Abstract
Rationale: Pulmonary arterial hypertension (PAH) is a pulmonary vasculopathy that leads to failure of the right ventricle and premature death.
Objectives: To determine whether the sublingual microcirculation is affected in patients with PAH compared with healthy age- and sex-matched control subjects.
Methods: Using the CapiScope Handheld Video Capillaroscope we measured the sublingual microvasculature density, flow index, tortuosity, and curvature. Videos were acquired immediately after right heart catheterization, and determinations were made off-line by investigators blinded to the group assignment or hemodynamics.
Measurements and Main Results: In this cross-sectional pilot study, we included 26 patients with PAH (age, mean ± SD, 56.7 ± 10 yr; 77% women) and 14 healthy control subjects (age, 53.1 ± 12 yr; 71% women). Sublingual microvasculature flow index was lower (2 ± 0.66 vs. 2.7 ± 0.37, P < 0.001) with higher heterogeneity index (0.63 ± 0.63 vs. 0.25 ± 0.25, P = 0.04) in patients with PAH than control subjects. Microvasculature density was similar between the groups, but tortuosity was more pronounced in patients than control subjects (tort 0: 45 ± 19 vs. 23.6 ± 12, P = 0.001 and tort 1: 0.2 ± 0.16 vs. 0.06 ± 0.04, P < 0.001).
Conclusions: Patients with PAH showed lower sublingual microvasculature flow index and higher tortuosity compared with healthy age- and sex-matched control subjects. Further investigations are needed to assess whether this methodology can provide information on disease prognosis and/or response to therapy in this condition.
Keywords: pulmonary arterial hypertension, capillaroscopy, microcirculation, vascular function
Pulmonary arterial hypertension (PAH) is a pulmonary vasculopathy that leads to elevated mean pulmonary arterial pressure (mPAP), failure of the right ventricle, and premature death (1). PAH is hemodynamically characterized by a resting mPAP greater than or equal to 25 mm Hg and a pulmonary arterial occlusion pressure less than or equal to 15 mm Hg (2). Right heart catheterization (RHC) is the gold standard for diagnosis of PAH, and it continues to be an important test for assessing disease severity. However, RHC is an invasive procedure that is not exempt from complications (2). Therefore, there is a pressing need to identify noninvasive methods to diagnose and assess the severity of PAH.
The microcirculation is an essential component of the cardiovascular system, as it permits the interaction between the blood and tissue to ensure oxygen supply. A few devices that directly display the microcirculation have been introduced to clinical practice. Orthogonal polarization spectral imaging, sidestream dark field imaging, and oblique profiled epi-illumination imaging provide high-contrast images of the microcirculation and are based on the principle that green light is absorbed by the hemoglobin of the red blood cells (RBC) within superficial vessels; meanwhile, nonabsorbed light illuminates the tissue background. These noninvasive methodologies allow the visualization of the microcirculation in vivo at the bedside (3). The sublingual (SL) area is frequently selected to assess the microcirculation because it is easily accessible and is considered a mirror of the splanchnic microvasculature due to the embryological and metabolic similarities (4, 5).
So far, SL capillaroscopy has been used predominantly in critically ill patients (i.e., sepsis, heart failure, and cardiogenic shock) to identify abnormalities of the microcirculation that could lead to effective interventions (3, 6–9). In severe sepsis, the restoration of the microcirculation was associated with improvements in organ function and survival (10). Conversely, a persistent microcirculatory abnormality was associated with an increased mortality (10, 11). In patients with acute heart failure, the sublingual microcirculation is abnormal, and the extent of this impairment may be related to survival (12) and treatment response (13, 14).
Pulmonary vascular disease is the hallmark of PAH; however, the extent of systemic vasculature involvement, if any, is unknown. A few studies suggest that patients with PAH have systemic vascular dysfunction when compared with control subjects as determined by forearm blood flow dilation after brachial artery occlusion (15, 16). A study in a small number of patients with systemic sclerosis reported that the nailfold microvasculature is significantly more affected in individuals who had PAH when compared with those who did not (17). However, no study to date has evaluated the microvasculature at the level of the sublingual mucosa in patients with PAH.
We hypothesized that the SL microcirculation is distorted in all types of patients with PAH compared with control subjects and that the abnormality correlates to disease severity. If proven of value, SL capillaroscopy may become a valuable noninvasive tool for the integrated (macrohemodynamics and systemic microcirculation) hemodynamic assessment of patients with PAH. Some of the results of this pilot study have been previously reported in the form of an abstract (18).
Methods
Subjects and Design
The research protocol was approved by the Cleveland Clinic Institutional Review Board (study #11-441), and all subjects provided written informed consent. Patients were included in the study if they met hemodynamic criteria for PAH during RHC (defined as mPAP ≥ 25 mm Hg, pulmonary arterial occlusion pressure ≤ 15 mm Hg, and pulmonary vascular resistance ≥ 3 Wood units) and had no other conditions that place them in pulmonary hypertension groups other than PAH (19). To determine the etiology of PH, each patient underwent a thorough clinical evaluation, including complete blood cell count, comprehensive metabolic panel, antinuclear antibody, sedimentation rate, hepatitis panel, HIV serology, thyroid-stimulating hormone, pulmonary function, 6-minute walk, chest radiography, ventilation/perfusion scan, echocardiography, and RHC. Further clinical testing was performed when initial tests were either positive or inconclusive. Age- and sex-matched control subjects were recruited using institutional review board–approved flyers. We conducted this cross-sectional study between March 2012 and May 2013.
Capillaroscopy
Imaging of the microvasculature was performed using the CapiScope Handheld Video Capillaroscope (HVCS) system (KK Technology, Honiton, England) (Figure 1). The CapiScope uses oblique (illumination comes from an angle outside the aperture of the objective) profiled (higher-intensity light travels into the tissue outside the field of view) epi-illumination (onto the surface) imaging. The microcirculation is illuminated with a green light and the backscattered light is filtered, creating a high-contrast image of flowing RBC in superficial vessels (3, 6, 20). The light guide in CapiScope HVCS imaging is surrounded by light-emitting diodes of 530-nm wavelength that provide oblique profiled epi-illumination in synchrony with the frame rate of the USB camera. RBCs are seen as dark flowing structures over a greyish background, and white blood cells appear as gray gaps inside the vessels (7). Although the vessel walls are not visualized directly, a faint contour is delineated by the RBCs (3, 21).
Figure 1.
CapiScope handheld device connected to a laptop computer. Reprinted by permission from KK Technology.
Sublingual capillaroscopy was performed immediately after the RHC in all subjects. Patients did not eat or drink for 12 hours before testing. After acclimatization for 20 minutes at stable room temperature (72°F), videos were acquired with patients in a sitting position. The CapiScope probe was fitted with sterile disposable plastic caps. The tongue was stabilized by asking the patient to gently push it against his/her superior incisors. We examined three to four sublingual areas lateral to the frenulum on the left side, approximately 3 to 4 cm anterior to the base of the tongue (13). To decrease the local pressure applied (which can collapse the microcirculation), the probe was retracted gradually from the sublingual surface, minimizing contact while still enabling visualization. We avoided areas where the microvasculature network formed loops, as this is suggestive of lingual rather than sublingual mucosa (4). When appropriate, we made small adjustments of the focus to better visualize the capillary network. Seven to 10 video sequences of 30 to 50 seconds each were recorded using a portable computer and the CapiScope software (14). Video files were given code numbers.
Measurements Protocol and Analytical Methods
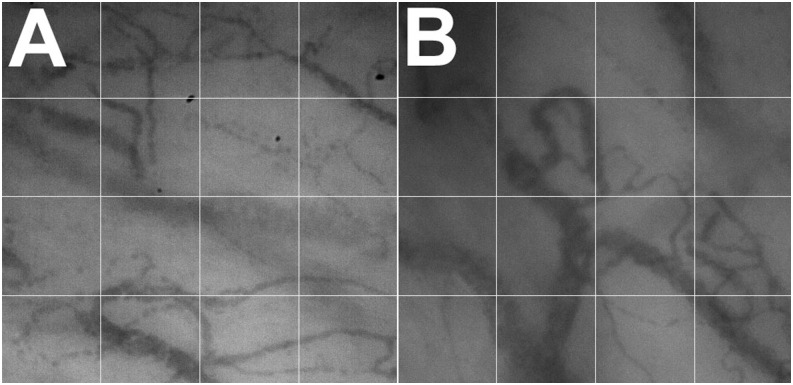
Two investigators (L.D and L.A.) blinded to the group assignment and other medical data analyzed off-line the digitally recorded sequences. We selected the best three videos for each subject. The more stable portion (10–15 s) of each video was preserved. The recordings that were affected by pressure artifacts were eliminated. Pressure artifact was considered present when the flow in the large venules (diameter > 30 μm) was sluggish or absent (5). Vascular size was ascertained by using a measuring tool in the CapiScope software. We discarded 16.6% of the videos, as they had some degree of pressure artifact. We focused our analysis to small vessels (<20 μm), which are mostly capillaries, and we calculated the flow index and density of the sublingual microvasculature following established guidelines (22). The microvasculature flow index assesses that quality of the flow and was measured semiquantitatively by averaging a score of 0 to 3 (0: absent flow; 1: intermittent; 2: continuous but sluggish; and 3: brisk/normal flow) from each of the four video quadrants (23). The heterogeneity flow index measures whether there are nonperfused areas next to well-perfused ones. It was calculated by subtracting the highest from the lowest flow score and dividing this result by the mean microvascular flow index (23). We estimated small vessel density using the De Backer score (22), a method based on the principle that the density of vessels is proportional to the number of vessels crossing arbitrary lines. For the arbitrary lines we used a grid of three equidistant horizontal and vertical lines (Figure 2). We estimated microvasculature density by dividing the number of vessels (<20 μm in diameter) crossing prespecified lines by the total length of the lines (3.6 mm) (4).
Figure 2.
Small vessel density of sublingual microvasculature in a control subject (A) and patient with pulmonary arterial hypertension (B). Microvascular density is measured by counting the small vessels (<20 μm) that cross the gridlines.
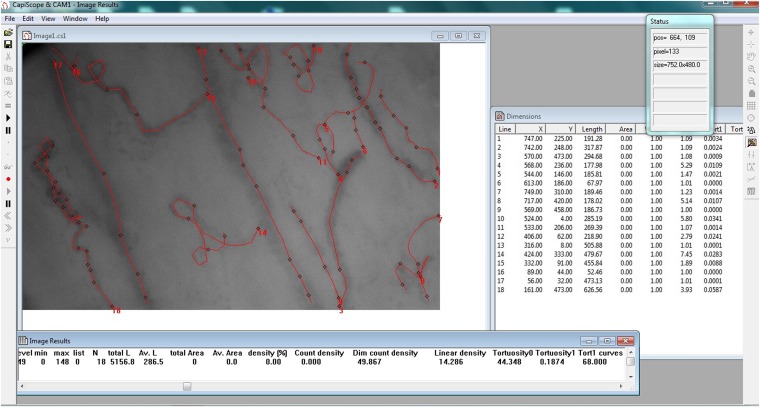
We used the CapiScope software to measure tortuosity and curvature of the sublingual microcirculation using a representative still image on each of the three selected videos (Figure 3). We drew a line over each microvessel (<20 μm in diameter) using the software tools (Figure 4). The software provided two measurements for tortuosity: tortuosity 0 (Tort 0), which is the ratio of the vessel over the chord length (shortest straight line between the two end points), and tortuosity 1 (Tort 1), which depends on the number of curves over the chord length and the sum of the ratios of curve length over chord length (24). Curvature was measured by counting the number of curves in all small vessels.
Figure 3.
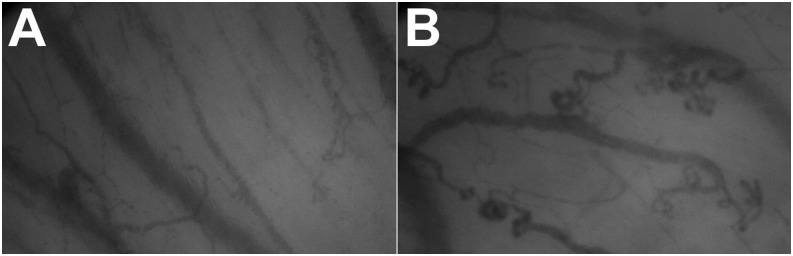
Tortuosity and curvature in a control subject (A) and patient with pulmonary arterial hypertension (B).
Figure 4.
CapiScope software used to measure the tortuosity and curvature of sublingual vessels. Red lines were drawn along the vessels to obtain the calculations provided.
We tested intrarater variability by randomly selecting 10 patients with PAH and evaluating three videos for each of them. Videos were presented and analyzed randomly on two occasions. Investigators picked the best three videos for microvasculature flow index determination and an image from each to measure microvasculature density, tortuosity, and curvature index. One investigator (L.D.) also measured the microvasculature flow index determination and microvasculature density in these 10 patients on two separate occasions (selected at random to prevent bias) to determine intrarater variability.
Functional, Echocardiographic, and Hemodynamic Measurements
We determine the severity of PAH by using New York Heart Association functional class, plasma brain natriuretic peptide, distance walked in the 6-minute-walk test, right ventricular function on echocardiography, and a variety of hemodynamic parameters during RHC (right atrial pressure, mPAP, cardiac output, cardiac index, pulmonary artery vascular resistance, and mixed venous oxygenation). In all cases, RHC was performed under local anesthesia (lidocaine 2%); no sedation or vasoactive medications were administered. Two patients underwent nitric oxide challenge with 40 ppm for 5 minutes. SL capillaroscopy was performed more than 20 minutes after this administration. Given the very short half-life of nitric oxide, this agent should not have affected the SL circulation at the time of capillaroscopy. Right ventricular function was subjectively measured by experienced cardiologists. We assessed quality of life with the Cambridge Pulmonary Hypertension Outcome Review questionnaire (25).
Statistical Analysis
Continuous data are presented as mean ± SD or median (interquartile range) where appropriate. Categorical data are summarized as discrete values and percentages (n [%]). Independent continuous and categorical samples were compared using Mann-Whitney and Fisher exact test, respectively. Relationships between sublingual capillaroscopy and hemodynamic parameters were tested using the Pearson correlation test. Analysis of covariance was applied to obtain means adjusted for covariates. Interrater and intrarater agreement for single measures was calculated using the intraclass correlation coefficients and their respective 95% confidence intervals. Each subject was rated by the same two raters. We tested for absolute agreement, as systematic differences are considered relevant. All P values are two tailed, and a value of less than 0.05 was considered significant. The statistical analyses were performed using the statistical package IBM SPSS, version 20 (IBM; Armonk, New York).
Results
Patient Characteristics
Twenty-six patients with PAH and 14 age- and sex-matched control subjects were included in the study. The mean age of the patients with PAH was 56.7 ± 10 years, and 77% were females. Etiology of PAH was idiopathic in nine (35%), heritable in four (15%), portopulmonary in seven (27%), scleroderma in five (19%), and HIV in one (4%) patient. Mean age of the control group was 53.1 ± 11.6 years, and 71% were women. Table 1 summarizes overall characteristics of patients with PAH and control subjects. The functional status, arterial blood gas, and echocardiographic and hemodynamic measurements of patients with PAH are presented in Table 2.
Table 1.
Overall characteristics of patients with pulmonary arterial hypertension and control subjects
| Patients with PAH | Control Subjects | |
|---|---|---|
| n | 26 | 14 |
| Age, yr | 56.7 (10) | 53.1 (11.6) |
| Sex | ||
| Female | 20 (77) | 10 (71) |
| Race | ||
| White | 24 (92) | 12 (86) |
| African American | 2 (8) | 2 (14) |
| BMI, kg/m2 | 29.1 (6) | 27.4 (5) |
| Etiology of PAH | ||
| Idiopathic | 9 (35) | |
| Heritable | 4 (15) | |
| Portopulmonary | 7 (27) | |
| Connective tissue disease | 5 (19) | |
| HIV | 1 (4) | |
| NYHA Class | ||
| I | 2 (8) | |
| II | 11 (42) | |
| III | 12 (46) | |
| IV | 1 (4) | |
| Other associated comorbidities | ||
| Controlled HTN | 2 (8) | 6 (43) |
| Controlled type 2 DM | 2 (8) | 3 (21) |
| Treated OSA | 6 (23) | |
| Treated COPD | 3 (12) | |
| PAH-specific treatment | ||
| No therapy (at time of diagnostic RHC) | 8 (31) | |
| Single therapy | 12 (46) | |
| Combination therapy | 6 (23) | |
| Phosphodiesterase-5 inhibitors | 9 | |
| Endothelin receptor antagonist | 7 | |
| Prostacyclin analogs | 10 |
Definition of abbreviations: BMI = body mass index; COPD = chronic obstructive pulmonary disease; DM = diabetes mellitus; HTN = hypertension; NYHA = New York Heart Association; OSA = obstructive sleep apnea; PAH = pulmonary arterial hypertension; RHC = right heart catheterization.
Data are presented as n (%) or mean (SD).
Table 2.
Functional, echocardiographic, and hemodynamic data in patients with pulmonary arterial hypertension
| n (%) or Mean (SD) | |
|---|---|
| 6-min-walk test | |
| Distance walked, m | 343 (126) |
| Distance walked, % predicted (47) | 66 (19) |
| Echocardiogram | |
| RVSP, mm Hg | 76 (28) |
| RV function | |
| Normal | 8 (31) |
| Mild | 6 (23) |
| Moderate | 6 (23) |
| Severe | 6 (23) |
| RV dilation | |
| Normal | 9 (35) |
| Mild | 5 (19) |
| Moderate | 7 (27) |
| Severe | 5 (19) |
| RHC | |
| Systolic ABP, mm Hg | 126 (23) |
| Diastolic ABP, mm Hg | 78 (11) |
| Heart rate, bpm | 78 (11) |
| SpO2, % | 94 (4) |
| RA pressure, mm Hg | 8 (5) |
| RV systolic pressure, mm Hg | 72 (21) |
| PA systolic pressure, mm Hg | 73 (22) |
| PA diastolic pressure, mm Hg | 32 (11) |
| PA mean pressure, mm Hg | 46 (14) |
| PAOP, mm Hg | 12 (6) |
| CO thermodilution, L/min | 6.1 (3) |
| CI thermodilution, L/min/m2 | 3.2 (1) |
| PVR, Wood units | 7.6 (6) |
| SvO2, % | 67 (9) |
Definition of abbreviations: ABP = arterial blood pressure; CI = cardiac index; CO = cardiac output; PA = pulmonary artery; PAH = pulmonary arterial hypertension; PAOP = pulmonary artery occlusion pressure; PVR = pulmonary vascular resistance; RA = right atrium; RHC = right heart catheterization; RSVP = right ventricular systolic pressure; RV = right ventricle; SpO2 = pulse oxygen saturation; SvO2 = mixed venous oxygenation.
Sublingual Capillaroscopy Determinations in Patients with PAH Versus Control Subjects
When compared with age- and sex-matched control subjects, patients with PAH had significantly lower SL microvasculature flow index (2 ± 0.66 vs. 2.7 ± 0.37, P < 0.001) with greater heterogeneity index (0.63 ± 0.63 vs. 0.25 ± 0.25, P = 0.04). The average SL microvasculature density was similar in the two groups; however, patients with PAH had more pronounced microvasculature tortuosity (tort 0 and 1) and curvature (Table 3). There was no statistical relationship between SL microvascular flow index, density, or tortuosity and sex, race, or the presence of comorbidities. There was no difference in control patients with or without systemic hypertension, and the results were not altered when we removed the two patients with PAH and diabetes mellitus from the analysis.
Table 3.
Capillaroscopy determinations in patients with pulmonary arterial hypertension and control subjects
| Patients with PAH, Mean (SD) | Control Subjects, Mean (SD) | P Value | |
|---|---|---|---|
| Microvasculature flow index | 2 (0.66) | 2.7 (0.37) | <0.001 |
| Microvasculature heterogeneity flow index | 0.63 (0.63) | 0.25 (0.25) | 0.039 |
| Microvasculature vessel density (per mm) | 8.1 (1) | 7.8 (2) | 0.58 |
| Microvasculature tortuosity | |||
| Tortuosity 0 | 45 (19) | 23.6 (12) | 0.001 |
| Tortuosity 1 | 0.2 (0.16) | 0.06 (0.04) | <0.001 |
| Curvature | 66.2 (20) | 35.6 (13) | <0.001 |
Definition of abbreviation: PAH = pulmonary arterial hypertension.
The intraclass correlation coefficients (95% confidence interval) for the same rater on SL microvasculature flow index and total microvasculature density (n = 30 videos) were 0.85 (0.7–0.92) and 0.78 (0.59–0.89), respectively. When averaging the results of the three videos for each individual (n = 10), the coefficients for microvasculature flow index and total microvasculature density were 0.96 (0.84–0.99) and 0.92 (0.72–0.98), respectively. The intraclass correlation coefficients for the two raters on SL microvasculature flow index and total microvasculature density (n = 30 videos) were 0.83 (0.33–0.94) and 0.75 (0.24–0.90), respectively. When averaging the results of the three videos of each individual (n = 10), the coefficients for microvasculature flow index and total microvasculature density were 0.72 (0.1–0.93) and 0.58 (0.1–0.88), respectively. The coefficients for tort 0, tort 1, and curvature (n = 5) were 0.58 (−0.53 to 0.95), 0.41 (−0.49 to 0.91), and 0.8 (0.1–0.98).
Sublingual Capillaroscopy Determinations in Patients with PAH in Relation to Treatment Status
We compared patients with PAH who were on PAH-specific therapies (n = 18) with treatment-naive subjects (n = 8). SL microvasculature flow index, heterogeneity flow index, total microvasculature density, tort 0, and number of curves were similar between the two groups (data not shown); however, tort 1 was greater in patients receiving PAH-specific therapies than those who were not (0.24 ± 0.18 vs. 0.11 ± 0.07, P = 0.02).
Association between Sublingual Capillaroscopy Determinations and Etiology or Severity of PAH Disease
There was no significant difference in SL microvasculature flow index, density, or tortuosity among the different etiologies of PAH (Table 4) or echocardiographic or hemodynamic predictors of PAH disease severity (Table 5).
Table 4.
Capillaroscopy determinations in different types of pulmonary arterial hypertension
| Idiopathic/Heritable PAH, Mean (SD) | PoPH, Mean (SD) | Scleroderma-associated PAH, Mean (SD) | P Value | |
|---|---|---|---|---|
| n | 13 | 7 | 5 | |
| Microvasculature flow index | 2 (0.6) | 2 (0.7) | 2 (1) | 0.99 |
| Microvasculature heterogeneity flow index | 0.52 (0.4) | 0.72 (0.6) | 0.89 (1) | 0.51 |
| Microvasculature vessel density (per mm) | 8.1 (1) | 8 (1) | 8.6 (1.7) | 0.75 |
| Microvasculature tortuosity | ||||
| Tortuosity 0 | 44 (20) | 45 (21) | 50 (19) | 0.87 |
| Tortuosity 1 | 0.19 (0.17) | 0.16 (0.12) | 0.30 (0.2) | 0.35 |
| Curvature | 61 (18) | 70 (19) | 74 (27) | 0.42 |
Definition of abbreviations: PAH = pulmonary arterial hypertension; PoPH = portopulmonary hypertension.
Table 5.
Association between predictors of pulmonary arterial hypertension severity and capillaroscopy determinations
| Right Atrial Pressure r (P Value) | Cardiac Index by Thermodilution r (P Value) | Pulmonary Vascular Resistance r (P Value) | |
|---|---|---|---|
| Microvasculature flow index | 0.02 (0.93) | 0.1 (0.62) | 0.01 (0.99) |
| Microvasculature heterogeneity flow index | −0.22 (0.28) | −0.03 (0.9) | −0.05 (0.82) |
| Microvasculature vessel density (per mm) | −0.19 (0.34) | −0.08 (0.7) | 0.08 (0.69) |
| Microvasculature tortuosity | |||
| Tortuosity 0 | −0.12 (0.56) | 0.11 (0.6) | −0.05 (0.83) |
| Tortuosity 1 | −0.11 (0.59) | −0.02 (0.93) | −0.01 (0.99) |
| Curvature | −0.26 (0.21) | 0.16 (0.43) | −0.24 (0.24) |
Definition of abbreviation: r = Pearson correlation coefficient.
Discussion
To our knowledge, this is the first study reporting direct visualization of the SL microcirculation in patients with PAH using oblique profiled epi-illumination imaging. Our preliminary data demonstrate that there is a reduced flow index and a greater heterogeneity flow index, tortuosity, and curvature of the SL microvasculature in patients with PAH when compared with age- and sex-matched control subjects. No associations were noted in the SL capillaroscopy indices among the etiologies of PAH or the severity of the PAH disease.
Capillaroscopy has been used to evaluate the SL microcirculation mainly in patients with severe sepsis (3, 6, 26). The oblique profiled epi-illumination imaging used in our study provided excellent images of the SL microcirculation. Capillaroscopy videos were easily acquired; however, artifacts were common, and the measurements performed were time consuming. The operators required substantial training in how to acquire and process the videos as well as detect artifacts and perform the measurements presented. These limitations have been previously noted by other authors (27). We paid particular attention to the presence of artifacts that could affect our determinations, such as motion, excessive local pressure, inadequate focus, or presence of oral secretions. We observed a good intra- and interrater agreement for microvasculature flow index, total microvasculature density, and curvature using an approach that is likely to be a closer reflection of clinical practice. Hubble and colleagues also reported a good interobserver reproducibility of SL microvascular density and flow index in healthy volunteers; more importantly, the authors observed no significant variability of SL microvasculature density and flow index determinations over time (4).
Our study showed that the SL microcirculation is significantly distorted in patients with PAH when compared with age- and sex-matched control subjects. Interestingly, the microvasculature flow index was lower in patients with PAH than healthy control subjects, a finding that may be associated with a reduced cardiac output in PAH. Nevertheless, we did not observe any association between this capillaroscopy measurement and cardiac index measured by thermodilution or indirect Fick, suggesting an alternative mechanism for the reduced microvasculature flow index. This lack of association between microvasculature flow index and cardiac output has been observed in patients with sepsis who have increased cardiac output but reduced microvasculature flow index (8, 23).
We also observed greater inequalities of the microvasculature flow in PAH compared with healthy control subjects. This is relevant, as the perfusion homogeneity is more important than blood flow velocity to assure an adequate tissue oxygenation (28). Trzeciak and colleagues found that patients with sepsis have greater microvasculature flow heterogeneities than control subjects (23) that originate in a selective vasodilation of some microcirculatory units with a consequent reduction of flow to other areas (7, 22, 29). This alteration may reflect an unequal expression of the inducible nitric oxide synthase in the SL microcirculation (30).
Interestingly, SL microvasculature tortuosity and curvature were more pronounced in subjects with PAH than control subjects. Similarly, Djaberi and colleagues noted that the labial capillaries of patients with diabetes had greater tortuosity compared with control subjects, potentially related to endothelial dysfunction caused by changes in vasa vasorum and hypoxia (31). It is possible that a lower PaO2 in patients with PAH might cause the vessels to become more tortuous (32, 33) by a more pronounced shear stress and neovascularization (32). We noted that patients with PAH receiving specific therapies had greater tortuosity than treatment-naive subjects, which could reflect a more advanced disease state.
We found no difference in the microvasculature density between patients with PAH and control subjects. Likewise, Edul and colleagues showed no difference in microvasculature density between patients with sepsis and control subjects (34); however, other investigations in sepsis showed a reduction in the microvascular density in patients with sepsis (8, 23).
We were not able to find a significant association between capillaroscopy measurements and hemodynamic findings. This is in line with other authors who demonstrated that macrohemodynamic determinations do not necessarily match microhemodynamic and organ function improvements (10, 26). In fact, experimental models of septic shock showed that microvascular perfusion is commonly affected even after normalization of systemic hemodynamics, supporting the concept that microvascular oxygen delivery cannot be predicted from systemic hemodynamic measurements (22).
A few studies have suggested the presence of peripheral endothelial dysfunction in patients with PAH (15, 16, 35–37). The biological mechanisms behind the SL microcirculatory abnormalities in PAH are not clear and need further investigation. Potential factors associated with systemic endothelial dysfunction in PAH include a reduction in nitric oxide synthesis, overexpression of endothelin in systemic endothelial cells (38, 39), modification of the serotonin pathways (40), increase in circulating inflammatory chemokines and cytokines (41), hypoxemia (42), sympathetic overactivity (43), or alterations of cellular bioenergetics (44).
Our study has limitations that should be mentioned. First, most of the patients were on PAH-specific therapies, reflecting the difficulties in finding treatment-naive patients with PAH in the current era. Second, we included patients with different types of PAH diseases, which could affect the results; however, subgroup analysis did not find any significant capillaroscopy difference among the groups. Third, a number of patients with PAH and control subjects had comorbidities such as hypertension and obstructive sleep apnea that may have affected the capillaroscopy determination; nonetheless, most of them were adequately treated, and the comorbidities were present in both groups. We did not test patients with collagen vascular diseases without PAH, uncontrolled hypertension, or diabetes; therefore, it is unknown whether SL capillaroscopy would be different in these subgroups of patients. Finally, we could have missed differences between groups or associations with hemodynamic variables due to the limited number of patients in our study (type II error).
In summary, we demonstrate that the SL microvasculature is significantly different in patients with PAH when compared with matched healthy control subjects. The determinations are time consuming, and improvements in image stabilization and automatic processing are needed to improve the usefulness of this tool in the evaluation of SL microcirculation. Interestingly, SL capillaroscopy parameters were not associated with traditional markers of disease severity, and this suggests that the microcirculatory changes likely represent a different aspect of the disease. It is possible that macrohemodynamic variables might not be sensitive surrogates of changes in the microcirculation, related clinical outcomes, and ability to predict treatment response in pulmonary hypertension (45, 46). Further investigations are needed to determine whether sequential evaluation of the SL microcirculation could provide prognostic information or assess treatment response to PAH-specific therapies.
Conclusions
Patients with PAH had significant changes in the sublingual microcirculation when compared with healthy control subjects. These changes are characterized by lower microvasculature flow index and greater vascular tortuosity. Further studies are needed to assess whether these noninvasive measurements of the microcirculation can provide information on disease severity, prognosis, and/or response to therapy in PAH.
Acknowledgments
Acknowledgment
The authors thank patients and volunteers for their participation in the study. They also thank Dr. Cynthia Beall for providing them with the CapiScope HVCS video capillaroscopy system and critically reviewing the present manuscript for important intellectual content.
Footnotes
Supported by the National Center for Research Resources, a component of the National Institutes of Health (NIH), Clinical and Translational Science Award KL2 grant TR000440 (A.R.T.) and P01HL107147 (R.A.D.) from the National Heart, Lung and Blood Institute.
Author Contributions: L.D. participated in the data collection, analysis and interpretation of the results, writing of the manuscript, revision of the manuscript for important intellectual content, and final approval of the manuscript submitted. F.C. participated in the data collection, analysis and interpretation of the results, critical revision of the manuscript for important intellectual content, and final approval of the manuscript submitted. L.A. participated in the data collection, analysis and interpretation of the results, critical revision of the manuscript for important intellectual content, and final approval of the manuscript submitted. R.A.D. participated in the conception and design of the study, critical revision of the manuscript for important intellectual content, and final approval of the manuscript submitted. A.R.T. participated in the conception and design of the study, data collection, statistical analysis and interpretation of the results, writing and critical revision of the manuscript for important intellectual content, and final approval of the manuscript submitted.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, et al. American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–S66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Goedhart PT, Khalilzada M, Bezemer R, Merza J, Ince C. Sidestream Dark Field (SDF) imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Express. 2007;15:15101–15114. doi: 10.1364/oe.15.015101. [DOI] [PubMed] [Google Scholar]
- 4.Hubble SM, Kyte HL, Gooding K, Shore AC. Variability in sublingual microvessel density and flow measurements in healthy volunteers. Microcirculation. 2009;16:183–191. doi: 10.1080/10739680802461935. [DOI] [PubMed] [Google Scholar]
- 5.Boerma EC, Mathura KR, van der Voort PH, Spronk PE, Ince C. Quantifying bedside-derived imaging of microcirculatory abnormalities in septic patients: a prospective validation study. Crit Care. 2005;9:R601–R606. doi: 10.1186/cc3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groner W, Winkelman JW, Harris AG, Ince C, Bouma GJ, Messmer K, Nadeau RG. Orthogonal polarization spectral imaging: a new method for study of the microcirculation. Nat Med. 1999;5:1209–1212. doi: 10.1038/13529. [DOI] [PubMed] [Google Scholar]
- 7.Ince C. The microcirculation is the motor of sepsis. Crit Care. 2005;9:S13–S19. doi: 10.1186/cc3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166:98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 9.Verdant CL, De Backer D, Bruhn A, Clausi CM, Su F, Wang Z, Rodriguez H, Pries AR, Vincent JL. Evaluation of sublingual and gut mucosal microcirculation in sepsis: a quantitative analysis. Crit Care Med. 2009;37:2875–2881. doi: 10.1097/CCM.0b013e3181b029c1. [DOI] [PubMed] [Google Scholar]
- 10.Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32:1825–1831. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 11.Top AP, Ince C, de Meij N, van Dijk M, Tibboel D. Persistent low microcirculatory vessel density in nonsurvivors of sepsis in pediatric intensive care. Crit Care Med. 2011;39:8–13. doi: 10.1097/CCM.0b013e3181fb7994. [DOI] [PubMed] [Google Scholar]
- 12.De Backer D, Creteur J, Dubois MJ, Sakr Y, Vincent JL. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J. 2004;147:91–99. doi: 10.1016/j.ahj.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 13.den Uil CA, Lagrand WK, Spronk PE, van der Ent M, Jewbali LS, Brugts JJ, Ince C, Simoons ML. Low-dose nitroglycerin improves microcirculation in hospitalized patients with acute heart failure. Eur J Heart Fail. 2009;11:386–390. doi: 10.1093/eurjhf/hfp021. [DOI] [PubMed] [Google Scholar]
- 14.Erol-Yilmaz A, Atasever B, Mathura K, Lindeboom J, Wilde A, Ince C, Tukkie R. Cardiac resynchronization improves microcirculation. J Card Fail. 2007;13:95–99. doi: 10.1016/j.cardfail.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Peled N, Bendayan D, Shitrit D, Fox B, Yehoshua L, Kramer MR. Peripheral endothelial dysfunction in patients with pulmonary arterial hypertension. Respir Med. 2008;102:1791–1796. doi: 10.1016/j.rmed.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Friedman D, Szmuszkovicz J, Rabai M, Detterich JA, Menteer J, Wood JC. Systemic endothelial dysfunction in children with idiopathic pulmonary arterial hypertension correlates with disease severity. J Heart Lung Transplant. 2012;31:642–647. doi: 10.1016/j.healun.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riccieri V, Vasile M, Iannace N, Stefanantoni K, Sciarra I, Vizza CD, Badagliacca R, Poscia R, Papa S, Mezzapesa M, et al. Systemic sclerosis patients with and without pulmonary arterial hypertension: a nailfold capillaroscopy study. Rheumatology (Oxford) 2013;52:1525–1528. doi: 10.1093/rheumatology/ket168. [DOI] [PubMed] [Google Scholar]
- 18.Dababneh L, Cikach F, Alkukhun L, Beall C, Dweik RA, Tonelli AR. Sublingual capillaroscopy in pulmonary arterial hypertension [abstract] Chest. 2013;144:856A. [Google Scholar]
- 19.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Stanclova M, Kokstein Z, Cerny V. Orthogonal polarization spectral (OPS)/sidestream dark field (SDF) imaging: a new method for the observation of the microcirculation in pediatrics. Appl Cardiopulm Pathophysiol. 2012;16:249–253. [Google Scholar]
- 21.Treu CM, Lupi O, Bottino DA, Bouskela E. Sidestream dark field imaging: the evolution of real-time visualization of cutaneous microcirculation and its potential application in dermatology. Arch Dermatol Res. 2011;303:69–78. doi: 10.1007/s00403-010-1087-7. [DOI] [PubMed] [Google Scholar]
- 22.De Backer D, Hollenberg S, Boerma C, Goedhart P, Büchele G, Ospina-Tascon G, Dobbe I, Ince C. How to evaluate the microcirculation: report of a round table conference. Crit Care. 2007;11:R101. doi: 10.1186/cc6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, Arnold RC, Colilla S, Zanotti S, Hollenberg SM. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2007;49:88–98. doi: 10.1016/j.annemergmed.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Grisan E, Foracchia M, Ruggeri A. A novel method for the automatic grading of retinal vessel tortuosity. IEEE Trans Med Imaging. 2008;27:310–319. doi: 10.1109/TMI.2007.904657. [DOI] [PubMed] [Google Scholar]
- 25.McKenna SP, Doughty N, Meads DM, Doward LC, Pepke-Zaba J. The Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR): a measure of health-related quality of life and quality of life for patients with pulmonary hypertension. Qual Life Res. 2006;15:103–115. doi: 10.1007/s11136-005-3513-4. [DOI] [PubMed] [Google Scholar]
- 26.Donati A, Domizi R, Damiani E, Adrario E, Pelaia P, Ince C.From macrohemodynamic to the microcirculation Crit Care Res Pract 2013. 2013:892710 [DOI] [PMC free article] [PubMed]
- 27.Sallisalmi M, Oksala N, Pettilä V, Tenhunen J. Evaluation of sublingual microcirculatory blood flow in the critically ill. Acta Anaesthesiol Scand. 2012;56:298–306. doi: 10.1111/j.1399-6576.2011.02569.x. [DOI] [PubMed] [Google Scholar]
- 28.Walley KR. Heterogeneity of oxygen delivery impairs oxygen extraction by peripheral tissues: theory. J Appl Physiol (1985) 1996;81:885–894. doi: 10.1152/jappl.1996.81.2.885. [DOI] [PubMed] [Google Scholar]
- 29.Ince C, Sinaasappel M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med. 1999;27:1369–1377. doi: 10.1097/00003246-199907000-00031. [DOI] [PubMed] [Google Scholar]
- 30.Morin MJ, Unno N, Hodin RA, Fink MP. Differential expression of inducible nitric oxide synthase messenger RNA along the longitudinal and crypt-villus axes of the intestine in endotoxemic rats. Crit Care Med. 1998;26:1258–1264. doi: 10.1097/00003246-199807000-00031. [DOI] [PubMed] [Google Scholar]
- 31.Djaberi R, Schuijf JD, de Koning EJ, Wijewickrama DC, Pereira AM, Smit JW, Kroft LJ, Roos Ad, Bax JJ, Rabelink TJ, et al. Non-invasive assessment of microcirculation by sidestream dark field imaging as a marker of coronary artery disease in diabetes. Diab Vasc Dis Res. 2013;10:123–134. doi: 10.1177/1479164112446302. [DOI] [PubMed] [Google Scholar]
- 32.Schweizer R, Merz K, Schlosser S, Spanholtz T, Contaldo C, Stein JV, Enzmann V, Giovanoli P, Erni D, Plock JA. Morphology and hemodynamics during vascular regeneration in critically ischemic murine skin studied by intravital microscopy techniques. Eur Surg Res. 2011;47:222–230. doi: 10.1159/000333088. [DOI] [PubMed] [Google Scholar]
- 33.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:261–272. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edul VS, Enrico C, Laviolle B, Vazquez AR, Ince C, Dubin A. Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit Care Med. 2012;40:1443–1448. doi: 10.1097/CCM.0b013e31823dae59. [DOI] [PubMed] [Google Scholar]
- 35.Hughes R, Tong J, Oates C, Lordan J, Corris PA. Evidence for systemic endothelial dysfunction in patients and first-order relatives with pulmonary arterial hypertension. Chest. 2005;128:617S. doi: 10.1378/chest.128.6_suppl.617S. [DOI] [PubMed] [Google Scholar]
- 36.Wolff B, Lodziewski S, Bollmann T, Opitz CF, Ewert R.Impaired peripheral endothelial function in severe idiopathic pulmonary hypertension correlates with the pulmonary vascular response to inhaled iloprost Am Heart J 20071531088.e1–7 [DOI] [PubMed] [Google Scholar]
- 37.Peled N, Shitrit D, Fox BD, Shlomi D, Amital A, Bendayan D, Kramer MR. Peripheral arterial stiffness and endothelial dysfunction in idiopathic and scleroderma associated pulmonary arterial hypertension. J Rheumatol. 2009;36:970–975. doi: 10.3899/jrheum.081088. [DOI] [PubMed] [Google Scholar]
- 38.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J. 2004;18:1746–1748. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- 39.Shao D, Park JE, Wort SJ. The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharmacol Res. 2011;63:504–511. doi: 10.1016/j.phrs.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Xu M, Xia J, Qin RY. Association between serotonin transporter (SERT) gene polymorphism and idiopathic pulmonary arterial hypertension: a meta-analysis and review of the literature. Metabolism. 2013;62:1867–1875. doi: 10.1016/j.metabol.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 41.El Chami H, Hassoun PM. Immune and inflammatory mechanisms in pulmonary arterial hypertension. Prog Cardiovasc Dis. 2012;55:218–228. doi: 10.1016/j.pcad.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimopoulos S, Tzanis G, Manetos C, Tasoulis A, Mpouchla A, Tseliou E, Vasileiadis I, Diakos N, Terrovitis J, Nanas S. Peripheral muscle microcirculatory alterations in patients with pulmonary arterial hypertension: a pilot study. Respir Care. 2013;58:2134–2141. doi: 10.4187/respcare.02113. [DOI] [PubMed] [Google Scholar]
- 43.Velez-Roa S, Ciarka A, Najem B, Vachiery JL, Naeije R, van de Borne P. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation. 2004;110:1308–1312. doi: 10.1161/01.CIR.0000140724.90898.D3. [DOI] [PubMed] [Google Scholar]
- 44.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, et al. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci USA. 2007;104:1342–1347. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ventetuolo CE, Benza RL, Peacock AJ, Zamanian RT, Badesch DB, Kawut SM. Surrogate and combined end points in pulmonary arterial hypertension. Proc Am Thorac Soc. 2008;5:617–622. doi: 10.1513/pats.200803-029SK. [DOI] [PubMed] [Google Scholar]
- 46.Steele P, Strange G, Wlodarczyk J, Dalton B, Stewart S, Gabbay E, Keogh A. Hemodynamics in pulmonary arterial hypertension (PAH): do they explain long-term clinical outcomes with PAH-specific therapy? BMC Cardiovasc Disord. 2010;10:9. doi: 10.1186/1471-2261-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]