Abstract
Rationale: Childhood obesity is a known risk factor for pulmonary diseases, likely due to obesity-mediated alteration of pulmonary function. Inflammation and mechanical fat load are two proposed causative mechanisms for altered pulmonary function among obese children; however, the association of metabolic abnormalities with pulmonary function among children is poorly understood.
Objectives: We investigated the independent association of truncal and general adiposity and metabolic abnormalities with pulmonary function in a sample of urban minority adolescents.
Methods: Spirometry and lung volume indices were compared between adolescents with general (body mass index [BMI] > 95th percentile) or truncal adiposity (waist circumference > 90th percentile) and normal-weight (BMI < 85th percentile or waist circumference ≤ 90th percentile) and between those with metabolic abnormalities (homeostatic model assessment of insulin resistance [HOMA-IR] in the top quartile or high-density lipoprotein [HDL] < 40 mg/dl) and those with a normal metabolic profile.
Measurements and Main Results: Obese adolescents had lower lung volumes, including residual volume (RV), RV/TLC ratio, expiratory reserve volume (ERV), and FRC, and higher inspiratory capacity (IC) than normal-weight adolescents, but did not differ in measures of lower airway obstruction, FEV1/FVC ratio, and mid-expiratory flow rate. Adolescents with high HOMA-IR had lower FEV1/FVC ratio, RV, RV/TLC ratio, ERV, and FRC and higher IC, whereas those with low HDL had lower FEV1/FVC and RV/TLC ratios. After adjusting for adiposity, HOMA-IR remained a predictor of ERV (β = −1.4; P = 0.02) and FEV1/FVC ratio (β = −0.5; P = 0.03), and HDL remained a predictor of FEV1/FVC ratio (β = 0.1; P = 0.01). General adiposity was a predictor of FRC (β = −0.5; P < 0.001), IC (β = 0.3; P < 0.001), RV (β = −0.8; P < 0.0001), and RV/TLC ratio (β = −0.2; P < 0.0001), and truncal adiposity was a predictor of RV (β = −20.3; P = 0.03) and FRC (β = −13.8; P = 0.004). Thus, adiposity and metabolic abnormalities were independent predictors of ERV, but only metabolic abnormalities independently predicted FEV1/FVC ratio. Although general adiposity predicted RV and RV/TLC ratio, truncal adiposity was predictive of RV and FRC, conferring additional risk above general adiposity.
Conclusions: These results suggest that metabolic abnormalities and adiposity are independently associated with pulmonary function deficits among urban adolescents. Metabolic assessment of obese adolescents may identify those at risk of developing obesity-associated pulmonary morbidity.
Keywords: obesity, metabolic abnormalities, pulmonary function, children
Increased prevalence of childhood obesity over the past three decades (1) has led to the identification of an epidemiologic association between obesity and pediatric pulmonary diseases (2–4). One of the proposed mechanisms for this association is obesity-mediated pulmonary function impairment (5). Obese children have more lower airway obstruction as measured by lower FEV1/FVC ratio and have lower lung volumes, including residual volume (RV), expiratory reserve volume (ERV), and FRC (6–8). However, the underlying pathogenesis for pulmonary function deficits observed among obese children and adolescents remains poorly understood.
Obesity has several systemic effects that may influence pulmonary function. Although inflammation (9) and adiposity associated with the mechanical load of truncal fat are proposed to be causative mechanisms for the alteration of pulmonary function among obese persons (5), there is emerging evidence that metabolic abnormalities may also influence pulmonary function (10, 11). Obesity is associated with metabolic abnormalities, including hyperinsulinemia, insulin resistance (12), and dyslipidemia (13). Insulin resistance and its surrogate clinical marker, acanthosis nigricans, are more prevalent in children with asthma than in children without asthma (14–16). Dyslipidemia has been associated with wheeze (17) and insulin resistance with incident asthma (18) and with lower airway obstruction among adults (10, 11). However, little is known about the association between metabolic abnormalities and pulmonary function among children.
Because the prevalence of childhood obesity (1) and its association with pulmonary diseases is greater in urban minority populations (4, 19), our objective was to investigate the association of adiposity and metabolic abnormalities with pulmonary function in a sample of urban minority adolescents. We hypothesized that metabolic abnormalities, including insulin resistance and dyslipidemia, will be predictors of pulmonary function among urban adolescents. We also hypothesized that these associations will be independent of adiposity, particularly the mechanical load of truncal fat. Homeostatic model assessment of insulin resistance (HOMA-IR) was used as a measure of insulin resistance, high-density lipoprotein (HDL) as a measure of dyslipidemia, body mass index (BMI) z-score as a measure of general adiposity, and waist circumference as a measure of truncal adiposity.
Methods
Study Population
A total of 168 Hispanic and African-American adolescents, 13 to 18 years of age, were recruited at primary care clinics affiliated with Children’s Hospital at Montefiore, a tertiary care teaching hospital located in the Bronx, New York, between July 2011 and May 2013. Obesity was defined as BMI > 95th percentile (BMI z-score > 1.64) and normal-weight as BMI < 85th percentile (BMI z-score < 1.04) for age and sex. Those who were overweight (BMI 85–95th percentile) and had a history of smoking or chronic inflammatory conditions, including rheumatologic, endocrine, gastrointestinal, or renal diseases, were excluded. Within the obese and normal-weight groups, adolescents were classified as having asthma, eczema, and allergic rhinitis based on physician diagnosis, the presence of ongoing symptoms, and medication use. Asthma diagnosis was made by the health care providers as per the National Asthma Education and Prevention Program guidelines (20). The diagnosis of allergic rhinitis was based on seasonal symptoms of rhinorrhea that were responsive to nasal steroids.
Health care providers at the primary care clinics notified research assistants of eligible participants based on review of their medical records and BMI. The research assistants obtained informed consent and scheduled the study visit within 2 days. Of the 220 eligible participants who agreed to participate, 168 returned for the study visit. Demographic characteristics did not differ between those who did and those who did not return for the study visit. The study was conducted with the approval of the Montefiore Medical Center Institutional Review Board. All study records were stored in compliance with Health Insurance Portability and Accountability Act regulations.
Study Measures
Anthropometric measures
All study visits took place in the Clinical Research Center located at the Montefiore Medical Center. The participants underwent measurement of height, weight, and waist and hip circumference. Waist circumference was measured at the upper most lateral border of the iliac crest, and hip circumference was measured at the level of maximum protrusion (21). BMI was calculated as weight (in kilograms)/height squared (in meters) and was used to calculate BMI z-score to adjust for sex and age differences (22). The participants also underwent a fasting blood draw from which serum was separated and stored at −80°C until further analysis.
Pulmonary function testing
All participants underwent pulmonary function testing, including spirometry and lung volume quantification by body plethysmography (SensorMedics, Yorba Linda, CA), as per the American Thoracic Society (ATS) criteria on the day of the study visit (23). National Health and Nutrition Examination Survey reference values were applied to calculate percent-predicted values of the spirometric indices (24) and those developed by the ATS workshop for lung volume indices (25). The following pulmonary function variables were included in the analysis: FVC, FEV1, FEV1/FVC ratio, mid-expiratory flow rates (FEF25–75%), TLC, residual volume (RV), RV/TLC ratio, ERV, FRC, and inspiratory capacity (IC).
Quantification of metabolic measures
Lipids (low-density lipoprotein [LDL], high-density lipoprotein [HDL], triglycerides, and cholesterol), glucose, and insulin levels were quantified in the serum separated from 10 ml of a fasting blood sample. Measurements for HDL, total cholesterol, and triglycerides were performed by enzymatic immunoassay and measured on an AU400 chemistry autoanalyzer (Beckman-Coulter Corp., Brea, CA) using commercially available enzymatic reagents. Triglycerides and total cholesterol were measured by direct enzymatic measurements. HDL was separately measured using two reagent homogenous systems with selective detergents to homogenize the lipoprotein of interest. LDL levels were calculated using the Friedewald equation. Insulin was quantified by radioimmunoassay (Millipore Corp., Billerica, MA) and quantified on a Wizard2 γ counter (Perkin Elmer Corp., Waltham, MA). HOMA-IR was calculated (glucose [mg/dl] × insulin [uU/ml]/405).
Statistical Analysis
The primary outcomes of interest were pulmonary function indices, reported as percent predicted values (except for FEV1/FVC, which is reported as a percentage), and the primary predictors of interest were adiposity and metabolic measures. BMI z-score was used as a measure of general adiposity, waist circumference as a measure of truncal adiposity, HOMA-IR as a measure of insulin resistance, and HDL as a measure of dyslipidemia. Because HOMA-IR varies by pubertal status during adolescence (26) and because there are no standard cut-offs to define insulin resistance among Hispanic and African-American adolescents, we compared pulmonary function between adolescents with HOMA-IR levels in the highest quartile with those with HOMA-IR levels in the lower three quartiles. Standard cutoffs were used to define dyslipidemia (HDL < 40 mg/dl) (15), general adiposity (BMI z-score > 1.64, corresponding to 95th percentile of BMI), and truncal adiposity (waist circumference > 90th percentile) (27).
We used t tests to compare pulmonary function indices between those with and without metabolic abnormalities or general or truncal adiposity; results are reported as mean ± SD. Categorical variables were compared using Pearson χ2 tests and reported as percentages. Correlations between variables were calculated using Pearson correlation.
Given the links between adiposity and metabolic abnormalities (28, 29), we conducted multivariate linear regression analysis to identify variables that were independent predictors of pulmonary function indices (FEV1/FVC ratio, RV, RV/TLC ratio, ERV, FRC, and IC). Demographic and clinical factors, including sex (30), ethnicity, and diagnosis of asthma (31), were included as covariates because of their known association with pulmonary function. BMI z-score and waist circumference were included as dichotomous variables of general and truncal adiposity. Thus, the regression models included HOMA-IR, HDL, waist circumference, and BMI as predictor variables and asthma status, sex, and ethnicity as pertinent covariates. Statistical significance was set a priori at P < 0.05. Statistical analysis was conducted on STATA statistical software v.12 (STATA, College Station, TX) (32).
Results
Study Population Characteristics
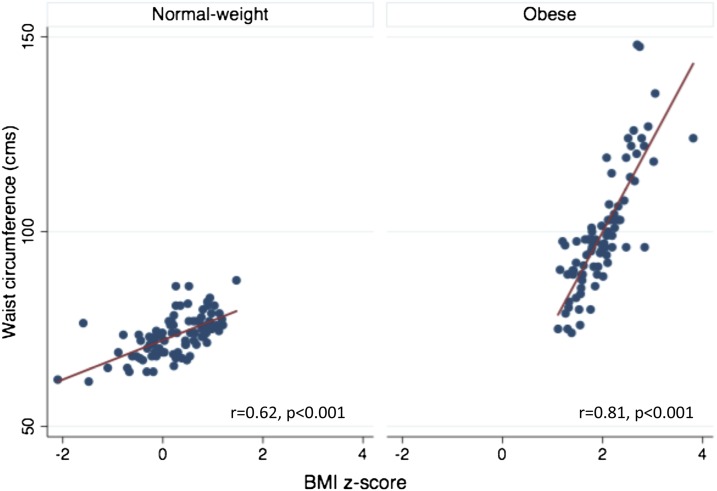
The demographic and clinical characteristics of the study population are summarized in Table 1. Obese and normal-weight participants did not differ by age, sex, ethnicity, or pubertal status or in the prevalence of asthma, eczema, and allergic rhinitis. There was no difference in the use of inhaled steroids (25% in each group) and leukotriene receptor antagonists (25 vs. 23.4%) among subjects with asthma in the obese and normal-weight groups. In keeping with higher body weight, metabolic measures of dyslipidemia, including lower HDL and higher LDL levels, and measures of insulin resistance with higher insulin, glucose, and HOMA-IR levels were more prevalent among the obese than the normal-weight adolescents. Moreover, increase in waist circumference with increase in BMI was greater among the obese than the normal-weight participants (Figure 1). Thus, waist circumference was > 90th percentile in 51.3% of obese participants but in none of the normal-weight participants.
Table 1.
Characteristics of the study population
| Variable | All Participants (n = 168) | Obese* (n = 82) | Normal Weight (n = 86) | P Value† |
|---|---|---|---|---|
| Age, yr | 16.1 ± 1.6 | 16.1 ± 1.6 | 16.2 ± 1.6 | 0.8 |
| Sex, % males | 44.6 | 46.3 | 43 | 0.7 |
| Ethnicity, % Hispanics | 57.9 | 58.5 | 55.8 | 0.7 |
| Asthma, % | 47.9 | 48.2 | 47.6 | 0.6 |
| Allergic rhinitis, % | 33.3 | 37.8 | 29.1 | 0.2 |
| Eczema, % | 21.4 | 23.2 | 19.8 | 0.6 |
| Passive smoke exposure | 21.4 | 26.8 | 16.3 | 0.1 |
| Tanner stage, % participants | 0.9 | |||
| 3 | 7.7 | 9.7 | 8.9 | |
| 4 | 36.9 | 37.8 | 36 | |
| 5 | 46.4 | 46.3 | 46.5 | |
| Height, cm | 166.7 ± 9.9 | 167 ± 9.8 | 166.9 ± 9.6 | 1 |
| Weight, kg | 75.9 ± 23.1 | 91.3 ± 22.6 | 60.9 ± 10.1 | <0.0001 |
| BMI, kg/m2 | 27.2 ± 7.8 | 32.6 ± 7 | 21.7 ± 2.4 | <0.0001 |
| BMI z-score | 1.1 ± 1.1 | 1.99 ± 0.5 | 0.3 ± 0.8 | <0.0001 |
| Waist circumference, cm | 86.2 ± 17.5 | 99.7 ± 15.5 | 73.4 ± 5.5 | <0.0001 |
| Waist–hip ratio | 0.82 ± 0.09 | 0.87 ± 0.08 | 0.78 ± 0.07 | <0.0001 |
| HDL, mg/dl | 49.1 ± 11.4 | 44.8 ± 9.6 | 53.2 ± 11.5 | <0.0001 |
| LDL, mg/dl | 81.6 ± 23.4 | 85.4 ± 23.1 | 78.1 ± 23.2 | 0.04 |
| Cholesterol, mg/dl | 147.2 ± 30 | 146.8 ± 26.9 | 147.6 ± 32.9 | 0.9 |
| Glucose, mg/dl | 89.6 ± 8.2 | 91.5 ± 8 | 87.7 ± 8 | 0.002 |
| Insulin, uU/ml | 25.1 ± 17.4 | 33 ± 20.8 | 17.6 ± 7.9 | <0.0001 |
| HOMA | 5.6 ± 4.1 | 7.5 ± 5 | 3.8 ± 1.9 | <0.0001 |
| FVC, % predicted | 94.8 ± 14 | 96.2 ± 15.8 | 93.4 ± 12 | 0.2 |
| FEV1, % predicted | 88.8 ± 12.5 | 90.2 ± 12.1 | 87.4 ± 12.8 | 0.1 |
| FEV1/FVC, % | 82.1 ± 7.9 | 81.7 ± 7.5 | 82.5 ± 8.3 | 0.5 |
| FEF25–75%‡ | 77.9 ± 22.3 | 79.8 ± 21.9 | 76.1 ± 22.7 | 0.3 |
| TLC, % predicted | 94.8 ± 13.5 | 96.3 ± 13 | 99.4 ± 13.8 | 0.2 |
| RV, % predicted | 105.6 ± 40.3 | 89.2 ± 32.6 | 122.1 ± 40.7 | <0.0001 |
| RV/TLC ratio, % | 24 ± 6.9 | 20.7 ± 5.5 | 27.2 ± 6.5 | <0.0001 |
| ERV, % predicted | 80.2 ± 22.9 | 75.1 ± 20.3 | 85.4 ± 24.4 | 0.004 |
| FRC, % predicted | 88.6 ± 21.6 | 79.3 ± 18.5 | 98 ± 20.6 | <0.0001 |
| IC, % predicted | 91.5 ± 17.2 | 97.7 ± 17.2 | 85.3 ± 14.3 | <0.0001 |
Definition of abbreviations: BMI = body mass index; ERV = expiratory reserve volume; FEF25–75% = mid-expiratory flow rate; HDL = high-density lipoprotein; HOMA = homeostatic model assessment; IC = inspiratory capacity; LDL = low-density lipoprotein; RV = residual volume;
BMI z-score was used to classify obese (z-score > 1.64) and normal-weight participants (z-score < 1.04).
P value derived from t test for continuous variables and χ2 for categorical variables to compare clinical characteristics between obese and normal-weight adolescents.
Figure 1.
Comparison of the association of waist circumference with body mass index (BMI) z-score in obese and normal-weight participants.
Association of Pulmonary Function with Adiposity and Metabolic Measures
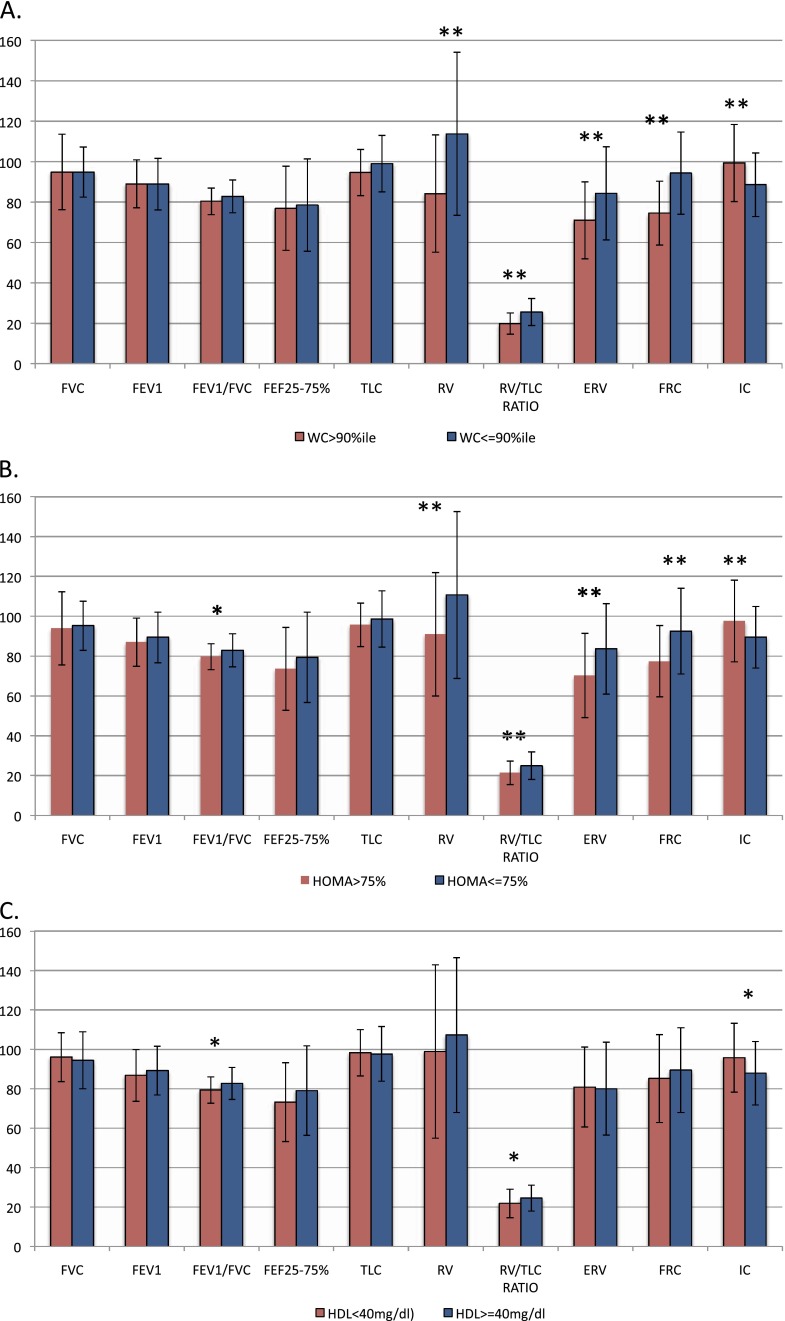
Although pulmonary function among all participants was in the normal range (Table 1), when characterized based on BMI (Table 1) and waist circumference (Figure 2A), adolescents with general or truncal adiposity had significantly lower percent predicted values of lung volumes, including RV, RV/TLC ratio, ERV, and FRC, and higher values of IC but did not differ in measures of lower airway obstruction (FEV1/FVC ratio and percent predicted FEF25–75%) compared with their normal-weight adolescents.
Figure 2.
Association of pulmonary function with truncal adiposity and metabolic abnormalities. Comparison of pulmonary function between those with truncal adiposity (waist circumference [WC] > 90th percentile) (A) and normal weight and those with abnormal homeostatic model assessment (HOMA) of insulin resistance (B) and high-density lipoprotein (HDL) (C) with metabolically normal participants. *P < 0.05. **P < 0.001. ERV = expiratory reserve volume; IC = inspiratory capacity.
Adolescents with high HOMA-IR (Figure 2B) had lower percent FEV1/FVC ratio in addition to lower RV, RV/TLC ratio, ERV, and FRC and higher IC and those with low HDL (Figure 2C), had lower percent FEV1/FVC ratio and RV/TLC ratio and higher IC. Thus, measures of adiposity and metabolic abnormalities were found to be predictors of pulmonary function in univariate analyses (Table 2).
Table 2.
Association of pulmonary function with adiposity and metabolic measures
| Metabolic or Adiposity Measure | FEV1/FVC |
RV |
RV/TLC |
ERV |
FRC |
IC |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β* (95% CI) | P Value | β* (95% CI) | P Value | β* (95% CI) | P Value | β* (95% CI) | P Value | β* (95% CI) | P Value | β* (95% CI) | P Value | |
| HOMA-IR | −0.3 (−0.6 to −0.01) | 0.04 | −1.6 (−3.1 to −0.1) | 0.03 | −0.3 (−0.6 to −0.1) | <0.001 | −1.7 (−2.5 to −0.9) | <0.001 | −1.5 (−2.3 to −0.7) | <0.001 | 1 (0.4 to 1.6) | 0.001 |
| HDL | 0.2 (0.1 to 0.3) | 0.002 | 0.6 (0.04 to 1.1) | 0.03 | 0.2 (0.1 to 0.3) | <0.0001 | −0.1 (−0.4 to 0.2) | 0.7 | 0.2 (−0.1 to 0.5) | 0.1 | −0.4 (−0.6 to −0.2) | <0.001 |
| BMI z-score | −1.1 (−2.2 to 0.02) | 0.05 | −15.1 (−20.4 to −9.8) | <0.0001 | −3 (−3.9 to −2.1) | <0.0001 | −6.2 (−9.4 to −3.1) | <0.001 | −9.5 (−12.2 to −6.7) | <0.0001 | 6.2 (3.9 to 8.4) | <0.0001 |
| WC, cm | −0.1 (−0.2 to −0.03) | 0.004 | −0.8 (−1.2 to −0.5) | <0.0001 | −0.2 (−0.2 to −0.1) | <0.0001 | −0.4 (−0.6 to −0.2) | <0.0001 | −0.6 (−0.7 to −0.4) | <0.0001 | 0.4 (0.3 to 0.5) | <0.001 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; ERV = expiratory reserve volume; HDL = high-density lipoprotein; HOMA-IR = homeostatic model assessment of insulin resistance; IC = inspiratory capacity; WC = waist circumference.
β denotes the change in each pulmonary function index (percent predicted or percent) with a unit increase in metabolic measures (HOMA-IR and HDL) or adiposity measures (BMI z-score and waist circumference).
Pulmonary function was also influenced by asthma status, sex, and ethnicity. Although adolescents with asthma had a lower FEV1/FVC ratio than those without asthma (79.7 ± 7.9 vs. 84.5 ± 7.2; P < 0.001), lung volume indices did not significantly differ by asthma status. Pulmonary function indices did not differ between those with or without allergic rhinitis or eczema. Percent predicted ERV was the only lung function index that differed by sex: it was lower in male subjects compared with female subjects (75.4 ± 22.5 vs. 84.2 ± 22.6; P = 0.01). Compared with African Americans, Hispanics had lower ERV (73.8 ± 21.1 vs. 85.1 ± 23.2; P = 0.002) and IC (85 ± 16.1 vs. 96.8 ± 16.2; P < 0.0001).
After adjusting for general and truncal adiposity in multivariate analysis (Table 3), HOMA-IR remained a significant predictor of FEV1/FVC ratio and ERV, but its associations with FRC, RV, RV/TLC ratio, and IC were rendered nonsignificant. Similarly, HDL remained a significant predictor of FEV1/FVC ratio, but its associations with ERV, FRC, RV, and RV/TLC ratio were rendered nonsignificant. Conversely, general adiposity was an independent predictor of IC, FRC, RV, and RV/TLC ratio, and truncal adiposity was an independent predictor of RV and FRC, but its associations with FEV1/FVC ratio, ERV, IC, and RV/TLC ratio were rendered nonsignificant. Of the other covariates, asthma was a significant predictor of FEV1/FVC ratio (β= −3.8; 95% confidence interval [CI], −6.1 to −1.5; P = 0.01), and female sex was a significant predictor of ERV (β = 7.2; 95% CI, 0.7–13.6; P = 0.03). Hispanic ethnicity was a significant predictor of ERV (β = −12.7; 95% CI, −19.4 to −6.1; P < 0.001), FRC (β = −8.2; 95% CI, −14.4 to −2.1; P < 0.01), and IC (β = −10.5; 95% CI, −15.6 to −5.6; P < 0.001).
Table 3.
Mutivariate regression analysis of association of pulmonary function with adiposity and metabolic measures
| Metabolic or Adiposity Measure | FEV1/FVC |
RV |
RV/TLC |
ERV |
FRC |
IC |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β* (95% CI) | P Value (R2† = 0.19; P = 0.0001) | β* (95% CI) | P Value (R2† = 0.2; P < 0.001) | β* (95% CI) | P Value (R2† = 0.25; P < 0.0001) | β* (95% CI) | P Value (R2† = 0.22; P < 0.0001) | β* (95% CI) | P Value (R2† = 0.25; P < 0.0001) | β* (95% CI) | P Value (R2† = 0.24; P < 0.0001) | |
| HOMA-IR, mg/dl | −0.5 (−0.9 to −0.1) | 0.03 | 1.4 (−0.7 to 3.5) | 0.2 | 0.2 (−0.1 to 0.6) | 0.2 | −1.4 (−2.6 to −0.2) | 0.02 | −0.05 (−1.1 to 1) | 0.9 | 0.1 (−0.7 to 1) | 0.8 |
| HDL, mg/dl | 0.1 (0.03 to 0.3) | 0.01 | 0.2 (−0.4 to 0.8) | 0.5 | 0.07 (−0.02 to 0.2) | 0.1 | −0.3 (−0.6 to 0.04) | 0.09 | −0.06 (−0.3 to 0.2) | 0.7 | −0.1 (−0.3 to 0.1) | 0.4 |
| BMI z-score‡ | 1.8 (−1.2 to 4.7) | 0.2 | −23.7 (−38.9 to −8.4) | 0.003 | −4.8 (−7.3 to −2.3) | <0.001 | −2.9 (−11.5 to 5.7,) | 0.5 | −10.7 (−18.5 to −2.8) | 0.008 | 9.6 (3.2 to 16) | 0.004 |
| WC‡ | 0.6 (−3 to 4.1) | 0.8 | −20.3 (−38.3 to −2.3) | 0.03 | −2.9 (−5.9 to 0.02) | 0.05 | −7.8 (−17.9 to 2.4) | 0.1 | −13.8 (−23.1 to −4.6) | 0.004 | 1.8 (−5.8 to 9.3) | 0.6 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; ERV = expiratory reserve volume; HDL = high-density lipoprotein; HOMA-IR = homeostatic model assessment of insulin resistance; IC = inspiratory capacity; WC = waist circumference.
β denotes the change in each pulmonary function index (percent predicted or percent) with a unit increase in metabolic measures (HOMA-IR and HDL) or in those with general (BMI > 95th percentile) or truncal (WC > 90th percentile) adiposity, adjusted for sex, ethnicity, and asthma diagnosis in multivariate linear regression analysis.
R2 denotes the variance of each pulmonary function index explained by the linear regression model.
Waist circumference and BMI z-score were included as dichotomous variables, comparing those with truncal or general adiposity with normal-weight counterparts.
Discussion
In a sample of urban minority adolescents, we found that elevated HOMA-IR, decreased HDL, and adiposity were associated with pulmonary function indices, including spirometric measures of lower airway obstruction and lung volumes. However, the relationship of adiposity and these metabolic abnormalities varied for different pulmonary function indices. Whereas HOMA-IR was an independent predictor of FEV1/FVC ratio and ERV, HDL was an independent predictor of FEV1/FVC ratio. General adiposity independently predicted RV and RV/TLC ratio, and truncal adiposity was predictive of RV and FRC, conferring additional risk above general adiposity. The univariate associations of insulin resistance and HDL with lung volumes, including IC, RV, and RV/TLC ratio, were no longer significant after adjustment for general and truncal adiposity. These results suggest that metabolic abnormalities and the mechanical load of fat are associated with pulmonary function deficits among urban adolescents. Although obesity is a known risk factor for pulmonary morbidity (33), not all obese children develop pulmonary diseases. However, the factors that lead to pulmonary disease in certain obese children are not known. In keeping with their links with diabetes and coronary artery disease (34, 35), we found that adiposity and associated metabolic abnormalities (36, 37) are linked with pulmonary function deficits in obese adolescents. These findings extend the associations between insulin resistance and pulmonary function reported in adults (10, 11) into the pediatric population.
Our findings of lower FEV1/FVC ratio (38, 39), FRC, and RV among obese minority adolescents corroborate with those reported among predominantly white obese adolescents (6–8). Higher IC in conjunction with low FEV1/FVC ratio, ERV, FRC, and RV have also been reported among obese adults with (40, 41) and without asthma (42, 43), where they correlated with the duration of obesity (44). In addition, we have previously reported lower FRC, RV, and RV/TLC ratio among urban obese preadolescent children (45). These studies suggest that pulmonary function deficits associated with obesity among urban children may develop as early as the preadolescent years and persist into adolescence. The presence of impaired pulmonary function among obese children and adolescents and its correlation with duration of obesity in adults (44) highlights the need for early detection of pulmonary morbidity among obese children to institute timely preventive and therapeutic interventions.
The independent associations of adiposity and metabolic abnormalities with pulmonary function elucidate additional mechanisms underlying an obesity-mediated effect on pulmonary mechanics in the pediatric population. It is known that obese adolescents with a clinical diagnosis of asthma have a lower FEV1/FVC ratio (31, 39) but do not display a higher degree of bronchial hyperreactivity than normal-weight adolescents with asthma (46, 47). Although few studies have measured lung volumes (6, 8, 47), ERV is the one pulmonary function index that is consistently reduced among obese subjects with pulmonary symptoms (5, 6, 48). Airway resistance in obese adults has been associated with lower ERV and FRC, rather than with an inherent decrease in FEV1 or FEV1/FVC ratio, because reduced ERV and FRC are associated with closure of dependent airways, decreased expiratory flows, increased airway resistance, and decreased lung compliance (48). Normalization of airway resistance in obese men, when adjusted for the corresponding lung volume at measurement, further supports the role of airway narrowing due to reduced lung volumes in obesity-mediated pulmonary diseases, rather than inherent bronchial hyperreactivity (48). Our observation of the inverse correlation between mechanical load of adiposity and ERV, FRC, RV, and RV/TLC ratio among adolescents suggests that similar mechanisms may be at play among adolescents. This concept is further supported by the improvement in pulmonary function after diet-induced weight loss in children (47) and surgically induced weight loss in adults (49, 50). However, the attenuated association of pulmonary function with adiposity when adjusted for insulin resistance and HDL suggests that adiposity influences pulmonary function not only by mechanically altering diaphragmatic movement (48) but also by its metabolic activity (28, 29).
The association of insulin resistance with pulmonary function offers a biologic explanation for its epidemiologic links with asthma among children (15) and incident asthma-like symptoms among adults (18). Moreover, insulin resistance inversely correlated with ERV after adjusting for general and truncal adiposity, suggesting that it may be associated with altered airway resistance due to impairment of lung volumes. However, the underlying mechanisms for this association are not known. Insulin resistance and dyslipidemia are associated with systemic inflammation (16) and with alteration of pulmonary immune and oxidative stress responses (51). The extent to which these mechanisms mediate the effect of metabolic abnormalities on pulmonary function needs further investigation. Moreover, although pulmonary function improvement after bariatric surgery has been investigated (50), future studies examining improvement in pulmonary function with change in metabolic and adiposity measures after weight loss may help elucidate the individual role of these two specific mechanisms.
We also found that asthma status correlated with lower airway obstruction but not with lung volume indices. The presence of atopic diseases, such as allergic rhinitis and eczema, was also not associated with altered pulmonary function. These findings corroborate those of Santamaria and colleagues, who did not find an association between pulmonary function deficits and atopy or asthma among a large cohort of obese subjects including children, adolescents, and adults (44). These findings, in conjunction with prior reports of lack of atopy (52) and absence of atopic airway inflammation (53, 54) among obese children with asthma, provide additional evidence to support the hypothesis that mechanisms other than the classic atopic airway inflammation play a role in obesity-mediated pulmonary diseases. Because FEV1/FVC ratio is decreased in the setting of reduced lung volumes, dysanapsis may be one such mechanism that may play a role in pulmonary morbidity in obese individuals. We did not observe a difference in FEF25–75%/FVC ratio, a standardized measure of dysanapsis (55), between obese and normal-weight children and between those with and without metabolic abnormalities. Whether there is a threshold effect of weight gain before the onset of dysanapsis, given the threshold effect of weight gain associated with onset of airway resistance in obese mice (56), needs further investigation.
We also found that Hispanic ethnicity was an independent predictor of low ERV and FRC and IC. Because Hispanics have more visceral adiposity than whites for the same BMI, which is also associated with higher asthma severity (52), these differences in body fat distribution may offer an explanation for the ethnic differences observed in lung volume measurements in our study. Thus, future studies investigating the association of pulmonary function with change in adiposity and metabolic measures are needed in the high-risk ethnic groups to define the utility of metabolic measures as early predictive tools to identify obese children at risk for development of pulmonary disease.
Our study has certain limitations. The percentage of asthma in our study population was higher than the prevalence reported for the Bronx population (57). Moreover, only 25% of the subjects with asthma in either study group were on preventative asthma medications. Therefore, it is possible that underlying asthma severity or control may have influenced pulmonary function in our study sample. However, because the proportion of adolescents with asthma, atopic diseases, or medication use did not differ between the obese and normal-weight groups, it is unlikely that there was a differential effect of asthma prevalence or low preventative medication use on pulmonary function comparison between the study groups. Further, we recognize that additional factors, including history of wheezing in early childhood, may influence pulmonary function (58), but this information was not available in our study. Similarly, duration of obesity in our adolescents, which is known to be associated with pulmonary function deficits, was not known in our sample. To address these limitations, longitudinal studies are needed in this vulnerable population to elucidate the effect of timing of onset and duration of obesity on pulmonary function deficits.
In summary, we identified that mechanical load of fat and certain metabolic abnormalities (HOMA-IR and HDL) were independent predictors of pulmonary function deficits among obese urban adolescents, an association that has been previously observed in adults. These associations suggest that early evaluation of metabolic risk profile among obese children may help to identify those at greater risk of developing pulmonary disease. They also identify the need for improved understanding of the underlying pathophysiology of obesity-mediated effect on pulmonary function in children.
Footnotes
This work was supported by grant 5 KL2 RR025749-04, NIH/NCRR CTSA Grant 1 UL1 TR001073-01, 1 TL1 TR001072-01, and 1 KL2 TR001071-01 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health.
Author Contributions: D.R. acquired and analyzed the data and prepared the manuscript. K.B. compiled the data and conducted analysis. C.B.H. and C.R.I. contributed to data interpretation and revised the manuscript for important intellectual content.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrell LN, Nguyen EA, Roth LA, Oh SS, Tcheurekdjian H, Sen S, Davis A, Farber HJ, Avila PC, Brigino-Buenaventura E, et al. Childhood obesity and asthma control in the GALA II and SAGE II studies. Am J Respir Crit Care Med. 2013;187:697–702. doi: 10.1164/rccm.201211-2116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang JE. Obesity, nutrition, and asthma in children. Pediatr Allergy Immunol Pulmonol. 2012;25:64–75. doi: 10.1089/ped.2011.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black MH, Zhou H, Takayanagi M, Jacobsen SJ, Koebnick C. Increased asthma risk and asthma-related health care complications associated with childhood obesity. Am J Epidemiol. 2013;178:1120–1128. doi: 10.1093/aje/kwt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, Celedón JC, Shore SA American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 6.Davidson WJ, Mackenzie-Rife KA, Witmans MB, Montgomery MD, Ball GD, Egbogah S, Eves ND. Obesity negatively impacts lung function in children and adolescents. Pediatr Pulmonol. In press doi: 10.1002/ppul.22915. [DOI] [PubMed] [Google Scholar]
- 7.Li AM, Chan D, Wong E, Yin J, Nelson EA, Fok TF. The effects of obesity on pulmonary function. Arch Dis Child. 2003;88:361–363. doi: 10.1136/adc.88.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson N, Johnston K, Bear N, Stick S, Logie K, Hall GL. Expiratory flow limitation and breathing strategies in overweight adolescents during submaximal exercise. Int J Obes (Lond) 2014;38:22–26. doi: 10.1038/ijo.2013.137. [DOI] [PubMed] [Google Scholar]
- 9.Ferrante AW., Jr Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 10.Naveed B, Weiden MD, Kwon S, Gracely EJ, Comfort AL, Ferrier N, Kasturiarachchi KJ, Cohen HW, Aldrich TK, Rom WN, et al. Metabolic syndrome biomarkers predict lung function impairment: a nested case-control study. Am J Respir Crit Care Med. 2012;185:392–399. doi: 10.1164/rccm.201109-1672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lecube A, Sampol G, Muñoz X, Lloberes P, Hernández C, Simó R. Insulin resistance is related to impaired lung function in morbidly obese women: a case-control study. Diabetes Metab Res Rev. 2010;26:639–645. doi: 10.1002/dmrr.1131. [DOI] [PubMed] [Google Scholar]
- 12.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiess W, Reich A, Müller G, Galler A, Kapellen T, Raile K, Böttner A, Seidel B, Kratzsch J. Obesity in childhood and adolescence: clinical diagnosis and management. J Pediatr Endocrinol Metab. 2001;14:1431–1440. [PubMed] [Google Scholar]
- 14.Al-Shawwa BA, Al-Huniti NH, DeMattia L, Gershan W. Asthma and insulin resistance in morbidly obese children and adolescents. J Asthma. 2007;44:469–473. doi: 10.1080/02770900701423597. [DOI] [PubMed] [Google Scholar]
- 15.Cottrell L, Neal WA, Ice C, Perez MK, Piedimonte G. Metabolic abnormalities in children with asthma. Am J Respir Crit Care Med. 2011;183:441–448. doi: 10.1164/rccm.201004-0603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arshi M, Cardinal J, Hill RJ, Davies PSW, Wainwright C. Asthma and insulin resistance in children. Respirology. 2010;15:779–784. doi: 10.1111/j.1440-1843.2010.01767.x. [DOI] [PubMed] [Google Scholar]
- 17.Fenger RV, Gonzalez-Quintela A, Linneberg A, Husemoen LL, Thuesen BH, Aadahl M, Vidal C, Skaaby T, Sainz JC, Calvo E. The relationship of serum triglycerides, serum HDL, and obesity to the risk of wheezing in 85,555 adults. Respir Med. 2013;107:816–824. doi: 10.1016/j.rmed.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Thuesen BH, Husemoen LLN, Hersoug LG, Pisinger C, Linneberg A. Insulin resistance as a predictor of incident asthma-like symptoms in adults. Clin Exp Allergy. 2009;39:700–707. doi: 10.1111/j.1365-2222.2008.03197.x. [DOI] [PubMed] [Google Scholar]
- 19.Luder E, Melnik TA, DiMaio M. Association of being overweight with greater asthma symptoms in inner city black and Hispanic children. J Pediatr. 1998;132:699–703. doi: 10.1016/s0022-3476(98)70363-4. [DOI] [PubMed] [Google Scholar]
- 20.National Heart, Lung and Blood Institute, National Asthma Education and Prevention Program. Bethesda, MD: National Institutes of Health: National Heart, Lung, and Blood Institute; 2007. Expert panel report 3: guidelines for the diagnosis and management of asthma. [Google Scholar]
- 21.McDowell MA, Fryar CD, Ogden CL, Flegal KM. Hyattsville, MD: National Center for Health Statistics; 2008. Anthropometric reference data for children and adults: United States, 2003–2006. National Health Statistics Reports, no 10. [PubMed] [Google Scholar]
- 22.Centers for Disease Control and PreventionEpi info version 7 [internet]. Available from: wwwn.cdc.gov/epiinfo
- 23.Brusasco V, Crapo R, Viegi G. ATS/ERS Task Force: Standardisation of lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 24.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 25.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop and Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur Respir J. 1995;8:492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 26.Kurtoğlu S, Hatipoğlu N, Mazıcıoğlu M, Kendirici M, Keskin M, Kondolot M. Insulin resistance in obese children and adolescents: HOMA-IR cut-off levels in the prepubertal and pubertal periods. J Clin Res Pediatr Endocrinol. 2010;2:100–106. doi: 10.4274/jcrpe.v2i3.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 28.Barat P, Gayard-Cros M, Andrew R, Corcuff JB, Jouret B, Barthe N, Perez P, Germain C, Tauber M, Walker BR, et al. Truncal distribution of fat mass, metabolic profile and hypothalamic-pituitary adrenal axis activity in prepubertal obese children. J Pediatr. 2007;150:535–539, e1. doi: 10.1016/j.jpeds.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 29.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91:4459–4466. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 30.Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, Kercsmar CM, Visness CM, Matsui EC, Steinbach SF, et al. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010;125:584–592. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vo P, Makker K, Matta-Arroyo E, Hall CB, Arens R, Rastogi D. The association of overweight and obesity with spirometric values in minority children referred for asthma evaluation. J Asthma. 2013;50:56–63. doi: 10.3109/02770903.2012.744035. [DOI] [PubMed] [Google Scholar]
- 32.StataCorp. College Station, TX: StatCorp; 2011. Stata statistical software: release 12. [Google Scholar]
- 33.Gilliland FD, Berhane K, Islam T, McConnell R, Gauderman WJ, Gilliland SS, Avol E, Peters JM. Obesity and the risk of newly diagnosed asthma in school-age children. Am J Epidemiol. 2003;158:406–415. doi: 10.1093/aje/kwg175. [DOI] [PubMed] [Google Scholar]
- 34.Klöting N, Fasshauer M, Dietrich A, Kovacs P, Schön MR, Kern M, Stumvoll M, Blüher M. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299:E506–E515. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 35.Canoy D, Boekholdt SM, Wareham N, Luben R, Welch A, Bingham S, Buchan I, Day N, Khaw KT. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation. 2007;116:2933–2943. doi: 10.1161/CIRCULATIONAHA.106.673756. [DOI] [PubMed] [Google Scholar]
- 36.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naukkarinen J, Heinonen S, Hakkarainen A, Lundbom J, Vuolteenaho K, Saarinen L, Hautaniemi S, Rodriguez A, Frühbeck G, Pajunen P, et al. Characterising metabolically healthy obesity in weight-discordant monozygotic twins. Diabetologia. 2014;57:167–176. doi: 10.1007/s00125-013-3066-y. [DOI] [PubMed] [Google Scholar]
- 38.Spathopoulos D, Paraskakis E, Trypsianis G, Tsalkidis A, Arvanitidou V, Emporiadou M, Bouros D, Chatzimichael A. The effect of obesity on pulmonary lung function of school aged children in Greece. Pediatr Pulmonol. 2009;44:273–280. doi: 10.1002/ppul.20995. [DOI] [PubMed] [Google Scholar]
- 39.Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL Childhood Asthma Management Program Research Group. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP) Thorax. 2003;58:1036–1041. doi: 10.1136/thorax.58.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deesomchok A, Fisher T, Webb KA, Ora J, Lam YM, Lougheed MD, O’Donnell DE. Effects of obesity on perceptual and mechanical responses to bronchoconstriction in asthma. Am J Respir Crit Care Med. 2010;181:125–133. doi: 10.1164/rccm.200906-0934OC. [DOI] [PubMed] [Google Scholar]
- 41.Canoy D, Luben R, Welch A, Bingham S, Wareham N, Day N, Khaw KT. Abdominal obesity and respiratory function in men and women in the EPIC-Norfolk Study, United Kingdom. Am J Epidemiol. 2004;159:1140–1149. doi: 10.1093/aje/kwh155. [DOI] [PubMed] [Google Scholar]
- 42.Watson RA, Pride NB. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J Appl Physiol (1985) 2005;98:512–517. doi: 10.1152/japplphysiol.00430.2004. [DOI] [PubMed] [Google Scholar]
- 43.Ceylan E, Cömlekçi A, Akkoçlu A, Ceylan C, Itil O, Ergör G, Yeşil S. The effects of body fat distribution on pulmonary function tests in the overweight and obese. South Med J. 2009;102:30–35. doi: 10.1097/SMJ.0b013e31818c9585. [DOI] [PubMed] [Google Scholar]
- 44.Santamaria F, Montella S, Greco L, Valerio G, Franzese A, Maniscalco M, Fiorentino G, Peroni D, Pietrobelli A, De Stefano S, et al. Obesity duration is associated to pulmonary function impairment in obese subjects. Obesity (Silver Spring) 2011;19:1623–1628. doi: 10.1038/oby.2011.1. [DOI] [PubMed] [Google Scholar]
- 45.Rastogi D, Canfield SM, Andrade A, Isasi CR, Hall CB, Rubinstein A, Arens R. Obesity-associated asthma in children: a distinct entity. Chest. 2012;141:895–905. doi: 10.1378/chest.11-0930. [DOI] [PubMed] [Google Scholar]
- 46.Mansell AL, Walders N, Wamboldt MZ, Carter R, Steele DW, Devin JA, Monica TH, Miller AL, Wamboldt FS. Effect of body mass index on response to methacholine bronchial provocation in healthy and asthmatic adolescents. Pediatr Pulmonol. 2006;41:434–440. doi: 10.1002/ppul.20368. [DOI] [PubMed] [Google Scholar]
- 47.Jensen ME, Gibson PG, Collins CE, Hilton JM, Wood LG. Diet-induced weight loss in obese children with asthma: a randomized controlled trial. Clin Exp Allergy. 2013;43:775–784. doi: 10.1111/cea.12115. [DOI] [PubMed] [Google Scholar]
- 48.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol (1985) 2010;108:206–211. doi: 10.1152/japplphysiol.00694.2009. [DOI] [PubMed] [Google Scholar]
- 49.Boulet LP, Turcotte H, Martin J, Poirier P. Effect of bariatric surgery on airway response and lung function in obese subjects with asthma. Respir Med. 2012;106:651–660. doi: 10.1016/j.rmed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508–515, e1–e2. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gowdy KM, Fessler MB. Emerging roles for cholesterol and lipoproteins in lung disease. Pulm Pharmacol Ther. 2013;26:430–437. doi: 10.1016/j.pupt.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Musaad SM, Patterson T, Ericksen M, Lindsey M, Dietrich K, Succop P, Khurana Hershey GK. Comparison of anthropometric measures of obesity in childhood allergic asthma: central obesity is most relevant. J Allergy Clin Immunol. 2009;123:1321–1327, e12. doi: 10.1016/j.jaci.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santamaria F, Montella S, De Stefano S, Sperlì F, Barbarano F, Spadaro R, Franzese A. Asthma, atopy, and airway inflammation in obese children. J Allergy Clin Immunol. 2007;120:965–967. doi: 10.1016/j.jaci.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Cibella F, Cuttitta G, La Grutta S, Melis MR, Bucchieri S, Viegi G. A cross-sectional study assessing the relationship between BMI, asthma, atopy, and eNO among schoolchildren. Ann Allergy Asthma Immunol. 2011;107:330–336. doi: 10.1016/j.anai.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Vilozni D, Lavie M, Sarouk I, Efrati O. Progressive flow-to-volume dysanapsis in cystic fibrosis: a predictor for lung transplantation? Am J Respir Crit Care Med. 2012;186:82–87. doi: 10.1164/rccm.201202-0272OC. [DOI] [PubMed] [Google Scholar]
- 56.Shore SA. Obesity and asthma: lessons from animal models. J Appl Physiol (1985) 2007;102:516–528. doi: 10.1152/japplphysiol.00847.2006. [DOI] [PubMed] [Google Scholar]
- 57.Public Health Information Group. Albany, NY: New York State Department of Health; 2009. New York State asthma surveillance summary report. [Google Scholar]
- 58.Guilbert TW, Singh AM, Danov Z, Evans MD, Jackson DJ, Burton R, Roberg KA, Anderson EL, Pappas TE, Gangnon R, et al. Decreased lung function after preschool wheezing rhinovirus illnesses in children at risk to develop asthma. J Allergy Clin Immunol. 2011;128:532–538, e1–e10. doi: 10.1016/j.jaci.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]