Abstract
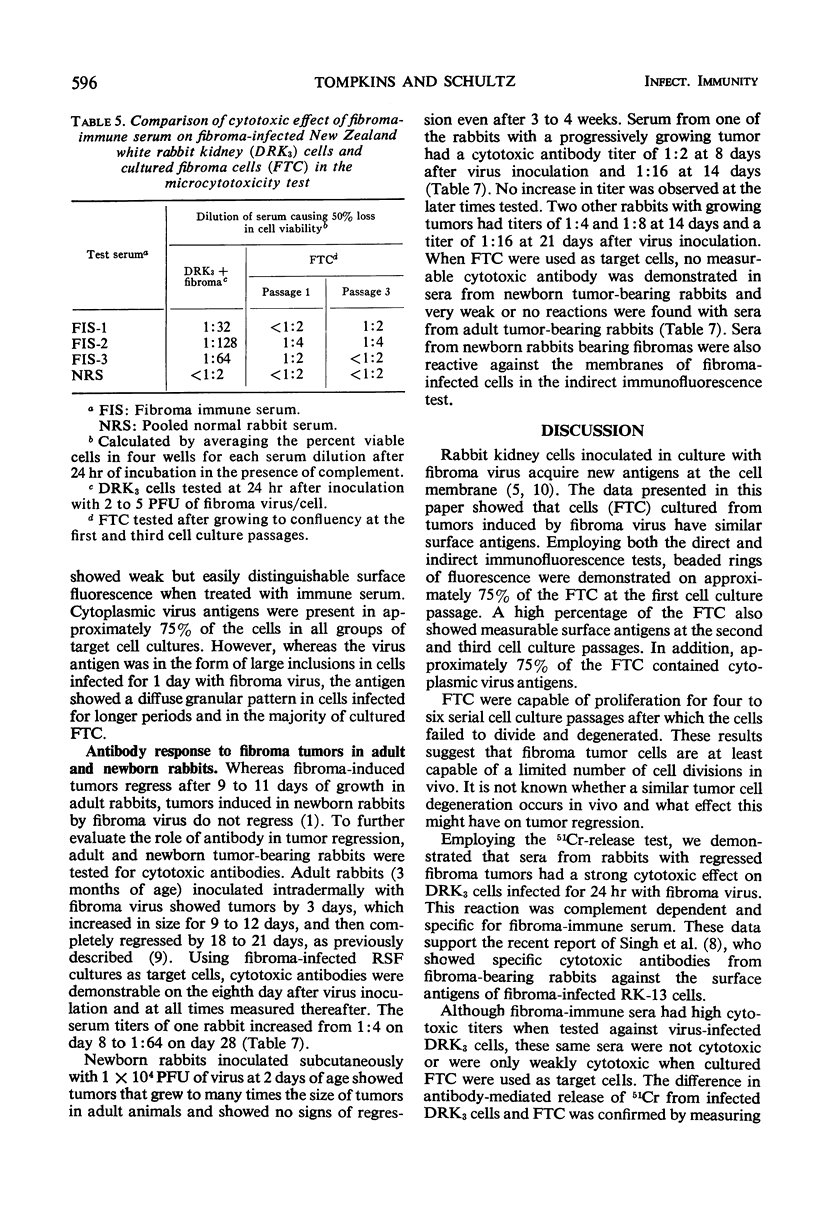
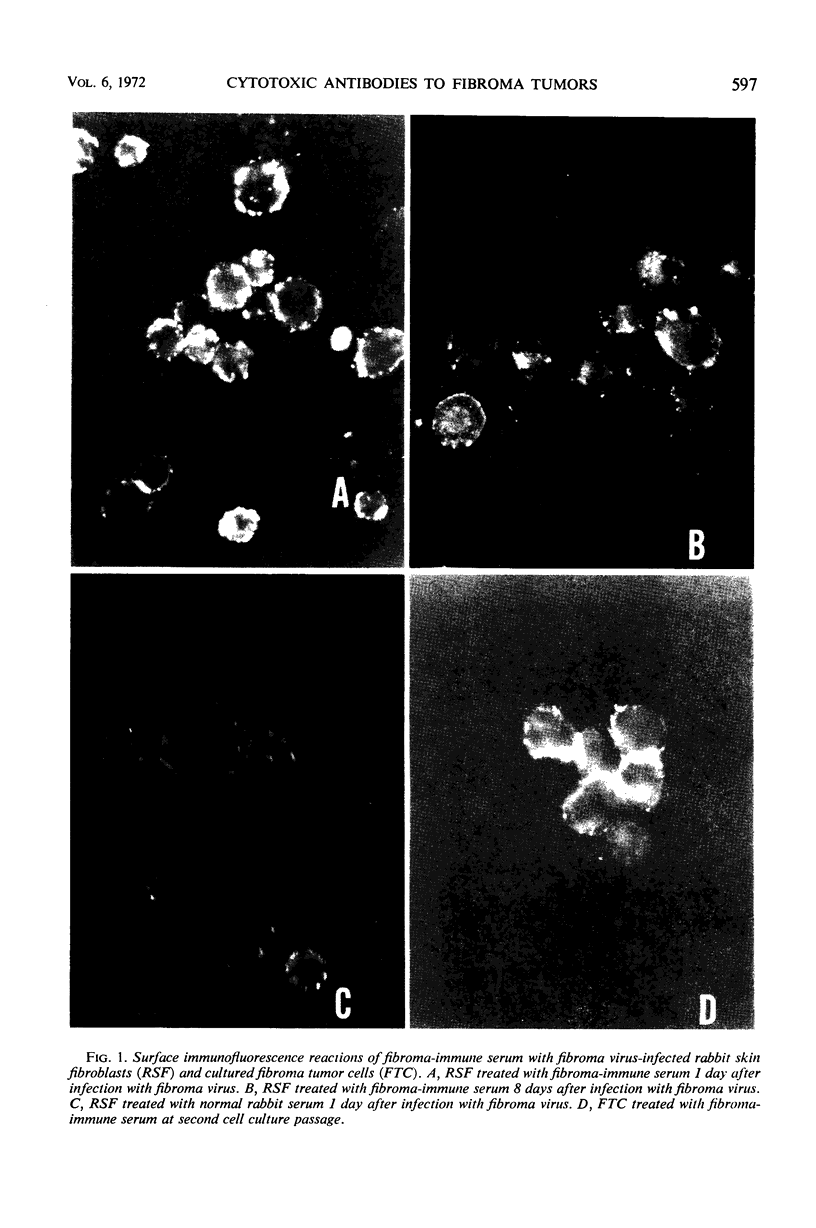
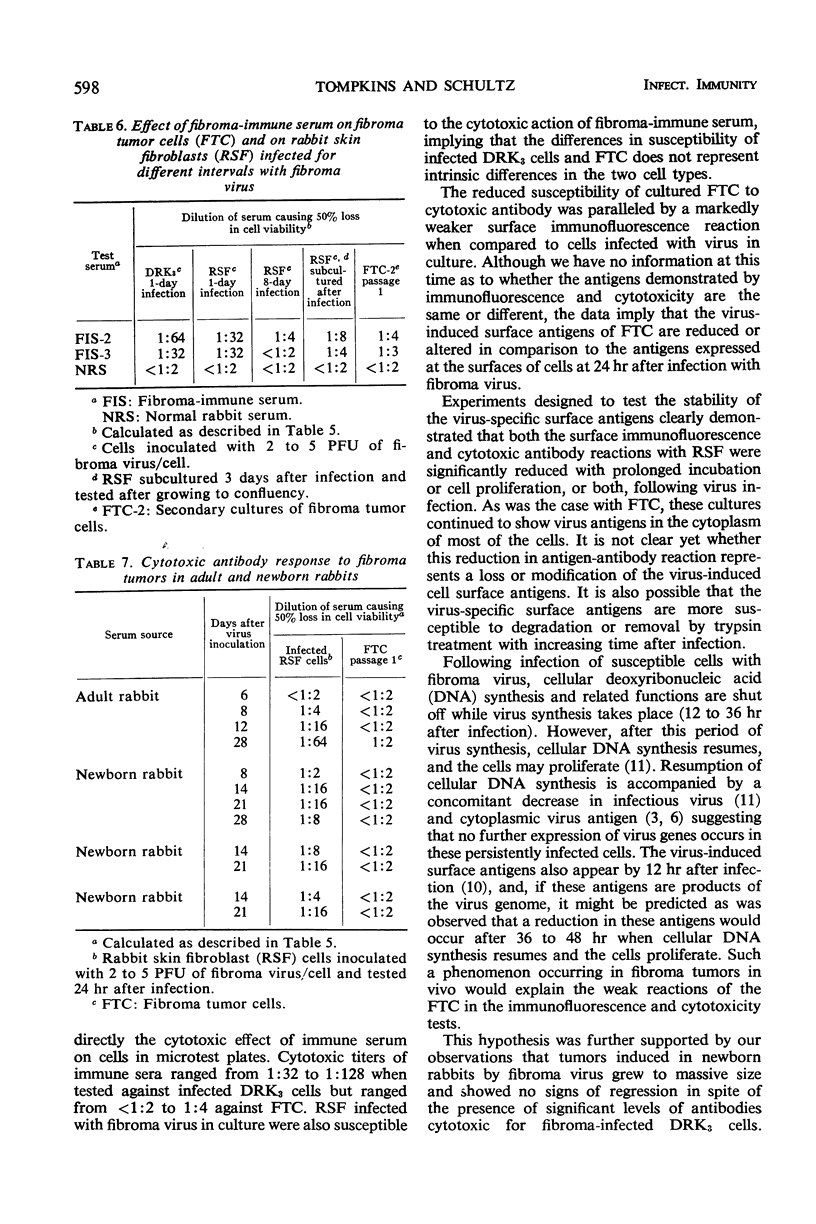
Cells cultured from tumors induced in rabbits by inoculation of fibroma virus possessed virus-specific cell surface and cytoplasmic antigens. Tumor cell cultures were capable of a limited number of cell divisions before degenerating. Employing the 51Cr-release test and the microcytotoxicity test, it was demonstrated that sera from rabbits with regressed fibroma tumors contained antibodies cytotoxic for cells infected in culture with fibroma virus. These sera were only weakly cytotoxic for cultured fibroma tumor cells. In addition, newborn rabbits bearing progressively growing tumors had serum antibodies cytotoxic for cells infected with fibroma virus in culture but not for fibroma tumor cells. Immunofluorescence studies also showed that the reaction of immune serum with the surface antigens of fibroma tumor cells was markedly weaker than with the surface antigens of cells infected with virus in culture. Furthermore, membrane cytotoxicity and immunofluorescence reaction were significantly reduced when cells were tested after prolonged incubation or cell division, or both, following infection with fibroma virus. The failure of tumors to regress in newborn rabbits in spite of the presence of cytotoxic antibodies is discussed in respect to a possible reduction or alteration of virus-specific surface antigens on proliferating tumor cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HINZE H. C., WALKER D. L. RESPONSE OF CULTURED RABBIT CELLS TO INFECTION WITH THE SHOPE FIBROMA VIRUS. I. PROLIFERATION AND MORPHOLOGICAL ALTERATION OF THE INFECTED CELLS. J Bacteriol. 1964 Oct;88:1185–1194. doi: 10.1128/jb.88.4.1185-1194.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan I. C., Loewi G. The effect of peripheral lymphocytes from patients with inflammatory joint disease on human target cells in vitro. Clin Exp Immunol. 1968 Jun;3(5):385–391. [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H., Kato S. Immune hemadsorption by cells infected with poxviruses. Biken J. 1968 Dec;11(4):343–353. [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Effect of persistent fibroma virus infection on susceptibility of cells to other viruses. J Virol. 1970 Feb;5(2):199–204. doi: 10.1128/jvi.5.2.199-204.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls W. E., Tompkins W. A., Melnick J. L. The association of herpesvirus type 2 and carcinoma of the uterine cervix. Am J Epidemiol. 1969 May;89(5):547–554. doi: 10.1093/oxfordjournals.aje.a120967. [DOI] [PubMed] [Google Scholar]
- Singh S. B., Smith J. W., Rawls W. E., Tevethia S. S. Demonstration of cytotoxic antibodies in rabbits bearing tumors induced by Shope fibroma virus. Infect Immun. 1972 Mar;5(3):352–358. doi: 10.1128/iai.5.3.352-358.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins W. A., Adams C., Rawls W. E. An in vitro measure of cellular immunity to fibroma virus. J Immunol. 1970 Feb;104(2):502–510. [PubMed] [Google Scholar]
- Tompkins W. A., Crouch N. A., Tevethia S. S., Rawls W. E. Characterization of surface antigen on cells infected by fibroma virus. J Immunol. 1970 Nov;105(5):1181–1189. [PubMed] [Google Scholar]
- Tompkins W. A., Walker D. L., Hinze H. C. Cellular deoxyribonucleic acid synthesis and loss of contact inhibition in irradiated and contact-inhibited cell cultures infected with fibroma virus. J Virol. 1969 Nov;4(5):603–609. doi: 10.1128/jvi.4.5.603-609.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]