 4. Twenty nine percent (7/24) of cases recurred. The time interval to recurrence from initial surgery ranged from 9.6 months to 22 months (range: 13.9 months). Sites of recurrence included - urinary bladder (5 cases), prostate into the prostatic ducts and seminal vesicles (1 case), prostate along with prostatic and membranous urethra (1 case), retroperitoneal soft tissue (1 case) and pubic bone (1 case). The breakdown of disease stage in these 7 patients was pT3a (3 cases) and pT3b (4 cases). 5 of these 7 patients eventually died of the disease (pT3a-2; pT3b-3). The other two remain alive with no evidence of disease, after 18.2 and 92.1 months follow-up. The first of these two underwent an extended partial cystectomy with umbilical resection and pelvic lymph node dissection, while the second had a partial cystectomy followed by multiple recurrences along the distal urothelial tract for which he subsequently received radiation therapy.
TABLE 2.
CLINICAL FEATURES OF INDIVIDUAL CASES OF URACHAL CARCINOMA
| Case | Age | Sex | Surgery performed | Local Recurrence (Number; Site) | Time initial surgery to recurrence (mos) | Mets (site) | Time (initial dx to mets) (mos) | Length of follow-up (mos) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | M | PC | NO | NA | No | NA | 9.1 | Alive; NED |
| 2 | 60 | F | EPC + U + PLND | 1; Urinary bladder | 10.6 | No | 18.2 | Alive; NED | |
| 3 | 62 | M | PC | NO | NA | No | 74.5 | Alive; NED | |
| 4 | 46 | M | EPC + U + PLND | NO | NA | No | 9 | Alive; NED | |
| 5 | 43 | M | PC | NO | NA | Yes; lymph node | 16.5 | 30.3 | Alive; NED |
| 6 | 61 | F | EPC + U + PLND | NO | NA | No | 50.5 | Alive; NED | |
| 7 | 29 | M | EPC + U | NO | NA | No | 17.2 | Alive; NED | |
| 8 | 61 | M | EPC + U + PLND | 1; Urinary bladder | 10.6 | Yes; lymph node, lung | 17.6 | 32.8 | DOD |
| 9 | 49 | M | Radical CP + Ant abd wall resection | NO | NA | No | NA | 18.1 | Alive |
| 10 | 65 | M | PC | 1; Prostate, seminal vesicles | 14.3 | No | NA | 92.1 | Alive; NED |
| 11 | 63 | M | EPC + U + PLND | NO | NA | Yes; peritoneal implants at surgery; lung mets 1 year later | 0 | 24.4 | DOD |
| 12 | 37 | M | EPC + U + PLND | 1; Urinary bladder | 16.3 | Yes; retroperitoneum, anterior abdominal wall, bone | 16.3 | 27.4 | DOD |
| 13 | 42 | M | Radical cystoprostatectomy + PLND | NO | NA | No | NA | 16.6 | Alive; NED |
| 14 | 26 | M | EPC + U + PLND | NO | NA | No | NA | 0.9 | Alive; NED |
| 15 | 44 | F | PC | NO | NA | Yes; lung | 24 | 40.1 | Alive |
| 16 | 60 | F | EPC + U + PLND | NO | NA | No | NA | 155.6 | Alive; NED |
| 17 | 35 | F | Local excision of presumptive urachal cyst | 1; Urinary bladder | 9.6 | Yes; Right ovary | 9.6 | 23.8 | DOD |
| 18 | 68 | F | PC + PLND | 2; Pubic bone, Urinary bladder | 22.00 | Yes; peritoneal implants small bowel | 29.1 | 55.1 | DOD |
| 19 | 59 | M | PC | NO | NA | Yes; soft tissue pubic symphysis | 12 | 40.7 | DOD |
| 20 | 55 | F | RC | NO | NA | No | NA | 23.5 | Alive; NED |
| 21 | 60 | F | EPC + U + PLND + TAHBSO | NO | NA | No | NA | 42.1 | Alive; NED |
| 22 | 50 | M | PC + PLND | NO | NA | Yes; perivesical lymph node at initial surgery; lung - 3 mos later | 0 | 10.8 | Alive; WD |
| 23 | 53 | M | EPC + U + PLND | NO | NA | No | NA | 82 | Alive; NED |
| 24 | 58 | M | PC + PLND | 5; Prostate, prostatic and membranous urethra, late recurrence in the retropubic region | 12 | Yes; perisplenic soft tissue | 132.8 | 139.3 | DOD |
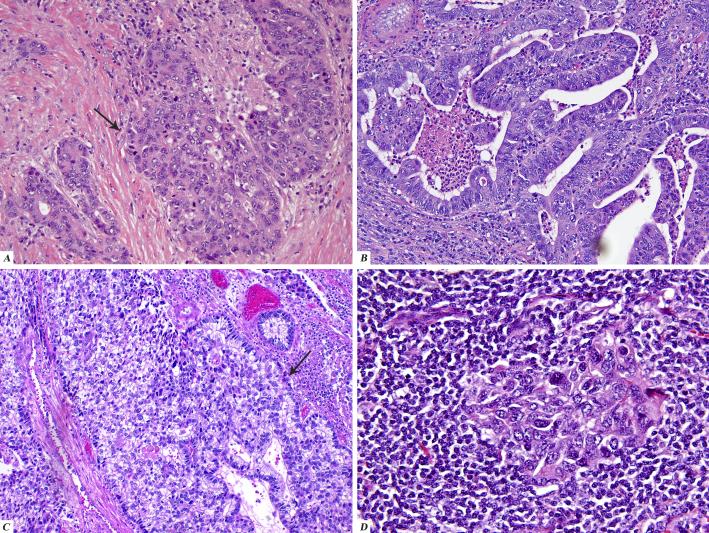
2.
Histologic patterns observed in urachal carcinoma
A. Urothelial carcinoma, NOS (arrow) with focal glandular differentiation on the left
B. Adenocarcinoma, NOS
C. Urothelial carcinoma with cytoplasmic clearing and focal glandular differentiation (arrow)
D. Lympho-epithelioma like carcinoma component
TABLE 3.
MORPHOLOGIC FEATURES IN URACHAL CARCINOMA
| SUBTYPE (24) | Adenocarcinoma, NOS | 13 |
| Adenocarcinoma, E | 9 | |
| Other | 2 | |
| URACHAL REMNANTS (15) | Benign “transitional” | 6* |
| Adenomatous | 9 | |
| INVASION PATTERN (24) | No invasion | 2 |
| Pushing | 2 | |
| Infiltrating | 20 | |
| CYSTITIS CYSTICA/CYSTITIS GLANDULARIS (9) | Cystitis cystica | 5 |
| Cystitis glandularis | 4 |
In one case the epithelium had focal glandular metaplasia
NOS – Not otherwise specified
E – Enteric (including mucinous adenocarcinomas)
Other – One case each of urothelial carcinoma with cytoplasmic clearing and lympho-epithelioma like differentiation
3.
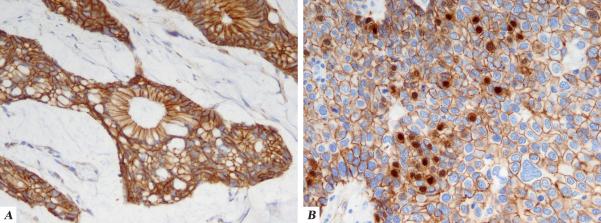
Immunohistochemical staining patterns in urachal carcinoma:
A. Strong, diffuse cytoplasmic membranous staining for beta-catenin
B. Focal nuclear staining for beta-catenin (seen in one case) in the background of diffuse cytoplasmic membranous staining
TABLE 4.
IMMUNOHISTOCHEMICAL PROFILE OF URACHAL CARCINOMA
Cytoplasmic
Nuclear
Distant metastases occurred in 9 patients (37.5%). In 4 of the 9 cases distant metastases occurred without prior local recurrence. Three patients were pT3a, 4 patients were stage pT3b, and 1 patient each was pT4a and pT4b. The most common sites of metastases were regional lymph node and lungs (4 instances each), followed by peritoneum and anterior abdominal wall, bone and soft tissue, and in one instance, the ovary. The time interval between initial diagnosis and metastases ranged from 0 months to 132.8 months (average 28.7 months).
Seven patients (29%) died of the disease. The breakdown of pathologic stage in this patient cohort is as follows: pT3a (3 cases), pT3b (3 cases) and pT4b (1 case). 5 of these 7 had local recurrences in the urinary bladder as well as distant metastases prior to death. The remaining two had only distant metastases before death. Of the other two patients with distant metastases, one was alive with disease and one had no evidence of disease.
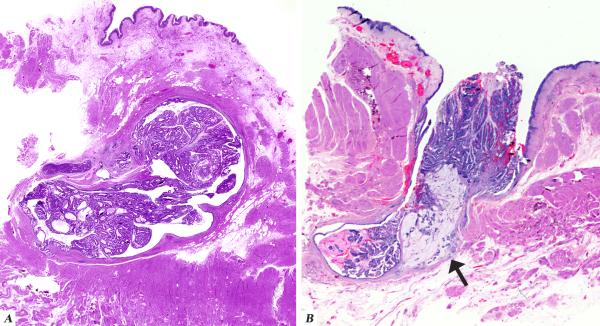
Interestingly, two patients had multiple local recurrences in the form of seeding in the genitourinary tract. The first patient (case 10) was a 65 year old male who was diagnosed with a urachal adenocarcinoma located in the dome of the bladder for which a partial cystectomy was performed. Surgical pathology evaluation showed surface involvement in the form of a villous adenoma. There was also a positive soft tissue margin for which the patient underwent external beam radiotherapy. Five years later the patient was found to have palpable disease in his prostate and a biopsy was consistent with adenocarcinoma morphologically similar to his urachal carcinoma. The patient was also found to have a nodule in the abdominal wall and subsequently underwent an en bloc resection of the abdominal wall recurrence and a cystoprostatectomy. Final pathology revealed extensive involvement of the prostate, prostatic urethra, as well as the seminal vesicles. The histology of the recurrent in-situ carcinoma was similar to that of the primary. Immunohistochemical studies demonstrated the tumor to be positive for CK20 and negative for CK7, PSA and PSAP, supporting the fact that it was not prostate cancer. The second patient (case 24) was a 62 year old male with a long standing history of prostatitis who presented with gross hematuria. A cystoscopic examination revealed a tumor in the dome of the bladder for which he underwent a partial cystectomy with retroperitoneal lymph node dissection. Pathologic examination showed a villous tumor arising within a urachal remnant in the muscularis propria of the dome protruding into the lumen of the urinary bladder. (FIGURE 1B) One year later, he once again had hematuria for which he underwent cystoscopy with local resection of five small papillary tumors. For the next seven years he had several episodes of bloody mucus discharges for which he was treated with transurethral resections. The histology in all of these recurrences was adenocarcinoma which was morphologically similar to the patient's original tumor. Since the tumor was purely non invasive, it appeared to represent seeding from the original tumor. An immunostain for PSA done on one of the specimens was negative, thus arguing against a prostatic origin. Eventually cystoprostatectomy with an ileal conduit placement was performed.
1.
A Low magnification image demonstrating carcinoma arising within and confined to the urachal remnant
Figure 1B Low magnification image demonstrating a villous tumor arising from the urachus and communicating with the lumen of the urinary bladder. Arrow indicates the invasive component
The pathology revealed moderately differentiated adenocarcinoma involving the prostatic urethra, the proximal membranous urethra and prostate gland, with extension along the prostatic ducts. The urinary bladder did not contain any tumor. There was no vascular invasion and the margins were negative. Three years later, he developed local recurrence in the right retropubic region, for which he received radiation therapy. Four months later follow up CT-scans revealed that he had stable disease in the right suprapubic region, but developed a lesion that was abutting the spleen, for which he received chemotherapy.
All 15 urachal tumors we studied were strongly and diffusely CK20 positive, while positive staining for CK7 was present in about half. Only 10 of the 15 tumors studied (66%) stained with 34BE12 , and in the majority of these only very focal staining was seen. CDX-2 stained all 15 tumors: 8 diffusely and the remaining 7 in a patchy manner. We observed strong and diffuse cytoplasmic membranous immunoreactivity for beta catenin in 14 of the 15 (93%) tumors studied. In one case (case 2), in addition to the adenocarcinoma component showing the typical cytoplasmic membranous pattern, the clear cell urothelial component demonstrated focal nuclear immunoreactivity in up to 15% of nuclei. CDX-2 was diffusely positive in both components. Of the 81 cases of colorectal adenocarcinoma evaluable with the 34BE12 stain, 72 (89%) were negative, while 9 (11%) showed rare or focal cytoplasmic reactivity. 73 cases of colorectal carcinoma were evaluable for the beta-catenin immunostain and of them, 55 (75%) showed only cytoplasmic membranous reactivity while 18 (25%) showed cytoplasmic membranous reactivity with focal or diffuse nuclear accentuation.
DISCUSSION
Herr and collaborators recently reported 50 cases of urachal carcinoma from our institution (9). However is was strictly a clinical paper, where all dome based tumors were considered ‘urachal’, the logic being that the therapeutic approach would be similar in all cases. In the current study, the case selection process was much more rigorous and took into account the topographic and pathologic features of each case, and therefore included a smaller number of cases. The pathologic criteria proposed by Sheldon et al (19) and Mostofi et al (14) for the diagnosis of urachal carcinoma are considered somewhat restrictive. Certainly a deviation from the first two, i.e., 1. tumor in the dome of the bladder, and 2. absence of cystitis cystica and cystitis glandularis, does not preclude a diagnosis of urachal carcinoma. This is supported by the fact that urachal remnants have been observed in the midline or vertex in only 54% of patients while they have reportedly been identified in the anterior wall in 2% (18). Schubert, Pavkovic and Bethke-Bedurftig (18) have also identified them in the posterior wall in 14%. However the latter is difficult to explain since the majority of tumors arising in these remnants, are known to be dome-based (10, 14), with a minority centered in the anterior wall. This was also true of our series, where in 23 cases, the location was the dome. Only one of the tumors additionally involved the anterior wall along with the dome. It is possible that remnants in some of the cases mentioned by Schubert et al may be other epithelial vestiges located in the posterior wall.
In the literature, whereas adenocarcinomas of the bladder have a relatively higher incidence in women as compared to urothelial carcinomas, most series on urachal carcinomas have reported a higher incidence in men (10, 11). Hematuria was the most common presenting symptom, followed by a palpable suprapubic mass and mucosuria (19). In our series, men were affected to a greater extent (15 men versus 9 women). The most common clinical presentation was gross hematuria (15 cases), which is similar to vesical adenocarcinoma. Less frequent presenting symptoms included bloody and mucoid urethral discharge, draining mucoid sinus to skin, recurrent urinary tract infections, abdominal mass and obstructive urinary symptoms.
In published series, the commonest histologic subtype of urachal carcinoma is adenocarcinoma with enteric features with or without mucin production (10, 17). Some of the adenocarcinomas have a signet ring component in the form of signet ring cells in association to extravasated mucin, and some have the morphology of colloid carcinomas. Squamous cell carcinoma, typical urothelial and anaplastic carcinomas may also arise in the urachus (6, 19). Villous adenomas of the urachus have been reported, sometimes associated with mucosuria, which suggests a continuity with the bladder lumen (4). Rarely mesenchymal neoplasms may also originate from these vestigial remnants (22), although we did not identify any. According to some investigators (10), there is no association between prognosis and tumor type (colloid versus enteric/ colonic), the degree of differentiation and the presence and amount of signet ring cells. Others (1) have reported tumors with lower grade to be strongly correlated with lower disease stage and better outcome.
In our series, the most frequent histologic subtype was adenocarcinoma, NOS (13 cases; 50%). Adenocarcinomas of the enteric type with a variable component of signet ring cells and mucinous features made up 9 cases ( 37.5%). No cases of “true” signet ring cell carcinoma (tumor with diffuse infiltrative growth, diffuse wall thickening and devoid of extracellular mucin) were identified. Within the adenocarcinoma category, the differentiation of the tumor, presence or absence of mucin production or the presence or absence of signet ring cells did not appear to influence outcome, although the number of cases were too small to draw any definite conclusions. We observed 2 cases in which the adenocarcinoma component was dominant, but a secondary component made up a significant portion of the tumor. In one of these the secondary component was a urothelial carcinoma with clear cell features (Fig 2C). Another case had a striking lymphoepithelioma-like carcinoma component (Fig 2D). Both tumors were high stage ( pT3a and pT3b respectively). In the case with lymphoepithelioma-like carcinoma features, the patient was node negative and remains disease free after initial extended partial cystectomy with umbilical resection and lymph node dissection after 42.1 months of follow-up. The small number of cases with unusual histology in this series does not make it possible to assess differences in biologic behavior based on histologic subtype of the tumor.
We noted the presence of cystitis cystica in 5 cases (21 %) and cystitis glandularis in 4 (17 %). In all of these cases except one, this finding was only focal and predominantly in the bladder overlying the urachal neoplasm. One case had exuberant cystitis glandularis in the transurethral resection but not in the partial cystectomy. In no instance did we find any dysplasia or transition to malignancy in these foci of proliferative cystitis. Other authors (10) have also reported the occasional presence of proliferative cystitis in cases with urachal carcinoma. It must be borne in mind that the changes of proliferative cystitis are very common in normal bladder, and are thought to be either a reactive phenomenon to local inflammation or variants of normal histology and possess virtually no malignant potential in this setting. It is thought that their presence in bladders with cancer may be coincidental or the cancer itself may be producing the local inflammatory insult that causes them (5).
The presence or absence of benign remnants has not been recorded consistently in the existing literature. Johnson et al (10) state that in their series of 14 patients, urachal rests were identified in none, and they cite the reason as the extent of the tumor. We identified definitive urachal remnants in 15 of our 24 cases (62.5 %) (Table 2). Two other cases had bulky tumors extending through the wall of the bladder partly obliterating circumscribed structures suggestive of urachal remnants. An additional problem, in our experience, is that seldom do the histological sections demonstrate the entire length of the median umbilical ligament as it traverses the bladder wall. Therefore we think that documentation of urachal remnants is helpful if present, but their absence, especially in widely invasive tumors, does not preclude a urachal origin.
Three cases in our series demonstrated spread of the tumor to the surface mucosa of the bladder. All three cases showed the presence of urachal remnants with transition from benign to adenomatous epithelium. The surface involvement by tumor, which can be attributed to direct extension to the bladder urothelium in continuity with the urachal orifice, would render it difficult to distinguish morphologically between a urachal carcinoma and a vesical adenocarcinoma with an in situ component showing glandular differentiation. In such a setting, the presence of underlying urachal remnants demonstrating adenomatous changes strongly favors a urachal origin. Finally, it is possible, and documented in at least one of our cases, that a urachal remnant based in the bladder wall may rarely communicate with the bladder lumen, a fact that makes sense based on embryologic findings.
There is a considerable overlap in the immunohistochemical staining patterns of colonic adenocarcinoma and primary vesical adenocarcinoma with enteric type differentiation (12, 21, 23). Colorectal adenocarcinoma, although classically of the CK7(-) CK20(+) phenotype, can on occasion be CK7 (+) CK20(+) (3). Positive immunoreactivity for CK7 was always found to be accompanied by positive staining for CK20. Wang et al suggest that nuclear beta-catenin expression is specific for colorectal adenocarcinoma (23). In their study, 81% of the examined colorectal adenocarcinomas had nuclear immunoreactivity, while none of the 17 primary vesical adenocarcinomas had nuclear immunoreactivity.
While all the tumors we studied were strongly and diffusely CK20 positive, positive staining for CK7 was present in about half. Only two-thirds stained with 34BE12, and in the majority of these only focal staining was seen. In contrast, only 11% of colonic adenocarcinoma showed reactivity for 34BE12, as rare cells with weak 34BE12 staining. The majority (93%) of the urachal tumors had strong and diffuse cytoplasmic membranous reactivity for beta catenin, with only one case showing focal nuclear accentuation. On the other hand, there was nuclear accentuation in a background of cytoplasmic membranous staining in 25% of colorectal adenocarcinoma. Therefore immunostains do not unequivocally discriminate between a urachal and a colonic malignancy, although diffuse strong immunoreactivity for 34BE12 would support and diffuse nuclear reactivity for beta catenin would militate against a diagnosis of urachal carcinoma.
The poor prognosis associated with urachal carcinoma is due to a high frequency of locally advanced disease at presentation. In our study, 7 of 24 (29%) patients recurred locally, of which 3 were pT3a and 4 were pT3b. This is in accordance with the literature where most of the tumors which recur locally are the tumors with high stage at presentation. In our study, the majority of recurrences were in the urinary bladder (5 cases), followed by the prostate and seminal vesicles (19), prostate with the prostatic and membranous urethra (19), retroperitoneal soft tissue (19) and pubic bone (19). In the literature the pelvis, urinary bladder and abdominal wall are preferred sites of local recurrence (8). Another interesting observation we make in our case series is that tumors that have an access to the vesical cavity have another mode of local relapse, namely, seeding and ‘drop metastases’ in the distal urothelial tract. In the instance of case 24, where the non-invasive villous-adenoma like component had a polypoid configuration and opened into the bladder lumen, the disease had an intractable clinical course characterized by repeated recurrences in the distal urothelial tract that were not amenable to local control and thus ultimately required radical surgery.
The surgical management differed in the recent large studies reported. In the M.D. Anderson cancer center series (20), only about half of patients undergoing primary surgical treatment had en bloc resection of the urachal ligament and umbilicus, with the majority of this group being long-term survivors. In the Mayo clinic series (1), there was a 48% survival among 60 surgically treated patients, of which more than half did not undergo en bloc resection of the urachus. However the authors observed that complete urachectomy and umbilectomy were significant predictors of survival on univariate analysis, and recommended their routine inclusion.
Three different staging systems for urachal carcinoma have been proposed: the Sheldon (19), the Mayo systems (1) and the Ontario systems (16). In the previous study from our institution by Herr et al, based on rate of cure after the standard surgery recommended in the same paper, it was recommended that staging of primary urachal tumors might better be simply dichotomized as urachal tumor confined to the urachus, bladder and perivesical tissue (surgical specimen) versus intraperitoneal spread of disease (a modification of the Ontario system). Only three cases in our study fit into the latter category. We are therefore reluctant to make the same staging recommendation based on our smaller patient cohort.
In the current study, all cases with locally recurrent and metastatic tumors and patients who were dead of disease belonged to Sheldon pathologic stage pT3 or higher. All 3 patients who were alive with evidence of disease at last follow-up belonged to stage pT3b or higher. The one case with a positive soft tissue margin after the initial surgical procedure was pT3b. The two cases with pT2 disease remained free of disease after initial surgical treatment with 155.6 and 23.5 months follow-up respectively. Although we were reluctant to perform a formal statistical evaluation with outcome correlation due to the limited sample size, our results are in accordance with the literature where outcome was strongly correlated with Sheldon stage (1, 19, 20).
In summary, urachal carcinomas are rare tumors that occur predominantly in the dome of the urinary bladder. The presence of surface urothelial colonization by urachal carcinoma and the presence of cystitis cystica and glandularis does not necessarily rule out this diagnosis. Benign and adenomatous urachal remnants are helpful if present, but their absence does not preclude a urachal origin. Based on this, we propose the following criteria (modified from Sheldon's original criteria) for the diagnosis of urachal carcinoma: 1. location of the tumor in the dome/ anterior wall; 2. epicenter of carcinoma in the bladder wall; 3. Absence of widespread cystitis cystica/ glandularis beyond the dome/ anterior wall, and; 4. Absence of a known primary elsewhere . Immunostains do not unequivocally discriminate between a urachal versus a colorectal origin, but diffuse 34BE12 positivity would support, and diffuse nuclear immunoreactivity for beta catenin would militate against a diagnosis of urachal carcinoma. The location of urachal carcinoma in the mid-outer bladder wall and frequently bulky nature account for a greater proclivity for extravesical extension into the space of Retzius and therefore for high stage disease. Hence the surgical procedure of choice is wide pelvic dissection to encompass the umbilicus, tumor and entire urachus, along with partial cystectomy , with achievement of negative bladder and soft tissue margins and pelvic lymph node dissection. A less well recognized mode of local recurrence, particularly in tumors with a superficial location in the bladder and access to the vesical cavity, is ‘seeding’ in the lower urothelial tract and urinary bladder. The most important determinant of prognosis is Sheldon pathologic stage.
TABLE 5.
CORRELATION OF SHELDON PATHOLOGIC STAGE WITH OUTCOME
| SHELDON STAGE | NED | AWD | DOD |
|---|---|---|---|
| pT1 (0) | 0 | 0 | 0 |
| pT2 (2) | 2 | 0 | 0 |
| pT3a (8) | 5 | 0 | 3* |
| pT3b (11) | 6* | 2 | 3 |
| pT3c (1) | 1 | 0 | 0 |
| pT4a (1) | 0 | 1 | 0 |
| pT4b (1) | 0 | 0 | 1 |
NED – No evidence of disease
AWD – Alive with disease
DOD – Dead of disease
Recurrences by ‘seeding’ in the urothelial tract in 2 patients
Footnotes
Abstract presented in part at the United States and Canadian Academy of Pathology annual meeting in Atlanta, GA, February 2006
References
- 1.Ashley RA, Inman BA, Sebo TJ, et al. Urachal carcinoma: clinicopathologic features and long-term outcomes of an aggressive malignancy. Cancer. 2006;107:712–720. doi: 10.1002/cncr.22060. [DOI] [PubMed] [Google Scholar]
- 2.Begg RC. The Urachus: its Anatomy, Histology and Development. J Anat. 1930;64:170–183. [PMC free article] [PubMed] [Google Scholar]
- 3.Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40:403–439. doi: 10.1046/j.1365-2559.2002.01387.x. [DOI] [PubMed] [Google Scholar]
- 4.Eble JN, Hull MT, Rowland RG, et al. Villous adenoma of the urachus with mucusuria: a light and electron microscopic study. J Urol. 1986;135:1240–1244. doi: 10.1016/s0022-5347(17)46056-3. [DOI] [PubMed] [Google Scholar]
- 5.Epstein JI, Amin MB, Reuter VE. Bladder biopsy interpretation. Lippincott Williams and Wilkins; Philadelphia: [Google Scholar]
- 6.Ghazizadeh M, Yamamoto S, Kurokawa K. Clinical features of urachal carcinoma in Japan: review of 157 patients. Urol Res. 1983;11:235–238. doi: 10.1007/BF00272286. [DOI] [PubMed] [Google Scholar]
- 7.Grignon DJ, Ro JY, Ayala AG, et al. Primary adenocarcinoma of the urinary bladder. A clinicopathologic analysis of 72 cases. Cancer. 1991;67:2165–2172. doi: 10.1002/1097-0142(19910415)67:8<2165::aid-cncr2820670827>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Herr HW. Urachal carcinoma: the case for extended partial cystectomy. J Urol. 1994;151:365–366. doi: 10.1016/s0022-5347(17)34950-9. [DOI] [PubMed] [Google Scholar]
- 9.Herr HW, Bochner BH, Sharp D, et al. Urachal carcinoma: contemporary surgical outcomes. J Urol. 2007;178:74–78. doi: 10.1016/j.juro.2007.03.022. discussion 78. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DE, Hodge GB, Abdul-Karim FW, et al. Urachal carcinoma. Urology. 1985;26:218–221. doi: 10.1016/0090-4295(85)90112-8. [DOI] [PubMed] [Google Scholar]
- 11.Kakizoe T, Matsumoto K, Andoh M, et al. Adenocarcinoma of urachus. Report of 7 cases and review of literature. Urology. 1983;21:360–366. doi: 10.1016/0090-4295(83)90152-8. [DOI] [PubMed] [Google Scholar]
- 12.McKenney JK, Amin MB. The role of immunohistochemistry in the diagnosis of urinary bladder neoplasms. Semin Diagn Pathol. 2005;22:69–87. doi: 10.1053/j.semdp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Molina JR, Quevedo JF, Furth AF, et al. Predictors of survival from urachal cancer: a Mayo Clinic study of 49 cases. Cancer. 2007;110:2434–2440. doi: 10.1002/cncr.23070. [DOI] [PubMed] [Google Scholar]
- 14.Mostofi FK, Thomson RV, Dean AL., Jr Mucous adenocarcinoma of the urinary bladder. Cancer. 1955;8:741–758. doi: 10.1002/1097-0142(1955)8:4<741::aid-cncr2820080417>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi K, Kawai T, Suzuki M, et al. Prognostic factors in urachal adenocarcinoma. A study in 41 specimens of DNA status, proliferating cell-nuclear antigen immunostaining, and argyrophilic nucleolar-organizer region counts. Hum Pathol. 1996;27:240–247. doi: 10.1016/s0046-8177(96)90063-5. [DOI] [PubMed] [Google Scholar]
- 16.Pinthus JH, Haddad R, Trachtenberg J, et al. Population based survival data on urachal tumors. J Urol. 2006;175:2042–2047. doi: 10.1016/S0022-5347(06)00263-1. discussion 2047. [DOI] [PubMed] [Google Scholar]
- 17.Reuter V. The urothelial tract: renal pelvis, ureter, urinary bladder and urethra. Lippincott Williams and Wilkins; Philadelphia: [Google Scholar]
- 18.Schubert GE, Pavkovic MB, Bethke-Bedurftig BA. Tubular urachal remnants in adult bladders. J Urol. 1982;127:40–42. doi: 10.1016/s0022-5347(17)53595-8. [DOI] [PubMed] [Google Scholar]
- 19.Sheldon CA, Clayman RV, Gonzalez R, et al. Malignant urachal lesions. J Urol. 1984;131:1–8. doi: 10.1016/s0022-5347(17)50167-6. [DOI] [PubMed] [Google Scholar]
- 20.Siefker-Radtke AO, Gee J, Shen Y, et al. Multimodality management of urachal carcinoma: the M. D. Anderson Cancer Center experience. J Urol. 2003;169:1295–1298. doi: 10.1097/01.ju.0000054646.49381.01. [DOI] [PubMed] [Google Scholar]
- 21.Suh N, Yang XJ, Tretiakova MS, et al. Value of CDX2, villin, and alpha-methylacyl coenzyme A racemase immunostains in the distinction between primary adenocarcinoma of the bladder and secondary colorectal adenocarcinoma. Mod Pathol. 2005;18:1217–1222. doi: 10.1038/modpathol.3800407. [DOI] [PubMed] [Google Scholar]
- 22.Wang BY, Boag AH, Idrees M, et al. Urachal malignant fibrous histiocytoma: a case report and review of the literature. Arch Pathol Lab Med. 2004;128:456–459. doi: 10.5858/2004-128-456-UMFHAC. [DOI] [PubMed] [Google Scholar]
- 23.Wang HL, Lu DW, Yerian LM, et al. Immunohistochemical distinction between primary adenocarcinoma of the bladder and secondary colorectal adenocarcinoma. Am J Surg Pathol. 2001;25:1380–1387. doi: 10.1097/00000478-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Wright JL, Porter MP, Li CI, et al. Differences in survival among patients with urachal and nonurachal adenocarcinomas of the bladder. Cancer. 2006;107:721–728. doi: 10.1002/cncr.22059. [DOI] [PubMed] [Google Scholar]