Abstract
Rationale: Cognitive and psychiatric impairments are threats to functional independence, general health, and quality of life. Evidence regarding these outcomes after lung transplantation is limited.
Objectives: Determine the frequency of cognitive and psychiatric impairment after lung transplantation and identify potential factors associated with cognitive impairment after lung transplantation.
Methods: In a retrospective cohort study, we assessed cognitive function, mental health, and health-related quality of life using a validated battery of standardized tests in 42 subjects post-transplantation. The battery assessed cognition, depression, anxiety, resilience, and post-traumatic stress disorder (PTSD). Cognitive function was assessed using the Montreal Cognitive Assessment, a validated screening test with a range of 0 to 30. We hypothesized that cognitive function post-transplantation would be associated with type of transplant, cardiopulmonary bypass, primary graft dysfunction, allograft ischemic time, and physical therapy post-transplantation. We used multivariable linear regression to examine the relationship between candidate risk factors and cognitive function post-transplantation.
Measurements and Main Results: Mild cognitive impairment (score, 18–25) was observed in 67% of post-transplant subjects (95% confidence interval [CI]: 50–80%) and moderate cognitive impairment (score, 10–17) was observed in 5% (95% CI, 1–16%) of post-transplant subjects. Symptoms of moderate to severe anxiety and depression were observed in 21 and 3% of post-transplant subjects, respectively. No transplant recipients reported symptoms of PTSD. Higher resilience correlated with less psychological distress in the domains of depression (P < 0.001) and PTSD (P = 0.02). Prolonged graft ischemic time was independently associated with worse cognitive performance after lung transplantation (P = 0.001). The functional gain in 6-minute-walk distance achieved at the end of post-transplant physical rehabilitation (P = 0.04) was independently associated with improved cognitive performance post-transplantation.
Conclusions: Mild cognitive impairment was present in the majority of patients after lung transplantation. Prolonged allograft ischemic time may be associated with cognitive impairment. Poor physical performance and cognitive impairment are linked, and physical rehabilitation post-transplant and psychological resilience may be protective against the development of long-term impairment. Further study is warranted to confirm these potential associations and to examine the trajectory of cognitive function after lung transplantation.
Keywords: lung transplantation, cognitive function, psychological function, quality of life, critical illness
Lung transplantation is an established therapy for advanced lung disease that improves quality of life and mortality (1, 2). It is estimated that 40,000 lung transplants have been performed, with increasing numbers of transplants being performed annually (3). Complications post-transplantation are common and frequently undermine the reasons for undergoing transplantation: prolonging and improving quality of life.
Cognitive and psychiatric impairments are major threats to functional independence, quality of life, and long-term health and survival (4–10). Care that results in cognitive impairment may be less acceptable to patients than death (11). Cognitive and psychiatric impairments are common in patients with advanced lung disease before transplantation (12–18). Recent evidence suggests that cognition may further decline after lung transplantation (19), yet the frequency and determinants of cognitive impairment after lung transplantation remain largely unknown.
In this cohort study, we examined cognitive function, mental health and the psychological trait of resilience (20), and health-related quality of life in relationship to lung transplantation. Our goals were to determine the frequency of cognitive and psychiatric impairment, to identify potential clinical risk factors associated with impaired cognitive function after lung transplantation, and to examine the relationship between cognitive function, mental health, resilience, and quality of life after lung transplantation.
Methods
Study Design
The Cognitive Outcomes after Lung Transplantation (COLT) study was a retrospective cohort study designed to examine cognitive function, mental health, and health-related quality of life after lung transplantation. The study was conducted at the Hospital of the University of Pennsylvania. The study was approved by the institutional review board of the University of Pennsylvania, and informed consent was obtained from all study participants.
Study Population
Patients were eligible for enrollment if they were 18 years of age or older and listed for lung transplantation or had received single or bilateral lung transplantation. Eligible subjects were recruited between July 2012 and April 2013. We focused our assessment on patients who had received lung transplantation (post-transplant). To determine feasibility and to examine cognition at different times after transplant, we enrolled 42 patients from hospital discharge to 64 months post-transplant. Using the same standardized test battery, we assessed 14 separate patients on the lung transplant waiting list (pretransplant control subjects) to contextualize the results of the post-transplant group.
Cognitive Function, Mental Health, and Quality of Life
We used a battery of validated and standardized tests to assess cognition (21–23), anxiety, depression, post-traumatic stress disorder (PTSD) (24–28), and health-related quality of life (30). In addition, given the inverse relationship between psychological distress and the psychological trait of resilience, we also assessed resilience (29). The battery was administered in person by one of two trained investigators to consenting subjects. The complete battery required approximately 30 minutes to complete. The instruments used in the battery are presented in the online supplement (Table E1). To reduce the burden on subjects tested before hospital discharge post-transplant (n = 4), we limited testing to the cognitive assessment in these instances.
Our primary outcome was cognitive function. We assessed cognition using the Montreal Cognitive Assessment (MoCA), a valid and sensitive screening tool with precedent in patients with advanced lung disease (21–23). The MoCA required approximately 10 minutes to complete. The MoCA yields an overall cognitive assessment and valid subscores for the individual cognitive domains of visuospatial and executive function, naming, attention, language, abstraction (abstract reasoning), delayed recall (memory), and orientation. We examined cognition function as a continuous variable in our primary analysis and categorized cognitive impairment as mild (score of 18–25), moderate (10–17), and severe (<10) in secondary analyses, with prior evidence suggesting that moderate to severe scores are similar to scores of patients with dementia (21–23; Z. Nasreddine, personal communication, July 2011). Mild cognitive impairment, as categorized based on the MoCA, is distinct from the diagnostic entity “Mild Cognitive Impairment” (9).
We assessed symptoms of depression and anxiety using the Zung Self-rating Depression Scale and the Beck Anxiety Inventory, respectively, and categorized symptoms as mild, moderate, or severe (24–26). We categorized results of the Post-Traumatic Stress Syndrome 10-Questions Inventory (PTSS-10) as normal or impaired (27, 28). Beginning in October 2012, we used the Connor-Davidson resilience scale to measure the modifiable psychological trait of resilience and categorized results as abnormal (<68), normal (68–92), or highly resilient (>92) based on population norms (29). The EuroQol (EQ-5D-3L), used to assess quality of life, yields a descriptive score for each of the five dimensions assessed (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) and a visual analog score (30).
Data Collection
Clinical parameters were collected prospectively using methods that we have previously described as part of the Lung Transplant Outcomes Group observational cohort study to examine primary graft dysfunction after lung transplantation (31). In addition, we reviewed subjects’ medical records to identify coexisting conditions at the time of testing, including clinically significant anxiety or depression requiring prescription of an antidepressant or anxiolytic. Last, we abstracted details retrospectively from post-transplantation physical therapy sessions.
Candidate Risk Factors for Cognitive Impairment
We hypothesized a priori that the type of transplant (single vs. double), use of cardiopulmonary bypass (32, 33), primary graft dysfunction (PGD) (31), and allograft ischemic time (34, 35) would be associated with worse cognitive function after lung transplantation. In addition, we hypothesized that physical therapy, as measured by gain of function after physical therapy and maximal function achieved in those having completed post-transplantation acute rehabilitation at the time of testing, would protect against cognitive decline (36). The post-transplant rehabilitation program is compulsory for transplant recipients at our center. It is held three times per week for 6 to 8 weeks and includes 1 hour of pulmonary conditioning (aerobic exercise) and 1 hour of physical therapy (strength and flexibility training). We considered cognitive reserve (age, level of education) (37), sex, etiology of advanced lung disease, coexisting conditions (as distinct conditions and summarized as Charlson Comorbidity Index score [38]), presence of pulmonary hypertension at transplantation, and time from transplantation to testing as potential confounders.
We defined PGD as grade 3 PGD within the first 72 hours post-transplant (39). Subjects met criteria for the diagnosis of grade 3 PGD if chest radiographs showed pulmonary infiltrates in the allograft(s) during the first 72 hours post-transplant and if the PaO2/FiO2 ratio was less than 200 (39). Two physicians, blinded to the assessment, independently reviewed chest radiographs, with adjudication by a third physician when required as part of the prospective Lung Transplant Outcomes Group cohort study (31, 39). Total allograft ischemic time was defined as the time interval (minutes) between aortic cross-clamp time during donor harvest and reperfusion in organ recipient; in bilateral lung transplantation, the longer of the two recorded times was defined as the total allograft ischemic time (34).
Statistical Analysis
We used the Student t test or the Wilcoxon rank-sum test to compare continuous variables and Pearson Chi-square test or Fisher exact test to compare categorical variables. We used the Spearman correlation coefficient to examine the relationship between cognitive function, psychiatric symptoms, resilience, and quality of life, given the ordinal nature of scores.
We used multivariable linear regression to examine the independent relationship between candidate risk factors and cognitive function after lung transplantation. We adjusted for each candidate risk factor and potential confounding variable with an α level of significance of less than 0.20 in bivariate analyses (40). We adjusted one covariate at a time, given the limited sample size. Multicollinearity was assessed using variance inflation factors; graft ischemic time was found to be collinear with cardiopulmonary bypass use and bypass time and cardiopulmonary bypass was used in each of the 28 bilateral lung transplant cases. We prespecified that our primary analysis would be limited to the 38 patients assessed within 24 months of lung transplantation to assess peritransplant risk factors. We performed sensitivity analyses, wherein we included the four long-term transplant survivors previously excluded to determine if the observed relationships persisted. We also categorized graft ischemic time based on the observed distribution and used a fractional polynomial regression model to assess for possible threshold effects in the relationship between graft ischemic time and cognition. We used Stata 12 to perform statistical analyses and we used two-sided tests with a type I error (α) set to 0.05 (StataCorp LP, College Station, TX).
Results
Enrollment
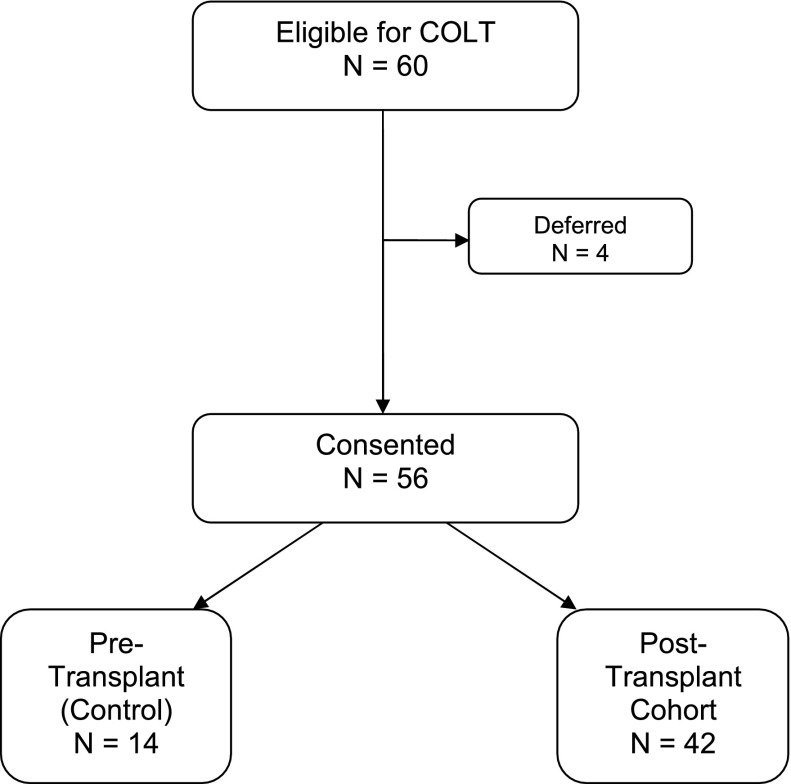
We approached 60 patients to participate; 4 deferred due to logistical reasons, and 56 consented and were tested (Figure 1). We tested 14 unique, pretransplant control subjects and 42 subjects post-transplant at a median of 8 months post-transplant (interquartile range, 2–16 mo). Baseline characteristics of the study cohort are reported in Table 1. In general, participants were middle-aged, female, well-educated, non-Hispanic whites. Compared with pretransplant subjects, post-transplant subjects were more likely to have chronic kidney disease (P = 0.02) and diabetes mellitus (P = 0.003), reflecting the effects of immunosuppressant medications post-transplant.
Figure 1.
Enrollment for Cognitive Outcomes after Lung Transplantation (COLT) study.
Table 1.
Characteristics of the study population, categorized as pre– and post–lung transplantation
| Variable | Pretransplant (N = 14) | Post-Transplant (N = 42) | P Value |
|---|---|---|---|
| Age, yr | 60 (56–65) | 60 (57–64) | 0.86 |
| Male sex, no. (%) | 4 (29) | 20 (48) | 0.21 |
| Level of education, yr | 13.5 (12–16) | 13 (12–16) | 0.50 |
| Race or ethnic group, no. (%) | 0.70 | ||
| White non-Hispanic | 12 (86) | 33 (78) | |
| Black non-Hispanic | 0 (0) | 1 (2) | |
| Hispanic | 2 (14) | 4 (10) | |
| Other | 0 (0) | 4 (10) | |
| Pulmonary diagnosis, no. (%) | 0.18 | ||
| COPD | 6 (43) | 17 (40) | |
| Idiopathic pulmonary fibrosis | 3 (21) | 19 (45) | |
| Cystic fibrosis | 1 (7) | 2 (5) | |
| Other | 4 (29) | 4 (10) | |
| Coexisting conditions, no. (%) | |||
| Anxiety | 3 (21) | 12 (29) | 0.74 |
| Depression | 5 (36) | 17 (40) | 0.75 |
| Chronic kidney disease | 1 (7) | 17 (40) | 0.02 |
| Coronary artery disease | 4 (29) | 10 (24) | 0.73 |
| Congestive heart failure | 1 (7) | 1 (2) | 0.44 |
| Diabetes mellitus | 3 (21) | 28 (67) | 0.003 |
| Hypertension | 5 (36) | 22 (52) | 0.28 |
| Hyperlipidemia | 5 (36) | 20 (48) | 0.44 |
| Dementia | 0 (0) | 0 (0) | — |
| Cerebrovascular disease | 0 (0) | 0 (0) | — |
| Charlson Comorbidity Index score | 3 (3–4) | 5 (3–5) | 0.02 |
| Pulmonary hypertension | 0.19 | ||
| Normal | 9 (64) | 28 (67) | |
| Mild, mPAP 25–40 | 2 (14) | 12 (29) | |
| Moderate, mPAP 41–55 | 2 (14) | 1 (2) | |
| Severe, mPAP > 55 | 1 (7) | 1 (2) | |
| 6-min-walk test pretransplant, m | 964 (800–1,200) | 996 (865–996) | 0.97 |
| Operative (transplantation) variables | |||
| Transplant type, single, no. (%)* | 14 (33) | ||
| Cardiopulmonary bypass, no. (%) | 29 (69) | ||
| Cardiopulmonary bypass time, min | 249 (238–286) | ||
| Primary graft dysfunction, no. (%) | 10 (24) | ||
| Graft ischemia time, min | 243 (217–272) | ||
| Postoperative variables* | |||
| Physical function | |||
| Initial 6-min-walk test post-transplant, m | 879 (634–1,054) | ||
| Final 6-min-walk test post-transplant, m | 1,347 (1,247–1,525) | ||
| Distance gained, m† | 423 (324–528) | ||
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; mPAP = mean pulmonary artery pressure.
Values expressed as a frequency (%) or median (interquartile range) unless otherwise specified.
One subject was status post repeat lung transplantation and one subject was status post heart-lung transplant.
Thirty-two subjects were tested after having completed physical therapy post-transplantation. Distance gained defined as final distance – initial distance (m).
Cognitive Function, Mental Health, Resilience, and Quality of Life Post-Transplantation
The test results are presented in Table 2. Mild cognitive impairment was observed in 67% (28 of 42) of post-transplant subjects (95% confidence interval [CI], 50–80%). Moderate cognitive impairment was rare, occurring in 5% (2 of 42) of post-transplant subjects (95% CI, 1–16%), and severe cognitive impairment was not observed. Compared with the pretransplant group, there were no statistically significant differences in cognitive scores or in the frequency of cognitive impairment; MoCA scores were, on average, 1 point higher (P = 0.24), and impairment was limited to the mild category in the pretransplant group.
Table 2.
Cognitive outcomes, mental health, resilience, and health-related quality of life
| Variable | Pretransplant (N = 14) | Post-Transplant (N = 42) | P Value |
|---|---|---|---|
| Cognition, MoCA score | 25 ± 2 | 24 ± 3 | 0.24 |
| Cognitive impairment, no. (%)* | 1.00 | ||
| None | 4 (29) | 12 (29) | |
| Mild | 10 (71) | 28 (67) | |
| Moderate or severe | 0 (0) | 2 (5) | |
| Anxiety score | 8 (5–17) | 7 (4–14) | 0.59 |
| Anxiety, no. (%) | 0.92 | ||
| Mild | 3 (21) | 9 (24) | |
| Moderate or severe | 4 (29) | 8 (21) | |
| Depression score | 40 (36–45) | 34 (29–40) | 0.05 |
| Depression, no. (%) | 0.62 | ||
| Mild | 1 (7) | 1 (3) | |
| Moderate or severe | 0 (0) | 1 (3) | |
| Posttraumatic stress score | 14 (13–17) | 16 (13–22) | 0.30 |
| Symptoms of PTSD, no. (%) | 1 (7) | 0 (0) | 0.27 |
| Resilience score† | 93 (82–93) | 87 (76–95) | 0.68 |
| Health-related quality of life, EuroQol | |||
| Descriptive score (5–15)‡ | 8 (7–8) | 6 (5–8) | 0.04 |
| Visual analog scale (0–100) | 52 (30–60) | 80 (60–90) | 0.001 |
Definition of abbreviations: MoCA = Montreal Cognitive Assessment; PTSD = posttraumatic stress disorder.
Mild cognitive impairment was defined as a score of 18–25 and moderate or severe impairment as <18. One point was added for patients with 12 or fewer years of education.
Resilience was assessed beginning in October 2012 (N = 30 subjects). Overall, 11 of 30 subjects (37%) were found to be highly resilient (score > 92) and 6 of 30 subjects (17%) had low (>1 SD below population mean scores) resilience.
Greater score represents more perceived problems in dimensions assessed.
Symptoms of moderate to severe anxiety and depression were observed in 21 and 3% of post-transplant subjects, respectively. None of the post-transplant subjects reported symptoms consistent with PTSD. Compared with the pretransplant group, there were no clinically or statistically significant differences in the frequency of anxiety, depression, or PTSD in the post-transplant group, although overall depression scores were significantly higher (P = 0.05) in the pretransplant group.
Health-related quality of life was low pretransplant and was significantly higher in the post-transplant group (Table 2). The higher quality of life after lung transplantation appeared to be driven by better scores in the dimension of usual activities (P = 0.005) and a trend toward better scores in the dimension of mobility (P = 0.06).
Overall, lung transplant candidates and recipients were found to be resilient, with a median score of 87 (IQR, 76–95) on the Connor-Davidson Resilience Scale-25. High resilience (>1 SD above normal population mean scores) was observed in 37% of subjects tested. Post-transplant, higher resilience values correlated with fewer symptoms of depression (n = 25, rho = −0.79, P < 0.001) and PTSD (n = 25, rho = −0.45, P = 0.02), but not anxiety (n = 25, rho = −0.34, P = 0.10), cognition (n = 25, rho = 0.19, P = 0.36), or quality of life (n = 25, rho = 0.32, P = 0.12).
Risk Factors for Cognitive Impairment Post-Transplantation
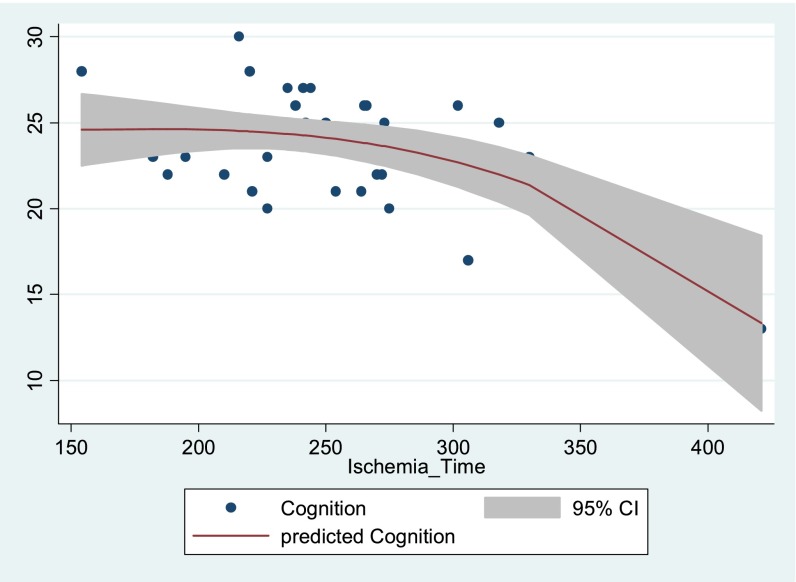
Prolonged total graft ischemia time was associated with worse cognitive performance after lung transplantation (P = 0.001), as were type of lung transplant (bilateral, P = 0.01) and use of cardiopulmonary bypass (P = 0.01) (Table 3). After adjustment for potential covariates, graft ischemic time was associated independently with cognition after lung transplantation, and the association remained after we included the long-term transplant survivors. When categorized by the observed distribution, the relationship between cognition and graft ischemic time appeared to suggest a threshold effect in the subjects in the highest quartile of graft ischemic time, which was consistent with the fitted relationship (Table 3, Figures 2 and E1). Prolonged graft ischemia was associated with worse performance in the domains of visuospatial and executive function (P = 0.04) and language (P = 0.01).
Table 3.
Association between risk factors and cognitive function in multivariable linear regression
| Cognitive Function |
|||
|---|---|---|---|
| Unadjusted Coefficient (95% CI) | P Value | Adjusted Coefficient (95% CI)* | |
| Candidate Risk Factors | |||
| Transplant type, single | 2.74 (0.70 to 4.78) | 0.01 | |
| Cardiopulmonary bypass | −2.69 (−0.59 to −4.79) | 0.01 | |
| Bypass time, min* | −0.58 (−0.19 to −0.97) | 0.005 | |
| Primary graft dysfunction | −1.15 (−3.62 to 1.32) | 0.35 | |
| Graft ischemic time, min† | −1.50 (−0.62 to −2.39) | 0.001 | −1.20 (−0.25 to −2.14) – −1.66 (−0.72 to −2.60) |
| Ischemic time, categorized | |||
| <217 (1st quartile) | Reference | Reference | |
| 217–242 | −0.27 (−2.66 to 2.13) | 0.82 | |
| >242 (4th quartile) | −3.1 (−0.38 to − 5.81) | 0.03 | |
| Post-transplant physical function gain, m‡ | 0.62 (0.03 to 1.20) | 0.04 | 0.61 (0.09 to 1.14) – 0.73 (0.14 to 1.32) |
| Potential covariates | |||
| Age, yr§ | 1.28 (0.22 to 2.33) | 0.02 | |
| Male sex, % | −1.19 (0.91 to −3.29) | 0.26 | |
| Pulmonary diagnosis | |||
| COPD | Reference | Reference | |
| IPF | 0.33 (−2.48 to 3.14) | 0.81 | |
| Cystic fibrosis | −1.77 (−6.85 to 3.31) | 0.48 | |
| Other | −0.31 (−2.93 to 2.32) | 0.81 | |
| Coexisting conditions | |||
| Anxiety | −0.84 (−3.11 to 1.43) | 0.46 | |
| Depression | −0.27 (−2.42 to 1.88) | 0.80 | |
| Chronic kidney disease | 0.99 (−1.19 to 3.17) | 0.36 | |
| Coronary artery disease | −1.30 (−3.88 to 1.26) | 0.31 | |
| Congestive heart failure | 6.51 (0.24 to 12.78) | 0.04 | |
| Diabetes mellitus | 0.41 (−1.93 to 2.76) | 0.72 | |
| Hypertension | −0.72 (−2.83 to 1.39) | 0.49 | |
| Hyperlipidemia | 0.62 (−1.50 to 2.74) | 0.56 | |
| Charlson Comorbidity Index score | 0.65 (−0.01 to 1.31) | 0.05 | |
| Preexisting mild pulmonary hypertension | −0.18 (−2.42 to 2.06) | 0.87 | |
| Level of education, yr | 0.21 (−0.25 to 0.68) | 0.36 | |
| Time from transplant to testing, mo | −0.002 (−0.15 to 0.15) | 0.98 | |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; IPF = idiopathic pulmonary fibrosis.
After adjustment for each candidate risk factor and potential confounder associated with cognition at significance level < 0.20, graft ischemia time and post-transplant physical function gain remained associated with cognitive function. Neither transplant type nor use of cardiopulmonary bypass remained significant after adjustment for age.
For each 50-unit decrease in ischemic time and cardiopulmonary bypass time.
For each 100-unit increase in 6-minute-walk distance (m) gained after physical therapy post-transplantation (final distance – initial distance)
For each 10-unit increase in age.
Figure 2.
Relationship between graft ischemic time (minutes) and cognitive scores (Montreal Cognitive Assessment score, range 0–30) depicted as scatter plot and fitted relationship of predicted cognitive scores using fractional polynomial regression. CI = confidence interval.
The functional gain in 6-minute-walk distance achieved at the end of post-transplant physical rehabilitation (P = 0.04), but not the maximal distance attained (P = 0.68), was associated with improved cognitive performance post-transplantation. The relationship between functional gain achieved through rehabilitation and cognitive performance remained after adjusting for potential covariates, although the association in the sensitivity analysis was attenuated to the null after we included the long-term transplant survivors (P = 0.10). These analyses suggest that a relationship may exist between physical rehabilitation and cognition after lung transplantation.
Discussion
In this observational cohort study, we examined long-term cognitive function, mental health, and health-related quality of life after lung transplantation. We found that the majority of lung transplant recipients experience long-term, mild cognitive impairment. In addition, we confirmed that a significant minority of recipients continues to experience psychological distress and identified the relationship between psychological distress and psychological resilience in this patient population. Last, we identified several novel factors that may modify the risk of cognitive decline after lung transplantation that warrant confirmation through further direct investigation.
Cognitive impairment is increasingly recognized as an important and potentially modifiable outcome after major cardiac surgery (41) and critical illness (42) that could undermine long-term health and quality of life after lung transplantation. In a recent study of patients with moderate to severe chronic obstructive pulmonary disease, 36% of these patients were found to have mild cognitive impairment using the MoCA compared with 12% of healthy subjects tested (23). In the initial study to examine the long-term cognitive effects of lung transplantation, Hoffman and colleagues found that mild cognitive impairment was common, occurring in 82% of patients pretransplant and 86% of patients 6 months post-transplant using a formal neuropsychological test battery (19). Although executive function appeared to improve in younger transplant recipients, 29% of subjects experienced significant cognitive decline after lung transplantation, and less cognitive reserve (increased age and lower level of education) was identified as a risk factor for post-transplant cognitive decline (19).
Using a brief, validated cognitive screening instrument, we confirmed that mild cognitive impairment appears to be common in both lung transplant candidates and recipients. In contrast to the study by Hoffman and colleagues, in which 22% of subjects were moderately to severely impaired based on a formal neuropsychological test battery at 6 months, we found that moderate to severe cognitive impairment was rare using a cognitive screening test. The most likely explanation for this difference is that the cognitive screening assessment used in the present study, although more sensitive to detect mild cognitive impairment than traditional screening tests (e.g., Mini Mental State Examination), is less sensitive than a formal neuropsychological battery. Future multicenter longitudinal studies should pair practical, sensitive screening tests with formal neuropsychological test batteries to better characterize the effects of transplant and related treatments on cognitive function over time.
We found that total allograft ischemic time, previously found to be associated with graft function and survival (34), may be a risk factor for worse cognitive performance after lung transplantation. We had proposed that allograft ischemic time may result in cognitive decline due to systemic effects of ischemia-reperfusion injury, mediated by oxidative stress and inflammation, which could lead to neuroinflammation (34, 35, 43, 44). Additionally, prolonged allograft ischemic time has been associated with impaired gas exchange (i.e., PaO2/FiO2) immediately postoperatively (34) and impaired long-term survival. Confirmatory studies, designed to examine markers of oxidative stress (34, 35), inflammation (e.g., IL-18) (35, 43–45), and oxygenation postoperatively as potential mediators of the observed relationships between allograft ischemic time, oxygenation, and cognitive function, are required to validate our observations. Related future studies are required to examine whether the previously identified relationship between prolonged allograft ischemic time and long-term survival (34) is mediated by cognitive impairment and the potential adverse events related to such impairment (i.e., functional impairment, medication nonadherence, infectious complications) (46, 47). Importantly, given the collinearity between graft ischemic time and cardiopulmonary bypass time and the standard use of cardiopulmonary bypass for bilateral lung transplantation at this center, the use and duration of cardiopulmonary bypass remains a potentially modifiable risk factor for cognitive decline after lung transplantation (32, 33). Future multicenter studies will need to be designed and powered to examine both of these potentially modifiable risk factors.
Functional gain achieved post-transplantation through physical therapy was associated with cognitive impairment. Whether physical therapy may serve to prevent cognitive decline after transplantation or accelerate recovery after an initial decline, and how allograft function may impact this relationship, are unknowns. The salutary effects of physical rehabilitation are extensive, and the neurobiological (e.g., release of neurotrophic factors) and neuroanatomical effects (e.g., increased brain volume) of exercise appear to have beneficial effects on brain health and cognition in general (36) and in patients with advanced lung disease (48). To explore this additional hypothesis-generating observation, studies designed to assess cognitive function and neuroanatomical changes longitudinally during the rehabilitation phase of recovery after lung transplantation are required.
Consistent with prior studies, we found that a significant minority of lung transplant recipients continue to experience symptoms of anxiety. We found that depressive symptoms were uncommon post-transplant and may improve after transplant. Given the frequency of antidepressant prescriptions, these findings suggest that symptom recognition and treatment can be used to effectively control symptoms in this patient population. Last, in contrast to prior studies of patients undergoing coronary artery bypass graft surgery or thoracic transplantation, which reported a rate of PTSD in the 15 to 18% range (49–51), none of the lung transplant recipients screened positive for PTSD symptoms. Related, we found that the psychological trait of resilience appeared to protect against psychological distress, an important observation given that resilience is a potentially modifiable trait and evidence exists that vital components of resilience (optimism and perceived social support) can be delivered to the lung transplant patient population through telephone-based coping skills training (52). Additional explanations to account for the lack of PTSD symptoms in the lung transplant recipients studied include: patient screening and selection, psychological preparation (including medical management of psychiatric symptoms and extensive psychosocial support), corticosteroid administration at induction (53, 54), and the benefits of protocolized post-transplant care that mimics the demonstrated efficacy of the intensive care unit diary to augment factual memories (55, 56).
Finally, consistent with prior studies (57–61), we found that health-related quality of life was higher after lung transplantation. The findings presented in our study extend our understanding of how lung transplantation improves quality of life, given the finding that the ability to resume one’s usual activities may explain higher health-related quality of life after lung transplantation.
There are several important potential limitations to our study. First, although our study is the first to examine risk factors at the time of transplantation, our sample size was small, and confirmatory studies are required to validate our findings and further characterize potentially modifiable risk factors (e.g., cardiopulmonary bypass [62]). Second, although we designed our study to permit comparison to a separate group of pretransplant (control) subjects using the same test battery, our design did not account for pretransplant cognitive function at the subject level. Future prospective longitudinal studies are required to examine the trajectory of cognitive function after lung transplant. Third, the potential for residual confounding exists, given our limited sample size and the inability to account for important, potential covariates (e.g., pretransplant cognitive function, apolipoprotein E genotype, pulmonary diagnosis leading to transplant), and perioperative (e.g., duration of delirium) and postoperative complications (e.g., severe sepsis episodes) (40, 41, 63). Future prospective studies should be designed to assess these and other important potential confounders and to elucidate the observed relationship between age and cognitive function. Finally, our results are potentially subject to survivor bias and may therefore understate the prevalence of cognitive and psychiatric impairment after transplant and underestimate the observed relationship between graft ischemic time and cognitive function post-transplant (34).
In conclusion, using a simple screening tool administered in the clinical setting, our findings provide evidence that mild cognitive impairment is common after lung transplantation. Prolonged graft ischemic time was a potential risk factor for long-term cognitive impairment after lung transplantation. In addition, physical rehabilitation post-transplant and psychological resilience are potential protective factors against the development of long-term cognitive and psychiatric impairment. These observations require validation in a longitudinal, multicenter study.
Acknowledgments
Acknowledgment
The authors thank the subjects and their family members for their participation to better understand the long-term effects of lung transplantation on cognition, mental health, and quality of life. They also thank the staff of the Penn Lung Transplant Center for their contributions and James C. Jackson, Psy.D., of Vanderbilt University, for providing his expertise during the developmental phase of the study.
Footnotes
Supported in part by the Center for Translational Lung Biology, National Institutes of Health (NIH), NHLBI, grant HL 115354 (J.D.C.), and the NIH NHLBI Loan Repayment Program and NIH (M.E.M.), National Institute of Neurological Disorders and Stroke Training Grant T32-NS-061779 (B.J.A.).
Author Contributions: Conception and design: D.G.C., J.D.C., R.O.H., M.E.M.; data collection: D.G.C., J.D.C., J.M.D., R.P.J., R.J.S., N.P.B., E.D., M.E.M.; analysis and interpretation of the data: D.G.C., J.D.C., B.J.A., J.M.D., R.J.S., E.C., S.L.B., N.P.B., E.D., R.O.H., M.E.M.; drafting of the manuscript: D.G.C., M.E.M.; critical revision of the article for important intellectual content: D.G.C., J.D.C., B.J.A., J.M.D., R.J.S., E.C., S.L.B., R.O.H., M.E.M.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Arcasoy SM, Kotloff RM. Lung transplantation. N Engl J Med. 1999;340:1081–1091. doi: 10.1056/NEJM199904083401406. [DOI] [PubMed] [Google Scholar]
- 2.Orens JB, Garrity ER. General overview of lung transplantation and review of organ allocation. Proc Am Thorac Soc. 2009;6:13–19. doi: 10.1513/pats.200807-072GO. [DOI] [PubMed] [Google Scholar]
- 3.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, Kirk R, Rahmel AO, Stehlik J. The registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report—2012. J Heart Lung Transplant. 2012;31:1073–1086. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Rothenhäusler HB, Ehrentraut S, Stoll C, Schelling G, Kapfhammer HP. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry. 2001;23:90–96. doi: 10.1016/s0163-8343(01)00123-2. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest. 2006;130:869–878. doi: 10.1378/chest.130.3.869. [DOI] [PubMed] [Google Scholar]
- 6.Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70:512–519. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikkelsen ME, Shull WH, Biester RC, Taichman DB, Lynch S, Demissie E, Hansen-Flaschen J, Christie JD. Cognitive, mood and quality of life impairments in a select population of ARDS survivors. Respirology. 2009;14:76–82. doi: 10.1111/j.1440-1843.2008.01419.x. [DOI] [PubMed] [Google Scholar]
- 8.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, Localio AR, Demissie E, Hopkins RO, Angus DC. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson RC. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 10.Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA. 2012;307:165–172. doi: 10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 12.Williams MA, LaMarche JA, Smith RL, Fielstein EM, Hardin JM, McGiffin DC, Zorn GL, Kirklin JA, Boll TJ. Neurocognitive and emotional functioning in lung transplant candidates: a preliminary study. J Clin Psychol Med Settings. 1997;4:79–90. [Google Scholar]
- 13.Ruchinskas RA, Broshek DK, Crews WD, Jr, Barth JT, Francis JP, Robbins MK. A neuropsychological normative database for lung transplant candidates. J Clin Psychol Med Settings. 2000;7:107–112. [Google Scholar]
- 14.Crews W. Neuropsychological dysfunction in patients with end-stage pulmonary disease: lung transplant evaluation. Arch Clin Neuropsychol. 2003;18:353–362. [PMC free article] [PubMed] [Google Scholar]
- 15.Parekh PI, Blumenthal JA, Babyak MA, LaCaille R, Rowe S, Dancel L, Carney RM, Davis RD, Palmer S. Gas exchange and exercise capacity affect neurocognitive performance in patients with lung disease. Psychosom Med. 2005;67:425–432. doi: 10.1097/01.psy.0000160479.99765.18. [DOI] [PubMed] [Google Scholar]
- 16.John C. Psychiatric aspects of lung transplant. Can J Psychiatry. 1990;35:759–764. doi: 10.1177/070674379003500907. [DOI] [PubMed] [Google Scholar]
- 17.Singer HK, Ruchinskas RA, Riley KC, Broshek DK, Barth JT. The psychological impact of end-stage lung disease. Chest. 2001;120:1246–1252. doi: 10.1378/chest.120.4.1246. [DOI] [PubMed] [Google Scholar]
- 18.Parekh PI, Blumenthal JA, Babyak MA, Merrill K, Carney RM, Davis RD, Palmer SM. Psychiatric disorder and quality of life in patients awaiting lung transplantation. Chest. 2003;124:1682–1688. doi: 10.1378/chest.124.5.1682. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman BM, Blumenthal JA, Carney RC, O’Hayer CVF, Freedland K, Smith PJ, Babyak MA, Davis RD, Mathew JP, Martinu T, et al. Changes in neurocognitive functioning following lung transplantation. Am J Transplant. 2012;12:2519–2525. doi: 10.1111/j.1600-6143.2012.04072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 21.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 22.Smith T, Gildeh N, Holmes C. The Montreal Cognitive Assessment; validity and utility in a memory clinic setting. Can J Psychol. 2007;52:329–332. doi: 10.1177/070674370705200508. [DOI] [PubMed] [Google Scholar]
- 23.Villeneuve S, Pepin V, Rahayel S, Bertrand JA, de Lorimier M, Rizk A, Desjardins C, Parenteau S, Beaucage F, Joncas S, et al. Mild cognitive impairment in moderate to severe COPD: a preliminary study. Chest. 2012;142:1516–1523. doi: 10.1378/chest.11-3035. [DOI] [PubMed] [Google Scholar]
- 24.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 25.Zung W. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 26.Biggs JT, Wylie LT, Ziegler VE. Validity of the Zung Self-rating Depression Scale. Br J Psychiatry. 1978;132:381–385. doi: 10.1192/bjp.132.4.381. [DOI] [PubMed] [Google Scholar]
- 27.Weisaeth L. Torture of a Norwegian ship’s crew: the torture, stress reactions and psychiatric after-effects. Acta Psychiatr Scand Suppl. 1989;355:63–72. [PubMed] [Google Scholar]
- 28.Jubran A, Lawm G, Duffner LA, Collins EG, Lanuza DM, Hoffman LA, Tobin MJ. Post-traumatic stress disorder after weaning from prolonged mechanical ventilation. Intensive Care Med. 2010;36:2030–2037. doi: 10.1007/s00134-010-1972-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Connor KM, Davidson JRT. Development of a new resilience scale: the Connor-Davidson resilience scale (CD-RISC) Depress Anxiety. 2003;18:76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- 30.EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 31.Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL, Lederer DJ, Cantu E, Kohl BA, Lama VN, et al. Lung Transplant Outcomes Group. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187:527–534. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA.Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery N Engl J Med 2001344395–402.Published erratum appears in N Engl J Med 344:1876 [DOI] [PubMed] [Google Scholar]
- 33.Selnes OA, Gottesman RF, Grega MA, Baumgartner WA, Zeger SL, McKhann GM. Cognitive and neurologic outcomes after coronary-artery bypass surgery. N Engl J Med. 2012;366:250–257. doi: 10.1056/NEJMra1100109. [DOI] [PubMed] [Google Scholar]
- 34.Thabut G, Mal H, Cerrina J, Dartevelle P, Dromer C, Velly JF, Stern M, Loirat P, Lesèche G, Bertocchi M, et al. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. Am J Respir Crit Care Med. 2005;171:786–791. doi: 10.1164/rccm.200409-1248OC. [DOI] [PubMed] [Google Scholar]
- 35.De Perrot M, Sekine Y, Fischer S, Waddell TK, McRae K, Liu M, Wigle DA, Keshavjee S. Interleukin-8 release during early reperfusion predicts graft function in human lung transplantation. Am J Respir Crit Care Med. 2002;165:211–215. doi: 10.1164/ajrccm.165.2.2011151. [DOI] [PubMed] [Google Scholar]
- 36.Hopkins RO, Suchyta MR, Farrer TJ, Needham DM. Improving post-ICU neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med. 2012;186:1220–1228. doi: 10.1164/rccm.201206-1022CP. [DOI] [PubMed] [Google Scholar]
- 37.Yaffe K, Weston A, Graff-Radford NR, Satterfield S, Simonsick EM, Younkin SG, Younkin LH, Kuller L, Ayonayon HN, Ding J, et al. Association of plasma β-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 2011;305:261–266. doi: 10.1001/jama.2010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 39.Diamond JM, Meyer NJ, Feng R, Rushefski M, Lederer DJ, Kawut SM, Lee JC, Cantu E, Shah RJ, Lama VN, et al. Lung Transplant Outcomes Group. Variation in PTX3 is associated with primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2012;186:546–552. doi: 10.1164/rccm.201204-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Amer J Epidemiol. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 41.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandharipane PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milbrandt EB, Angus DC. Bench-to-bedside review: critical illness-associated cognitive dysfunction—mechanisms, markers, and emerging therapeutics. Crit Care. 2006;10:238. doi: 10.1186/cc5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simen AA, Bordner KA, Martin MP, Moy LA, Barry LC. Cognitive dysfunction with aging and the role of inflammation. Ther Adv Chronic Dis. 2011;2:175–195. doi: 10.1177/2040622311399145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alboni S, Cervia D, Sugama S, Conti B. Interleukin 18 in the CNS. J Neuroinflammation. 2010;7:9. doi: 10.1186/1742-2094-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18:S19–S25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah FA, Pike F, Alvarez K, Angus D, Newman AB, Lopez O, Tate J, Kapur V, Wilsdon A, Krishnan JA, et al. Bidirectional relationship between cognitive function and pneumonia. Am J Respir Crit Care Med. 2013;188:586–592. doi: 10.1164/rccm.201212-2154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emery CF, Green MR, Suh S. Neuropsychiatric function in chronic lung disease: the role of pulmonary rehabilitation. Respir Care. 2008;53:1208–1216. [PubMed] [Google Scholar]
- 49.Barbour KA, Blumenthal JA, Palmer SM. Psychosocial issues in the assessment and management of patients undergoing lung transplantation. Chest. 2006;129:1367–1374. doi: 10.1378/chest.129.5.1367. [DOI] [PubMed] [Google Scholar]
- 50.Tully PJ, Baker RA. Depression, anxiety, and cardiac morbidity outcomes after coronary artery bypass surgery: a contemporary and practical review. J Geriatr Cardiol. 2012;9:197–208. doi: 10.3724/SP.J.1263.2011.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kollner V, Schade I, Maulhardt T, Maercker A, Joraschky P, Gulielmos V. Posttraumatic stress disorder and quality of life after heart or lung transplantaiton. Transplant Proc. 2002;34:2192–2193. doi: 10.1016/s0041-1345(02)03198-6. [DOI] [PubMed] [Google Scholar]
- 52.Blumenthal JA, Babyak MA, Keefe FJ, David RD, Lacaille RA, Carney RM, Freedland KE, Trulock E, Palmer SM. Telephone-based coping skills training for patients awaiting lung transplantation. J Consult Clin Psychol. 2006;74:535–544. doi: 10.1037/0022-006X.74.3.535. [DOI] [PubMed] [Google Scholar]
- 53.Schelling G, Kilger E, Roozendaal B, de Quervain DJ, Briegel J, Dagge A, Rothenhausler HB, Krauseneck T, Nollert G, Kapfhammer HP. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Biol Psychiatry. 2004;55:627–633. doi: 10.1016/j.biopsych.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 54.Weis F, Kilger E, Roozendaal B, de Quervain DJ, Lamm P, Schmidt M, Schmolz M, Briegel J, Schelling G. Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: a randomized study. J Thorac Cardiovasc Surg. 2006;131:277–282. doi: 10.1016/j.jtcvs.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 55.Garrouste-Orgeas M, Coquet I, Perier A, Timsit JF, Pochard F, Lancrin F, Philippart F, Vesin A, Bruel C, Blel Y, et al. Impact of an intensive care unit diary on psychological distress in patients and relatives. Crit Care Med. 2012;40:2033–2040. doi: 10.1097/CCM.0b013e31824e1b43. [DOI] [PubMed] [Google Scholar]
- 56.Jones C, Backman C, Capuzzo M, Egerod I, Flaatten H, Granja C, Rylander C, Griffiths RD. Intensive care diaries reduce new onset post traumatic stress disorder following critical illness: a randomized, controlled trial. Crit Care. 2010;14:R168. doi: 10.1186/cc9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Busschbach JJ, Horikx PE, Van den Bosch JM, Brutel de la Rivière A, De Charro FT. Measuring the quality of life before and after bilateral lung transplantation in patients with cystic fibrosis. Chest. 1994;105:911–917. doi: 10.1378/chest.105.3.911. [DOI] [PubMed] [Google Scholar]
- 58.Gross CR, Savik K, Bolman RM, Hertz MI. Long-term health status and quality of life outcomes of lung transplant recipients. Chest. 1995;108:1587–1593. doi: 10.1378/chest.108.6.1587. [DOI] [PubMed] [Google Scholar]
- 59.Anyanwu AC, McGuire A, Rogers CA, Murday AJ. Assessment of quality of life in lung transplantation using a simple generic tool. Thorax. 2001;56:218–222. doi: 10.1136/thorax.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smeritschnig B, Jaksch P, Kocher A, Seebacher G, Aigner C, Mazhar S, Klepetko W. Quality of life after lung transplantation: a cross-sectional study. J Heart Lung Transplant. 2005;24:474–480. doi: 10.1016/j.healun.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 61.Eskander A, Waddell TK, Faughnan ME, Chowdhury N, Singer LG. BODE index and quality of life in advanced chronic obstructive pulmonary disease before and after lung transplantation. J Heart Lung Transplant. 2011;30:1334–1341. doi: 10.1016/j.healun.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 62.Aykut K, Albayrak G, Guzeloglu M, Hazan E, Tufekci M, Erdogan I. Pulsatile versus non-pulsatile flow to reduce cognitive decline after coronary artery bypass surgery: a randomized prospective clinical trial. J Cardiovasc Dis Res. 2013;4:127–129. doi: 10.1016/j.jcdr.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]