Abstract
Right ventricular (RV) failure occurs when the RV fails to maintain enough blood flow through the pulmonary circulation to achieve adequate left ventricular filling. This can occur suddenly in a previously healthy heart due to massive pulmonary embolism or right-sided myocardial infarction, but many cases encountered in the intensive care unit involve worsening of compensated RV failure in the setting of chronic heart and lung disease. Management of RV failure is directed at optimizing right-sided filling pressures and reducing afterload. Due to a lower level of vascular tone, vasoactive medications have less salient effects on reducing vascular resistance in the pulmonary than in the systemic circulation. Successful management requires reversal of any conditions that heighten pulmonary vascular tone and the use of selective pulmonary vasodilators at doses that do not induce systemic hypotension or worsening of oxygenation. Systemic systolic arterial pressure should be kept close to RV systolic pressure to maintain RV perfusion. When these efforts fail, the judicious use of inotropic agents may help improve RV contractility enough to maintain cardiac output. Extracorporeal life support is increasingly being used to support patients with acute RV failure who fail to respond to medical management while the underlying cause of their RV failure is addressed.
Keywords: right-sided heart failure, pulmonary hypertension, critical care
Several developments have led to a greater focus on right ventricular (RV) function in critically ill patients. These include the rapid growth in our understanding of pulmonary vascular biology and the subsequent development of several new classes of pulmonary vasodilator medications. The widespread treatment of patients with pulmonary arterial hypertension has also enhanced our clinical expertise in managing acute RV failure toward the end of their disease. Finally, technical advances in extracorporeal life support have provided us the ability to support patients with acute RV failure through their illness and enhance our understanding of how the failing RV should be managed. This review discusses an approach to management of acute RV failure in the intensive care unit with emphasis on fluid management, afterload reduction, and augmentation of contractility using vasoactive medications, and an introduction to the rapidly expanding field of extracorporeal life support.
The Normal Pulmonary Circulation and RV
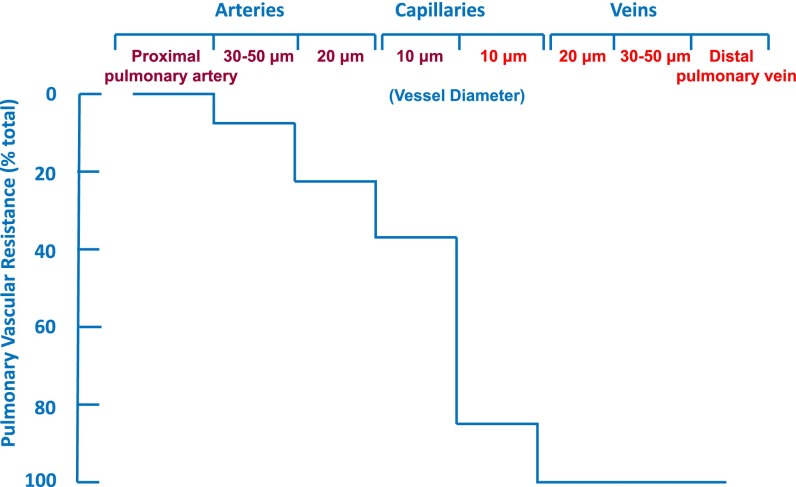
The pulmonary circulation is a low-pressure circuit both at rest and during exercise. Despite a rise in cardiac output during heavy exertion that may be fourfold above baseline, pulmonary arterial pressure increases minimally and pulmonary vascular resistance falls. This is in contrast to the systemic circulation, where increased cardiac output during exercise is associated with a significant increase in blood pressure. The difference between the two circulations is based on the ability of the lung to recruit partially collapsed or unused vessels as cardiac output increases and the relatively low degree of vascular motor tone in the proximal pulmonary vascular bed. In the systemic circulation, a large drop between arterial and venous pressure is created by muscularized arterioles that act as resistors and are used to redistribute blood flow to different organs as needed. In contrast, the difference between mean arterial and venous pressure in the pulmonary circulation is less than 10 mm Hg, and the greatest drop in pressure occurs across the nonmuscularized microcirculation and alveolar capillaries (1) (Figure 1).
Figure 1.
Drop in mean vascular pressure between different segments of the arterial and venous portions of the isolated dog lung as measured by micropuncture technique. Note the small decrease in pressure as pulmonary arterial diameter decreases compared with the large drop in resistance between precapillary arterioles and post-capillary venuoles. Adapted by permission from Reference 1.
Muscularized resistor vessels allow for marked alterations in systemic vascular resistance via sympathetic stimulation or release of endogenous catecholamines or vasodilators, such as nitric oxide or prostaglandins. A variety of vasoactive drugs can also be used to sharply increase or decrease systemic vascular tone. In contrast, the pulmonary circulation has relatively low vascular tone, making it difficult to acutely increase or decrease pulmonary artery pressure. Even under settings of acute hypoxia, where the majority of pulmonary vascular smooth muscle is constricted, pulmonary vascular resistance remains lower than basal systemic vascular resistance.
The pump responsible for perfusing the pulmonary circulation is also considerably different than its counterpart in the systemic circulation. Unlike the left ventricle (LV), which can be considered a four-walled chamber, the RV consists essentially of its thin free wall wrapped around the more muscularized medial wall of the LV. At end diastole, the RV free wall is only 2–3 mm in thickness, compared with 8–11 mm in the LV free wall (2). Although its smaller mass produces less contractile force, the RV has a greater end-diastolic volume and surface area per volume of blood. Flattening of the RV free wall during systole produces a large decrease in volume without much change in free wall area, allowing the RV to achieve a similar stroke volume as the LV with lower energy expenditure (3).
There are also considerable differences between ventricles in how contractile forces are generated. Myocytes within the interventricular septum and LV free wall are oriented circumferentially, resulting in concentric narrowing of the LV lumen during systole. The RV free wall, on the other hand, is comprised of a superficial, transversely oriented muscle layer and a deeper layer where the fibers are arranged longitudinally from apex to base. Systolic pressures are generated by longitudinal contraction with the apex moving toward the tricuspid valve. The inflow region of the RV contracts about 25–50 milliseconds before the outflow region (4). Dilatation of the outflow region expands the proximal pulmonary artery, priming it to receive the RV stroke volume and facilitating RV output. Continued ejection of RV stroke volume is further facilitated by the normally high capacitance and low resistance of the pulmonary vascular bed. Indeed, RV stroke volume continues to move into the proximal pulmonary artery even during diastole (5). Pulmonary blood flow during RV diastole falls sharply with an increase in RV afterload.
These structural and functional differences result in differing responses to preload and afterload (Figure 2). The more muscular LV tolerates abrupt increases in afterload fairly well, but has difficulty handling sudden increases in preload. On the other hand, the more compliant RV is better suited to accommodate large increases in right-sided venous return but tolerates acute increases in afterload poorly (6–8). As RV afterload increases, the RV begins to dilate limiting its ability to increase contractility. As illustrated in Figure 2, LV stroke volume falls only about 10% as mean aortic pressure increases from 100–140 mm Hg, whereas RV stroke volume falls sharply in response to increasing mean pulmonary artery pressure between 10 and 30 mm Hg. At the same time, an enlarging RV begins to impede LV filling by moving the interventricular septum toward the LV in a process known as interventricular dependence. Because they share a common septum, abnormalities in the function of one ventricle can adversely affect the function of the other. LV end-diastolic pressure normally exceeds RV end-diastolic pressure, allowing the septum to bow toward the RV during diastole. When RV end-diastolic pressure exceeds LV end-diastolic pressure, the septum shifts toward the LV during diastole, creating a flattened septum and a “D” shape when the heart is viewed in cross-section (Figure 3). The degree of septum shift is dependent on the difference between RV and LV end-diastolic pressure. When LV end-diastolic pressure falls from decreased intravascular volume, RV end-diastolic pressure is also reduced, and the difference between RV and LV end-diastolic pressure is maintained. Similarly, increased RV preload increases RV output, thereby raising LV end-diastolic pressure and maintaining the right-to-left end-diastolic pressure difference. As pulmonary vascular resistance increases, however, the RV becomes incapable of maintaining LV filling pressure. RV end-diastolic pressure begins to rise as LV end-diastolic pressure falls, sending the interventricular septum toward the LV during diastole (9). Reduced LV filling in this setting is often refractory to intravascular volume expansion, as raising central venous pressure only increases the difference between RV end-diastolic pressure and LV end-diastolic pressure, and further compromises LV filling. In one study of patients with chronic obstructive pulmonary disease and pulmonary hypertension, volume loading was shown to actually decrease LV end-diastolic volume (10). Similar findings have been demonstrated in animal models of acute pulmonary embolism (11, 12).
Figure 2.
Differences between right ventricular (RV) and left ventricular (LV) response to increasing afterload (left panel) and increasing preload (right panel). RV stroke volume falls sharply as mean vascular pressure () is increased from 20 to 30 mm Hg in the pulmonary artery, but LV stroke volume stays fairly constant as mean aortic pressure is increased from 100 to 140 mm Hg. In contrast, LV stroke work increases rapidly as left atrial pressure is raised from 10 to 20 cm H2O, while the increase in RV stroke work in response to the same elevation or right atrial pressure is much more modest. Reproduced by permission from Reference 113.
Figure 3.
The position of the interventricular septum during the cardiac cycle is determined by the difference between right ventricular (RV) and left ventricular (LV) pressure. Under normal conditions, LV end-diastolic pressure is greater than RV end-diastolic pressure, and the septum bows toward the RV during diastole. As the RV fails, RV end-diastolic pressure begins to exceed that of the left ventricle (LV) and the septum bows toward the LV during diastole forming a “D”-shaped pattern and impaired LV filling (left panel). When RV failure occurs due to elevated pulmonary vascular resistance, the combination of high RV systolic pressure and decreased LV filling may lead to near obliteration of the LV at end systole (right panel).
Detection and Monitoring of RV Function in Critically Ill Patients
Assessment of RV function in the critically ill patient begins with physical exam and consideration of the patient’s presentation and medical history. Evidence of elevated right-sided filling pressures, as suggested by jugular venous distension and peripheral edema, may be the most recognizable signs. With the head of the bed elevated to about 30°, jugular venous pulsations can usually be observed in the lateral neck where the medial and lateral bodies of the sternocleidomastoid muscle meet, or just posterior to the lateral belly. Jugular vein wave forms have a gradual upslope and a sharp collapse. This is the opposite of the carotid pulse, which has a sharp upslope and a gentle collapse. By palpating the carotid artery on the opposite side of the neck, the atrial (a wave) and ventricular (v wave) waves can be appreciated. The a wave comes just before the peak of the carotid pulse, and the v wave follows. In the setting of severe tricuspid insufficiency, as often occurs in RV failure, the v wave can be pronounced.
RV dilation can cause splitting of the second heart sound and produce a tricuspid regurgitation murmur heard best over the right sternal border. An RV heave may also be felt in the same location. Signs of RV failure may be evident on chest X-ray, although the anterior position of the RV makes it difficult to evaluate RV size unless a lateral film can be obtained. Contrast-enhanced computed tomography studies of the chest can provide a better assessment of RV size and position of the interventricular septum (13). Widely used biomarkers, such as plasma brain natriuretic peptide or cardiac troponin levels, are not specific for RV failure, but can signal RV dysfunction in the absence of left-sided heart disease.
Transthoracic echocardiography provides estimates of RV size and function, and may help elucidate possible precipitating factors for RV failure, including LV dysfunction and valvular heart disease. RV systolic function can be assessed by measuring the longitudinal systolic displacement of the RV base toward the RV apex. This is commonly referred to as the tricuspid annular plane systolic excursion (14) and is relatively simple to perform, highly reproducible, and has been shown to correlate well with RV ejection fraction derived from radionuclide angiography (14, 15). A normal tricuspid annular plane systolic excursion is about 2.4–2.7 cm (16, 17), whereas a value below 1.8 cm has been shown to have an 87% accuracy at predicting a stroke volume index less than 29 ml/m2 (18). Decreased tricuspid annular plane systolic excursion has been associated with increased hospitalization rates for right heart failure and decreased survival in patients with pulmonary arterial hypertension (18).
In pulmonary arterial hypertension, right atrial enlargement, pericardial effusion, low tricuspid annular plane systolic excursion, and septal displacement are poor prognostic indicators, but their use in risk stratification for critically ill patients with RV failure has not been systematically studied (19–21). Estimates of RV systolic pressure obtained by echocardiogram correlate well with measurements made by right heart catheterization, but the variance between measures can be greater than 10 mm Hg in up to 50% of cases (22), and the frequency with which echocardiogram over- or underestimates RV systolic pressure is greater in patients with chronic lung disease (23). Positive-pressure ventilation also makes estimates of right atrial pressure inaccurate, and can contribute to inaccurate estimates of pulmonary artery pressure in patients who are mechanically ventilated. When reliable measurement of pulmonary hemodynamics is needed, pulmonary artery catheterization provides valuable information on RV and LV filling pressures, cardiac output, and pulmonary artery pressure.
Approach to Management of Acute RV Failure in the Critically Ill Patient
Causes of RV failure in critical illness can be divided into three main categories: (1) excessive preload; (2) excessive afterload; and (3) insufficient myocardial contractility. In most cases, acute RV failure in the intensive care unit is a combination of established pulmonary vascular disease complicated by acute derangements in one or more of these three main categories. Examples include the patient with cor pulmonale from emphysema who develops severe pneumonia, or the patient with chronic right heart failure from pulmonary arterial hypertension who becomes septic. In these situations, the conditions responsible for chronic RV failure cannot be reversed, and management should be directed toward optimizing RV function while reducing the cause of RV failure. At other times, acute RV failure is the result of a sudden increase in RV afterload, such as occurs with massive pulmonary embolism. In this situation, the first priority should be relieving the increase in afterload.
RV Preload
Proper fluid management is critical for successful management of RV failure. In the early stages of critical illnesses, intravascular volume can fall rapidly in response to bleeding, increased vascular permeability, and insensible losses. Sedatives and analgesics blunt sympathetic vasoconstriction of the systemic venous circulation, leading to decreased venous tone and reduced right-sided return. Positive pressure ventilation can also impede RV preload by increasing intrathoracic pressure and reducing RV transmural filling pressure. Adequate right-sided filling pressure is essential in maintaining cardiac output in patients with acute RV failure (24). If low intravascular volume is suspected, volume resuscitation should be instituted as quickly as possible. However, RV preload requirements differ substantially based on whether afterload is normal or increased. When RV failure occurs in the setting of normal pulmonary vascular resistance, such as in right-sided myocardial infarction, RV end-diastolic pressure often needs to be increased above normal levels to maintain cardiac output. However, when RV failure occurs in the setting of increased RV afterload, volume loading can result in displacement of the interventricular septum toward the LV and impaired LV diastolic filling. At the same time, RV dilation increases free wall tension, resulting in increased oxygen demand and decreased RV perfusion. In this setting, intravascular volume may need to be decreased. Initial attempts at volume reduction may have little effect. The RV has a flatter Starling curve than the LV, meaning there is less of a change in RV contractility over a wide range of filling pressures. Hence, a considerable amount of volume unloading may be necessary before any improvement in RV function is seen. At the same time, care must be exercised not to allow RV preload to become too low. Optimal right-sided filling pressure may vary considerably between individual patients based on RV contractility and afterload. In general, preload goals should be to keep RV transmural filling pressures in a moderately elevated range, typically 8–12 mm Hg, and then adjusted from there to optimize RV function and cardiac output. A central venous line that provides access to superior vena cava oxygen saturation (SvO2) and central venous pressure can help with assessment of right-sided filling pressures and oxygen delivery. Normal SvO2 is 70–80%, and lower values, in the setting of normal arterial oxygenation, can be suggestive of reduced cardiac output. If RV preload is too high, reductions in central venous pressure via diuresis or dialysis should be accompanied by improvement in cardiac output, as assessed by SvO2 or systemic organ perfusion. Echocardiography may also be helpful. Evidence of RV dilation and impingement on LV filling suggest that further reduction in preload may be necessary. If these assessments of RV function are inadequate, placement of a pulmonary artery catheter may be necessary. Although the routine use of a pulmonary artery catheter has not been found to improve outcome in the management of severe sepsis (25–30), and has not been well studied in the management of acute RV failure (31), serial measurement of hemodynamics may be helpful in guiding clinical decision making. In our practice, we do not typically place Swan-Ganz–style catheters for continuous monitoring of RV function, but often send patients for right heart catheterization to determine the severity and etiology of their RV failure and then remove the catheter. If a patient is found to have a condition in which RV function is greatly dependent on small changes in preload or afterload, we may elect to leave the catheter in place for longer-term management.
RV Afterload
Excessive afterload plays some role in nearly all cases of acute RV failure, and decreasing it is usually the most effective way of improving RV function. Unfortunately, many cases of acute RV failure are associated with chronic heart or lung diseases that cannot easily be reversed. In these situations, efforts should be focused on removing any factors that can contribute to increased pulmonary vascular tone followed by the judicious use of selective pulmonary vasodilators.
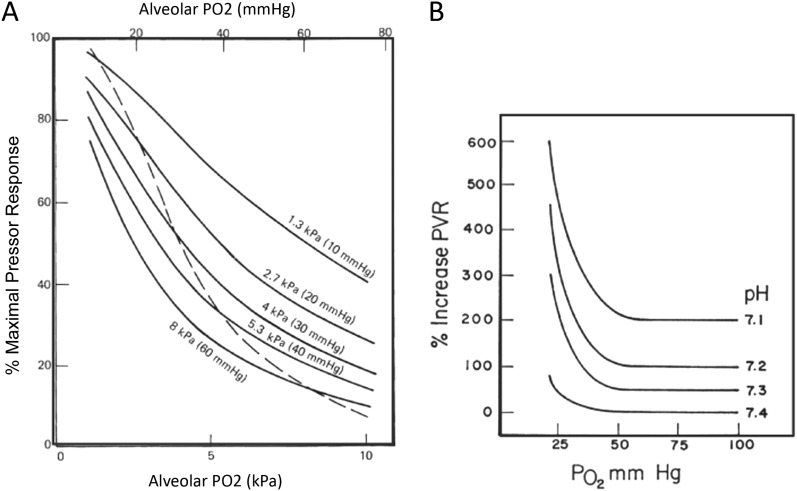
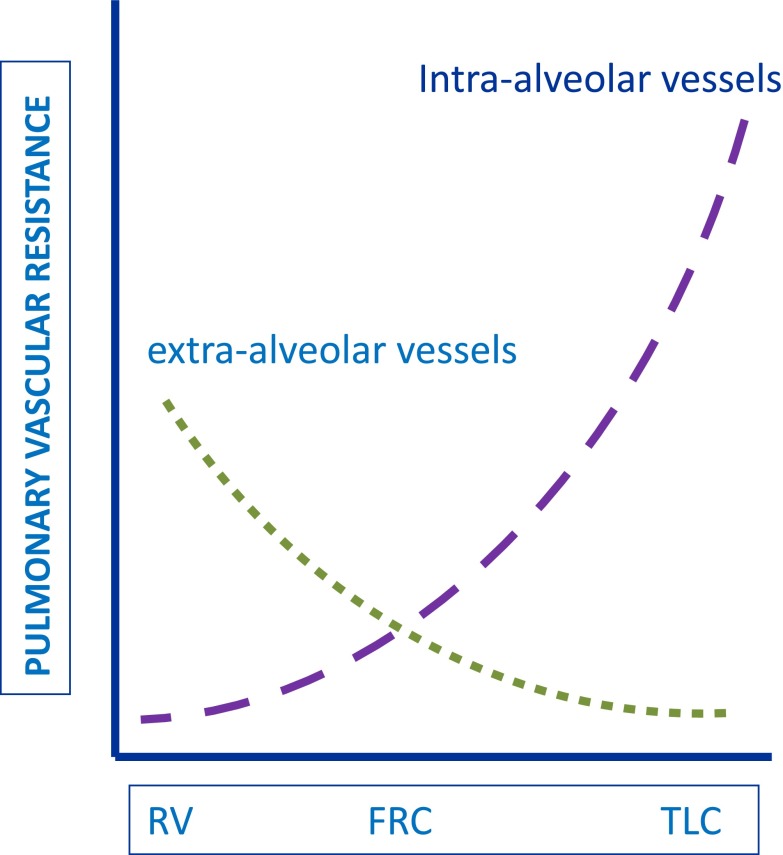
A number of adverse conditions associated with critical illness lead to increased pulmonary vascular resistance and, thereby, a rise in RV afterload. Hypoxic pulmonary vasoconstriction can occur in response to decreases in oxygen tension in the alveoli, pulmonary arterial blood, or bronchial arterial blood, and is enhanced by hypercapnea or acidemia (32, 33) (Figure 4). The vasoconstrictive response is greatest to decreases in oxygen tension in alveolar air, but for any degree of alveolar hypoxia, hypoxic pulmonary vasoconstriction is greater in the presence of decreased pulmonary arterial O2 (34). Adequate systemic SaO2 routinely monitored by pulse oximetry in the intensive care unit effectively excludes alveolar hypoxia, but is not a reliable indicator of pulmonary arterial oxygenation. High or low lung volume can worsen RV afterload, because pulmonary vascular resistance tends to be lowest when the lung is near functional residual capacity (Figure 5) (35).
Figure 4.
Effect of oxygen tension and acidemia on pulmonary vasoconstriction. (A) Pulmonary vasoconstriction increases as alveolar O2 tension (PAO2) falls while keeping mixed venous oxygenation constant (mixed venous Po2 indicated for each solid line from 60 to 10 mm Hg). The combined effect of allowing PAO2 and mixed venous Po2 to fall is shown by the dashed line where PAO2 and mixed venous oxygen saturation O2 are the same (34). Maximal pulmonary vasoconstrictor response was defined as the difference between baseline pulmonary artery pressure when ventilating with an FiO2 of 0.21 and perfusate FIO2 of 0.06, and the pulmonary artery pressure when both the inspired and the perfusate FiO2 was zero. The pressure response at all other combinations of inspired and perfusate FiO2 were expressed as a percent of this 10 maximum (%Rmax). (B) Pulmonary vasoconstriction in newborn calves as a function of inspired Po2 under conditions of different levels of arterial blood pH. Hypoxic pulmonary vasoconstriction is increased and occurs at a higher level of inspired O2 as arterial pH is decreased. PVR = pulmonary vascular resistance. Reproduced by permission from Reference 34.
Figure 5.
Pulmonary vascular resistance in intra- and extra-alveolar vessels relative to lung volume. Interstitial pressure becomes more negative during lung expansion in the spontaneously breathing patient, favoring enlargement of most pulmonary vessels and a fall in their resistance to blood flow. Intra-avleolar vessels, however, become compressed by expansion of surrounding alveoli during lung inflation, leading to increased vascular resistance in these vessels. Total pulmonary vascular resistance is lowest at functional residual capacity, where vascular resistance in both intra- and extra-alveolar vessels is intermediate. TLC = total lung capacity. Reproduced by permission from Reference 114.
Several vasoactive factors that have been implicated in the pathogenesis of pulmonary arterial hypertension, such as endothelin and thromboxane, are elevated during sepsis, and have been shown to correlate inversely with cardiac output (36, 37). Serotonin and IL-6 are also up-regulated in sepsis and the acute respiratory distress syndrome (38, 39). Decreased production of nitric oxide in the lung contributes to increased pulmonary vascular resistance in sepsis (40), and endotoxin can increase pulmonary vascular resistance via suppression of nitric oxide synthesis (41). Finally, any injury that damages the pulmonary vascular endothelium can precipitate thrombosis in situ and raise pulmonary vascular resistance further (42).
Interventions aimed at reducing RV afterload should begin with correction of hypercapnea, acidemia, and alveolar hypoxia. Ideally, SaO2 should be kept above 92%, and ventilator settings should be adjusted to achieve a lung volume near functional residual capacity and a Pco2 and pH that are as close to normal as possible. These goals are at odds with contemporary approaches to ventilation in critically ill patients, particularly the low lung volumes and permissive hypercapnea used in patients with acute respiratory distress syndrome (43). Considering the favorable effects of low-volume ventilation on survival (43), it seems prudent to use this approach in patients with acute respiratory distress syndrome, although it is interesting to note that elevated pulmonary artery pressure has been associated with worse outcomes in this setting (44).
When adjustment of preload and afterload do not achieve satisfactory improvement in RV function, administration of pulmonary vasodilators may be appropriate. Several classes of pulmonary vasodilators have been developed over the last 20 years for the treatment of pulmonary arterial hypertension (Table 1). These drugs target cellular pathways that have been implicated in the pathogenesis of pulmonary arterial hypertension, but none has been approved for the treatment of RV failure in critically ill patients. Each drug has systemic as well as pulmonary vasorelaxant properties, and is capable of causing hypotension. Furthermore, systemic administration can worsen gas exchange by blunting hypoxic pulmonary vasoconstriction and impairing matching (45, 46).
Table 1.
Currently available pulmonary vasodilator medications
| Name | Drug Class | Action | Route of Administration | Terminal Half-Life |
|---|---|---|---|---|
| Ambrisentan | Endothelin receptor antagonist | Blocks endothelin receptor A | Oral | 15 h |
| Bosentan | Endothelin receptor antagonist | Blocks endothelin receptor A and B | Oral | 5.4 h |
| Macitentan | Endothelin receptor antagonist | Blocks endothelin receptor A | Oral | 14–18 h |
| Sildenafil | Phosphodiesterase type-5 inhibitor | Slows metabolism of intracellular cGMP | Oral or intravenous | 4 h orally |
| Tadalafil | Phosphodiesterase type-5 inhibitor | Slows metabolism of intracellular cGMP | Oral | 17.5 h |
| Epoprostenol | Prostacyclin | Increases intracellular cAMP | Intravenous or inhaled* | <6 min |
| Treprostinil | Prostacyclin derivative | Increases intracellular cAMP | Intravenous, subcutaneous, inhaled, or oral | 4 h |
| Iloprost | Prostacyclin derivative | Increases intracellular cAMP | Inhaled | 20–30 min |
| Nitric oxide | Soluble guanylate cyclase stimulator | Increases intracellular cGMP | Inhaled | Seconds |
| Riociguat | Soluble guanylate cyclase stimulator | Increases intracellular cGMP | Oral | 7–12 h |
Not U.S. Food and Drug Administration approved for this route of administration.
Inhaled nitric oxide is a potent pulmonary vasodilator (47–49) with a rapid onset of action and an extremely short half-life, making it an ideal agent for attempting to unload the RV in the intensive care unit. Furthermore, its greater effect on blood vessels in well ventilated lung can improve oxygenation by stealing blood flow away from areas of very low or shunt (50). Although inhaled nitric oxide does not improve outcome in acute respiratory distress syndrome (51), it has been shown to improve RV ejection fraction and end-diastolic volume in these patients (52) and improve pulmonary hemodynamics and mixed venous oxygen saturation in patients with acute RV failure (53).
Three prostacyclin derivatives are currently available for treatment of pulmonary arterial hypertension in the United States. Like inhaled nitric oxide, these drugs are potent pulmonary vasodilators with rapid onset of action and short half-lives. They exert their vasodilator effects by increasing intracellular cAMP levels, and, thus, may also provide inotropic effects on cardiac function. All three presently available drugs can be delivered by inhalation, thereby minimizing systemic vasodilator effects and mismatch. Inhaled epoprostenol has been used successfully to manage patients with RV failure after cardiac surgery (54) and improve gastric mucosal pH in patients with sepsis and pulmonary hypertension (55).
Phosphodiesterase (PDE) 5 inhibitors reduce pulmonary vascular resistance and may improve RV contractility, but little is known about their use in critical illness (56–59). These agents inhibit the metabolism of cGMP, the second messenger that mediates the vasodilatory effects of nitric oxide and the natriuretic peptides. In animal studies, PDE5 inhibitors increase contractility in hypertrophied RV, but not in normal RV (60). Thus, these agents may improve RV function in patients with chronic pulmonary hypertension who develop acute RV failure. PDE5 inhibitors must be used cautiously in patients who are hemodynamically unstable (61, 62), because they have systemic vasodilator effects that can lower blood pressure, and their terminal half-life ranges from 4 to 18 hours. An intravenous form of sildenafil with shorter half-life is also available.
The use of other currently available pulmonary vasodilators, such as the endothelin receptor antagonists and the recently approved soluble guanylate cyclase stimulator, riociguat, should probably be avoided in acute RV failure. Endothelin receptor antagonists have been associated with increased mortality in left heart failure, and riociguat may have significant systemic vasodilator effects, especially under conditions such as sepsis, where endogenous nitric oxide production may be increased. Calcium channel blockers should also be avoided, because they have negative inotropic effects, and have been shown to increase RV stroke work index (63).
Insufficient RV Contractility
Loss of RV contractile force in acute RV failure is induced primarily by three interrelated factors: (1) overstretching of the RV free wall, placing the myocytes at a mechanical disadvantage; (2) derangements in cellular metabolism, leading to decreased myocardial contractile forces; and (3) insufficient oxygen delivery due to decreased coronary arterial perfusion. The interplay between RV dilation and ischemia is shown in Figure 6. Increases in RV preload and/or afterload increases free wall tension and O2 demand, while impeding LV filling, reducing LV output, and decreasing coronary artery pressure. Metabolic derangements from a variety of insults, including acid/base disturbances, generation of reactive oxygen species, and inflammatory cytokines, impair oxygen utilization and contribute to RV failure in critically ill patients (64, 65). At the same time, sepsis and other critical illnesses associated with increased metabolic demand result in the need for increased oxygen delivery, and may exceed the ability of the RV to maintain adequate cardiac output.
Figure 6.
Cycle of right ventricular (RV) decompensation. RV dysfunction begins with excessive increases in preload or afterload, or injury that results in decreased contractility. Tachycardia and increased stroke work lead to increased RV free wall tension, resulting in increased oxygen demand. Eventually, cardiac output begins to fall, leading to systemic hypotension. RV ischemia ensues from the combination of increased RV work load, free wall tension, and decreased coronary perfusion. Inadequate delivery of oxygen to the RV myocardium worsens myocyte contractility, leading to greater RV dilation and wall tension (left-sided arrow) and decreased O2 delivery, due to systemic hypotension, lower mixed venous oxygen saturation, and increased right-to-left-side intracardiac shunting as RV pressure rises (right-sided arrow). The pulmonary vascular disease associated with pulmonary arterial hypertension (PAH) poses a particularly difficult situation for RV function, because it increases RV afterload while decreasing oxygenation of pulmonary venous blood leading to systemic (including coronary arterial) hypoxemia. PFO = patent foramen ovale. Reproduced by permission from Reference 115.
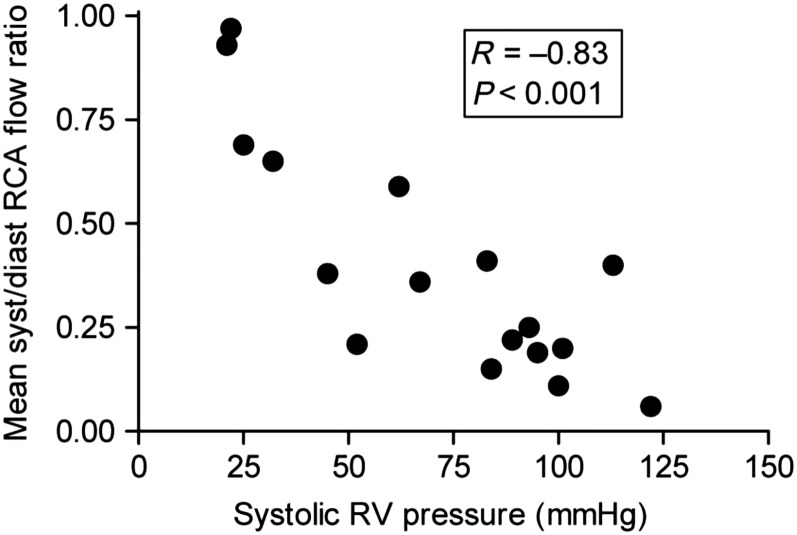
Perfusion of the RV free wall is determined by the difference in RV free wall tension and coronary artery pressure (66). Coronary perfusion of the RV decreases during systole in patients with pulmonary arterial hypertension and normal systemic pressures (Figure 7) (67), and a similar impairment is likely to occur when acute RV failure occurs in the setting of systemic hypotension. Fluid resuscitation that results in ventricular enlargement and increases in RV free wall tension without improving systemic arterial pressure can actually decrease RV perfusion (68, 69). Patients with acute RV failure associated with chronic pulmonary vascular disease may have RV systolic pressures that approach or exceed systemic pressure. In this situation, the first goal of vasopressor therapy is to restore systemic blood pressure to levels above RV systolic pressure (70, 71). Drugs that increase myocardial contractility should be withheld until this first goal is achieved.
Figure 7.
Ratio of blood flow during systole to blood flow during diastole in the right coronary artery (RCA) as a function of right ventricular (RV) systolic pressure in patients with pulmonary hypertension. When RV systolic pressure is near normal levels, the amount of RCA blood flow is similar during systole and diastole. As RV systolic pressure increases, RCA blood flow during systole falls. Blood flow in the RCA during systole is inversely proportional to RV systolic pressure, such that RCA blood flow during systole approaches zero as RV systolic pressure approaches systemic levels. Reproduced by permission from Reference 67.
Vasopressors
Several vasoactive drugs have been used to manage RV failure in the intensive care unit (Table 2). The ideal vasopressor for use in acute RV failure would be an agent that increases systemic arterial pressure and RV contractility without raising pulmonary vascular resistance. Norepinephrine primarily targets the α1 receptor, causing vasoconstriction with limited β1 receptor stimulation and cardiac inotropy (72). However, the β1 effects on contractility have been shown to improve pulmonary artery/RV coupling in animal models of RV dysfunction (73–75). In a small study of patients with sepsis with right heart failure, norepinephrine use was associated with improved RV myocardial oxygen delivery (via an increase in systemic vascular resistance), although pulmonary vascular resistance was increased, and no change was seen in RV ejection fraction (76). Phenylephrine is a pure α1 receptor agonist that augments right coronary artery perfusion, but does not impact RV contractility, and can increase pulmonary vascular resistance (71, 74, 77). It can also cause reflex bradycardia, which can be troublesome in the face of reduced RV stroke volume. Epinephrine is a mixed α/β receptor agonist that can induce vasoconstriction and increase inotropy. Epinephrine improved cardiac output without detrimental effects on pulmonary vascular resistance in an animal study (78), and improved RV contractility in a small study of patients with septic shock (79). Vasopressin binds to V1 receptors on vascular smooth muscle cells (72). At lower doses (e.g., 0.01–0.03 U/min), it causes pulmonary vasodilatation via stimulation of endothelial nitric oxide, but, at higher doses, it increases responsiveness to catecholamines and causes pulmonary and coronary artery vasoconstriction (24, 79–82). Taken together, norepinephrine is a reasonable agent in hypotensive patients with acute RV failure and is often the initial pressor used in our institution for this purpose.
Table 2.
Vasoactive drugs for management of acute right ventricular failure and their mechanism of action
| Receptor Binding |
||||||
|---|---|---|---|---|---|---|
| Agent | α1 | β1 | β2 | D | V1 | Notes |
| Norepinephrine | ++ | + | Improves PA/RV coupling in animals (73–75) | |||
| Phenylephrine | ++ | Increases PVR (71, 74, 77); may induce reflex bradycardia | ||||
| Epinephrine | ++ | ++ | + | (79) | ||
| Vasopressin | + | Dose dependent pulmonary vasodilatation (0.01–0.03 U/min) and vasoconstriction (24, 82, 83) | ||||
| Dopamine | Risk of arrhythmias | |||||
| Low (<5 μg/kg/min) | + | ++ | ||||
| Medium (>10 μg/kg/min) | + | ++ | ++ | |||
| High (>10 μg/kg/min) | ++ | ++ | ++ | |||
| Dobutamine | ++ | + | β2-mediated drop in SVR (31); risk of arrhythmias | |||
| Milrinone | Phosphodiesterase-3 inhibitor; inotropy and pulmonary vasodilatation; drop in LVEDP and SVR (72, 84, 89); risk of arrhythmias | |||||
Definition of abbreviations: D = dopaminergic receptor; LVEDP = left ventricular end-diastolic pressure; PA = pulmonary artery; PVR = pulmonary vascular resistance; RV = right ventricle; SVR = systemic vascular resistance; V1 = vasopressin receptor 1. + = low to moderate affinity, ++ = moderate to high affinity.
Inotropes
Low-dose dopamine is a reasonable option to improve RV contractility in patients with RV failure. Dopamine has dose-dependent effects on various receptors, activating dopaminergic receptors at low doses (<5 μg kg−1 min−1), β1 receptors at medium doses (5–10 μg kg−1 min−1), and α1 receptors at high doses (>10 μg kg−1 min−1). At doses below 16 μg kg−1 min−1, dopamine increases cardiac output without compromising pulmonary vascular resistance (83, 84). Dobutamine is another inotrope that acts via β1 receptor stimulation, but may also cause vasodilatation due to β2 effects (72). At low doses (5–10 μg kg−1 min−1), dobutamine improves pulmonary artery/RV coupling in animal studies and improves myocardial contractility and pulmonary vascular resistance in patients with left heart failure (73, 85). Dobutamine has been shown to improve hemodynamics in patients with pulmonary hypertension at liver transplantation and after RV infarction (86, 87). Higher doses should be avoided because of the risk of β2-mediated vasodilatation and hypotension (32).
Milrinone, a selective PDE-3 inhibitor that slows intracellular cAMP metabolism, is an appealing agent for use in pulmonary vascular disease, because it can improve inotropy and pulmonary vasodilatation (72, 88, 89). Milrinone is frequently the agent of choice in patients with pulmonary hypertension from biventricular failure, and in those recovering after ventricular assist or cardiac transplantation (90–92). Several small studies have also examined the use of inhaled milrinone in patients with pulmonary vascular disease to avoid systemic hypotension (93–96).
Inotropes increase the risk of tachyarrhythmias, and should generally be considered only when there is evidence of inadequate oxygen delivery despite the correction of abnormalities in RV preload, afterload, and ischemia. Sinus tachycardia is a normal compensatory mechanism for insufficient cardiac output. As contractility decreases or becomes inadequate to keep up with demand for oxygen delivery, heart rate increases. As a result, sinus tachycardia is often a poor prognostic indicator in RV failure. At the same time, it is important to avoid increasing cardiac output above normal levels, because this can increase pulmonary artery pressures and thereby increase RV workload. For this reason, some intensivists prefer to use milrinone for inotropic support, because it may have less of a chronotropic effect than dobutamine.
Calcium sensitizers, such as levosimendan, that enhance myocardial contractility without increasing cytosolic calcium and oxygen demand, have been used to increase cardiac contractility in heart failure. Randomized placebo-controlled studies have shown improvement in RV systolic and diastolic function in patients with left heart failure (97), and recent reports describe improved RV function in response to levosimendan in patients with RV failure associated with chronic thromboembolic pulmonary hypertension and heart transplantation (98, 99).
Mechanical Support
When medical therapy for acute RV failure in the intensive care unit is ineffective mechanical support may be considered. Extracorporeal life support, specifically venovenous and venoarterial extracorporeal membrane oxygenation, has been used successfully in patients with RV failure due to massive pulmonary embolus, chronic thromboembolic pulmonary hypertension, and pulmonary arterial hypertension, usually as a bridge to endarterectomy or lung transplantation (100–107). Unlike venovenous extracorporeal membrane oxygenation that oxygenates venous blood, but requires the RV to pump the entire cardiac output through the pulmonary circulation, venoarterial extracorporeal membrane oxygenation pumps enough blood from the venous to the arterial circulation to unload the RV while maintaining systemic oxygenation. Venoarterial extracorporeal membrane oxygenation improves RV function and oxygen delivery, and has been used successfully in awake, spontaneously breathing patients (107). Recent reports describe the use of venoarterial extracorporeal membrane oxygenation to support RV function during initiation of pulmonary vasodilator therapy in a treatment naive patient with pulmonary arterial hypertension presenting in severe RV failure (108), and as a bridge to recovery in pulmonary arterial hypertension patients presenting with worsening pulmonary hypertension (109). Complications include bleeding, infection, thromboembolism, and neurologic sequela (110). More recently, pumpless lung-assist devices have been developed that connect the pulmonary artery to the left atrium with a low-resistance membrane oxygenator. Pulmonary blood flow through the low-resistance circuit unloads the RV and enhances LV filling (111, 112).
Conclusions
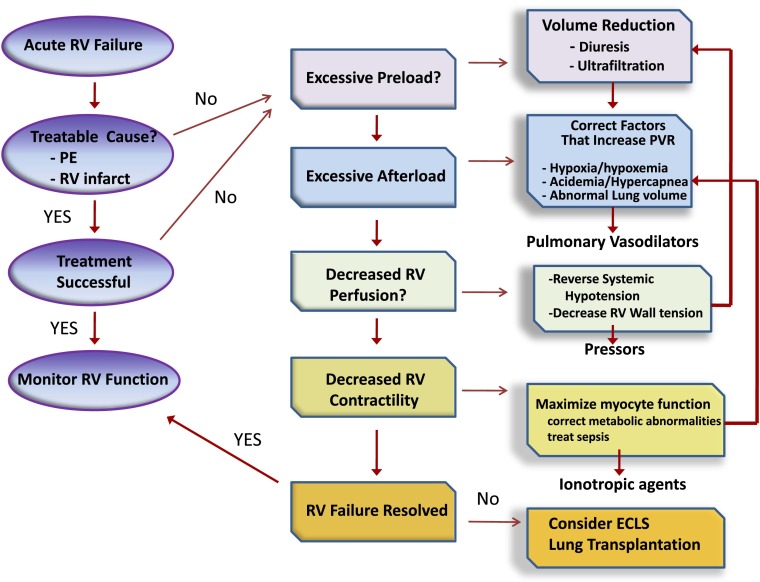
Acute RV failure is seen with increasing frequency in the intensive care unit and, when severe, can contribute to hemodynamic instability and insufficient oxygen delivery. An overall approach to management is outlined in Figure 8. Initial management should focus on determining the cause of RV decompensation and reversing it. When reversal is not possible, efforts should be directed toward optimizing RV preload and mitigating any factors that can increase pulmonary vascular resistance. If RV failure persists, short-acting pulmonary vasodilators should be used, preferably via inhalation in an attempt to lower RV afterload. When these efforts fail, the judicious use of vasopressors and inotropes should be considered in an attempt to improve RV perfusion and contractility. Extracorporeal life support is effective at restoring circulatory support when other measures fail, and may be indicated in patients who are expected to recover from the cause of their RV failure, or as a bridge to surgical interventions, such as pulmonary endarterectomy or lung transplantation.
Figure 8.
Approach to management of acute right ventricular (RV) failure. Patients should be assessed for acute cause of increased RV afterload or decreased contractility, such as pulmonary embolism or right-sided infarction. If no readily reversible cause is identified, efforts should be directed at optimizing RV preload and reducing RV afterload. The latter should include reversal of factors known to increase pulmonary vascular resistance and then the use of selective pulmonary vasodilator drugs. Metabolic conditions that reduce cardiac contractility, such as sepsis and acidemia, should be addressed. Systemic arterial pressure should be kept above RV systolic pressure to maintain RV perfusion. If these efforts fail, ionotropic agents can be tried to improve RV contractility. Measures to correct metabolic abnormalities and reduce RV dilation will also aid RV contractility. Extracorporeal life support should be considered when medical therapy is unsuccessful in a patient who has a reversible cause of RV failure or who is being prepared for lung transplantation. ECLS = extracorporeal life support; PE = pulmonary embolism; PVR = pulmonary vascular resistance.
Acknowledgments
Acknowledgment
The authors thank Anthony Cucci and Tim Lahm, Indiana University Medical School, for use of Figure 6, and Susan Barnaby for help with preparation of figures.
Footnotes
Supported by National Institutes of General Medical Sciences grant P20 GM103652 and American Heart Association grant 11FTF7400032.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bhattacharya J, Staub NC. Direct measurement of microvascular pressures in the isolated perfused dog lung. Science. 1980;210:327–328. doi: 10.1126/science.7423192. [DOI] [PubMed] [Google Scholar]
- 2.Dell’Italia LJ. The right ventricle: anatomy, physiology, and clinical importance. Curr Probl Cardiol. 1991;16:653–720. doi: 10.1016/0146-2806(91)90009-y. [DOI] [PubMed] [Google Scholar]
- 3.Greyson CR. Pathophysiology of right ventricular failure. Crit Care Med. 2008;36(1) Suppl:S57–S65. doi: 10.1097/01.CCM.0000296265.52518.70. [DOI] [PubMed] [Google Scholar]
- 4.James TN. Anatomy of the crista supraventricularis: its importance for understanding right ventricular function, right ventricular infarction and related conditions. J Am Coll Cardiol. 1985;6:1083–1095. doi: 10.1016/s0735-1097(85)80313-2. [DOI] [PubMed] [Google Scholar]
- 5.Pouleur H, Lefèvre J, Van Mechelen H, Charlier AA. Free-wall shortening and relaxation during ejection in the canine right ventricle. Am J Physiol. 1980;239:H601–H613. doi: 10.1152/ajpheart.1980.239.5.H601. [DOI] [PubMed] [Google Scholar]
- 6.Maughan WL, Shoukas AA, Sagawa K, Weisfeldt ML. Instantaneous pressure–volume relationship of the canine right ventricle. Circ Res. 1979;44:309–315. doi: 10.1161/01.res.44.3.309. [DOI] [PubMed] [Google Scholar]
- 7.Hurtford WE, Barlai-Kovach M, Strauss HW, Zapol WM, Lowenstein E. Canine biventricular performance during acute progressive pulmonary microembolization: regional myocardial perfusion and fatty acid uptake. J Crit Care. 1987;2:270–281. [Google Scholar]
- 8.Hurford WE, Zapol WM. The right ventricle and critical illness: a review of anatomy, physiology, and clinical evaluation of its function. Intensive Care Med. 1988;14:448–457. doi: 10.1007/BF00256958. [DOI] [PubMed] [Google Scholar]
- 9.Weyman AE, Wann S, Feigenbaum H, Dillon JC. Mechanism of abnormal septal motion in patients with right ventricular volume overload: a cross-sectional echocardiographic study. Circulation. 1976;54:179–186. doi: 10.1161/01.cir.54.2.179. [DOI] [PubMed] [Google Scholar]
- 10.Jardin F, Gueret P, Prost JF, Farcot JC, Ozier Y, Bourdarias JP. Two-dimensional echocardiographic assessment of left ventricular function in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1984;129:135–142. doi: 10.1164/arrd.1984.129.1.135. [DOI] [PubMed] [Google Scholar]
- 11.Belenkie I, Dani R, Smith ER, Tyberg JV. Ventricular interaction during experimental acute pulmonary embolism. Circulation. 1988;78:761–768. doi: 10.1161/01.cir.78.3.761. [DOI] [PubMed] [Google Scholar]
- 12.Belenkie I, Dani R, Smith ER, Tyberg JV. Effects of volume loading during experimental acute pulmonary embolism. Circulation. 1989;80:178–188. doi: 10.1161/01.cir.80.1.178. [DOI] [PubMed] [Google Scholar]
- 13.Boxt LM. Radiology of the right ventricle. Radiol Clin North Am. 1999;37:379–400. doi: 10.1016/s0033-8389(05)70100-7. [DOI] [PubMed] [Google Scholar]
- 14.Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984;107:526–531. doi: 10.1016/0002-8703(84)90095-4. [DOI] [PubMed] [Google Scholar]
- 15.Lee CY, Chang SM, Hsiao SH, Tseng JC, Lin SK, Liu CP. Right heart function and scleroderma: insights from tricuspid annular plane systolic excursion. Echocardiography. 2007;24:118–125. doi: 10.1111/j.1540-8175.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 16.Hammarström E, Wranne B, Pinto FJ, Puryear J, Popp RL. Tricuspid annular motion. J Am Soc Echocardiogr. 1991;4:131–139. doi: 10.1016/s0894-7317(14)80524-5. [DOI] [PubMed] [Google Scholar]
- 17.López-Candales A, Dohi K, Rajagopalan N, Edelman K, Gulyasy B, Bazaz R. Defining normal variables of right ventricular size and function in pulmonary hypertension: an echocardiographic study. Postgrad Med J. 2008;84:40–45. doi: 10.1136/pgmj.2007.059642. [DOI] [PubMed] [Google Scholar]
- 18.Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034–1041. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 19.Ghio S, Klersy C, Magrini G, D’Armini AM, Scelsi L, Raineri C, Pasotti M, Serio A, Campana C, Viganò M. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2010;140:272–278. doi: 10.1016/j.ijcard.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 20.López-Candales A, Bazaz R, Edelman K, Gulyasy B. Apical systolic eccentricity index: a better marker of right ventricular compromise in pulmonary hypertension. Echocardiography. 2010;27:534–538. doi: 10.1111/j.1540-8175.2009.01045.x. [DOI] [PubMed] [Google Scholar]
- 21.Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, de Boisblanc B, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002;39:1214–1219. doi: 10.1016/s0735-1097(02)01744-8. [DOI] [PubMed] [Google Scholar]
- 22.Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988–993. doi: 10.1378/chest.10-1269. [DOI] [PubMed] [Google Scholar]
- 23.Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, Pochettino A, Kotloff RM. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167:735–740. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- 24.Zamanian RT, Haddad F, Doyle RL, Weinacker AB. Management strategies for patients with pulmonary hypertension in the intensive care unit. Crit Care Med. 2007;35:2037–2050. doi: 10.1097/01.ccm.0000280433.74246.9e. [DOI] [PubMed] [Google Scholar]
- 25.Connors AF, Jr, Speroff T, Dawson NV, Thomas C, Harrell FE, Jr, Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM, et al. SUPPORT Investigators. The effectiveness of right heart catheterization in the initial care of critically ill patients. JAMA. 1996;276:889–897. doi: 10.1001/jama.276.11.889. [DOI] [PubMed] [Google Scholar]
- 26.Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, Brampton W, Williams D, Young D, Rowan K PAC-Man study collaboration. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet. 2005;366:472–477. doi: 10.1016/S0140-6736(05)67061-4. [DOI] [PubMed] [Google Scholar]
- 27.Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 28.Richard C, Warszawski J, Anguel N, Deye N, Combes A, Barnoud D, Boulain T, Lefort Y, Fartoukh M, Baud F, et al. French Pulmonary Artery Catheter Study Group. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2003;290:2713–2720. doi: 10.1001/jama.290.20.2713. [DOI] [PubMed] [Google Scholar]
- 29.Hadian M, Pinsky MR. Evidence-based review of the use of the pulmonary artery catheter: impact data and complications. Crit Care. 2006;10:S8. doi: 10.1186/cc4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, Laporta DP, Viner S, Passerini L, Devitt H, et al. Canadian Critical Care Clinical Trials Group. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5–14. doi: 10.1056/NEJMoa021108. [DOI] [PubMed] [Google Scholar]
- 31.Price LC, Wort SJ, Finney SJ, Marino PS, Brett SJ. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care. 2010;14:R169. doi: 10.1186/cc9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvey RM, Enson Y, Betti R, Lewis ML, Rochester DF, Ferrer MI. Further observations on the effect of hydrogen ion on the pulmonary circulation. Circulation. 1967;35:1019–1027. doi: 10.1161/01.cir.35.6.1019. [DOI] [PubMed] [Google Scholar]
- 33.Rudolph AM, Yuan S. Response of the pulmonary vasculature to hypoxia and H+ ion concentration changes. J Clin Invest. 1966;45:399–411. doi: 10.1172/JCI105355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lumb AB. The pulmonary circulation. In: Nunn's applied respiratory physiologyWoburn: Butterworth-Heinemann2000p152 [Google Scholar]
- 35.Howell JB, Permutt S, Proctor DF, Riley RL. Effect of inflation of the lung on different parts of pulmonary vascular bed. J Appl Physiol. 1961;16:71–76. doi: 10.1152/jappl.1961.16.1.71. [DOI] [PubMed] [Google Scholar]
- 36.Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med. 1991;114:464–469. doi: 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- 37.Pittet JF, Morel DR, Hemsen A, Gunning K, Lacroix JS, Suter PM, Lundberg JM. Elevated plasma endothelin-1 concentrations are associated with the severity of illness in patients with sepsis. Ann Surg. 1991;213:261–264. doi: 10.1097/00000658-199103000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sibbald W, Peters S, Lindsay RM. Serotonin and pulmonary hypertension in human septic ARDS. Crit Care Med. 1980;8:490–494. doi: 10.1097/00003246-198009000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Hack CE, De Groot ER, Felt-Bersma RJ, Nuijens JH, Strack Van Schijndel RJ, Eerenberg-Belmer AJ, Thijs LG, Aarden LA. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989;74:1704–1710. [PubMed] [Google Scholar]
- 40.Ogata M, Ohe M, Katayose D, Takishima T. Modulatory role of EDRF in hypoxic contraction of isolated porcine pulmonary arteries. Am J Physiol. 1992;262:H691–H697. doi: 10.1152/ajpheart.1992.262.3.H691. [DOI] [PubMed] [Google Scholar]
- 41.Myers PR, Wright TF, Tanner MA, Adams HR. EDRF and nitric oxide production in cultured endothelial cells: direct inhibition by E. coli endotoxin. Am J Physiol. 1992;262:H710–H718. doi: 10.1152/ajpheart.1992.262.3.H710. [DOI] [PubMed] [Google Scholar]
- 42.Aihara M, Nakazawa T, Dobashi K, Joshita T, Kojima M, Onai M, Mori M. A selective pulmonary thrombosis associated with sepsis-induced disseminated intravascular coagulation. Intern Med. 1997;36:97–101. doi: 10.2169/internalmedicine.36.97. [DOI] [PubMed] [Google Scholar]
- 43.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 44.Bull TM, Clark B, McFann K, Moss M National Institutes of Health/National Heart, Lung, and Blood Institute ARDS Network. Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med. 2010;182:1123–1128. doi: 10.1164/rccm.201002-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stolz D, Rasch H, Linka A, Di Valentino M, Meyer A, Brutsche M, Tamm M. A randomised, controlled trial of bosentan in severe COPD. Eur Respir J. 2008;32:619–628. doi: 10.1183/09031936.00011308. [DOI] [PubMed] [Google Scholar]
- 46.Blanco I, Gimeno E, Munoz PA, Pizarro S, Gistau C, Rodriguez-Roisin R, Roca J, Barberà JA. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med. 2010;181:270–278. doi: 10.1164/rccm.200907-0988OC. [DOI] [PubMed] [Google Scholar]
- 47.Rimar S, Gillis CN. Pulmonary vasodilation by inhaled nitric oxide after endothelial injury. J Appl Physiol (1985) 1992;73:2179–2183. doi: 10.1152/jappl.1992.73.5.2179. [DOI] [PubMed] [Google Scholar]
- 48.Rich GF, Roos CM, Anderson SM, Urich DC, Daugherty MO, Johns RA. Inhaled nitric oxide: dose response and the effects of blood in the isolated rat lung. J Appl Physiol (1985) 1993;75:1278–1284. doi: 10.1152/jappl.1993.75.3.1278. [DOI] [PubMed] [Google Scholar]
- 49.Walmrath D, Schermuly R, Pilch J, Grimminger F, Seeger W. Effects of inhaled versus intravenous vasodilators in experimental pulmonary hypertension. Eur Respir J. 1997;10:1084–1092. doi: 10.1183/09031936.97.10051084. [DOI] [PubMed] [Google Scholar]
- 50.Dembinski R, Max M, Lopez F, Kuhlen R, Sünner M, Rossaint R. Effect of inhaled nitric oxide in combination with almitrine on ventilation–perfusion distributions in experimental lung injury. Intensive Care Med. 2000;26:221–228. doi: 10.1007/s001340050051. [DOI] [PubMed] [Google Scholar]
- 51.Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis K, Jr, Kelly KM, Smith TC, Small RJ Inhaled Nitric Oxide in ARDS Study Group. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291:1603–1609. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 52.Rossaint R, Slama K, Steudel W, Gerlach H, Pappert D, Veit S, Falke K. Effects of inhaled nitric oxide on right ventricular function in severe acute respiratory distress syndrome. Intensive Care Med. 1995;21:197–203. doi: 10.1007/BF01701472. [DOI] [PubMed] [Google Scholar]
- 53.Bhorade S, Christenson J, O’connor M, Lavoie A, Pohlman A, Hall JB. Response to inhaled nitric oxide in patients with acute right heart syndrome. Am J Respir Crit Care Med. 1999;159:571–579. doi: 10.1164/ajrccm.159.2.9804127. [DOI] [PubMed] [Google Scholar]
- 54.Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Inhaled prostacyclin is safe, effective, and affordable in patients with pulmonary hypertension, right-heart dysfunction, and refractory hypoxemia after cardiothoracic surgery. J Thorac Cardiovasc Surg. 2004;128:949–950. doi: 10.1016/j.jtcvs.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 55.Eichelbrönner O, Reinelt H, Wiedeck H, Mezödy M, Rossaint R, Georgieff M, Radermacher P. Aerosolized prostacyclin and inhaled nitric oxide in septic shock—different effects on splanchnic oxygenation? Intensive Care Med. 1996;22:880–887. doi: 10.1007/BF02044111. [DOI] [PubMed] [Google Scholar]
- 56.Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A, et al. Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) Study Group Tadalafil therapy for pulmonary arterial hypertension Circulation 20091192894–2903.[Published erratum appears in Circulation 124:e279. Dosage error in article text.] [DOI] [PubMed] [Google Scholar]
- 57.Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, et al. Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 58.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 59.Galiè N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB, et al. Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy Studies (ARIES) Group. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 60.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB, et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 61.Vachiery JL, Huez S, Gillies H, Layton G, Hayashi N, Gao X, Naeije R. Safety, tolerability and pharmacokinetics of an intravenous bolus of sildenafil in patients with pulmonary arterial hypertension. Br J Clin Pharmacol. 2011;71:289–292. doi: 10.1111/j.1365-2125.2010.03831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cornet AD, Hofstra JJ, Swart EL, Girbes AR, Juffermans NP. Sildenafil attenuates pulmonary arterial pressure but does not improve oxygenation during ARDS. Intensive Care Med. 2010;36:758–764. doi: 10.1007/s00134-010-1754-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Packer M, Medina N, Yushak M. Adverse hemodynamic and clinical effects of calcium channel blockade in pulmonary hypertension secondary to obliterative pulmonary vascular disease. J Am Coll Cardiol. 1984;4:890–901. doi: 10.1016/s0735-1097(84)80048-0. [DOI] [PubMed] [Google Scholar]
- 64.Hoffman MJ, Lazar JG, Sugerman HF, Tatum JL. Unsuspected right ventricular dysfunction in shock and sepsis. Ann Surg. 1983;198:307–318. doi: 10.1097/00000658-198309000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitsuo T, Shimazaki S, Matsuda H. Right ventricular dysfunction in septic patients. Crit Care Med. 1992;20:630–634. doi: 10.1097/00003246-199205000-00014. [DOI] [PubMed] [Google Scholar]
- 66.Urabe Y, Tomoike H, Ohzono K, Koyanagi S, Nakamura M. Role of afterload in determining regional right ventricular performance during coronary underperfusion in dogs. Circ Res. 1985;57:96–104. doi: 10.1161/01.res.57.1.96. [DOI] [PubMed] [Google Scholar]
- 67.van Wolferen SA, Marcus JT, Westerhof N, Spreeuwenberg MD, Marques KM, Bronzwaer JG, Henkens IR, Gan CT, Boonstra A, Postmus PE, et al. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur Heart J. 2008;29:120–127. doi: 10.1093/eurheartj/ehm567. [DOI] [PubMed] [Google Scholar]
- 68.Kleinman WM, Krause SM, Hess ML. Differential subendocardial perfusion and injury during the course of gram-negative endotoxemia. Adv Shock Res. 1980;4:139–152. [PubMed] [Google Scholar]
- 69.Gold FL, Bache RJ. Transmural right ventricular blood flow during acute pulmonary artery hypertension in the sedated dog:evidence for subendocardial ischemia despite residual vasodilator reserve. Circ Res. 1982;51:196–204. doi: 10.1161/01.res.51.2.196. [DOI] [PubMed] [Google Scholar]
- 70.Lowensohn HS, Khouri EM, Gregg DE, Pyle RL, Patterson RE. Phasic right coronary artery blood flow in conscious dogs with normal and elevated right ventricular pressures. Circ Res. 1976;39:760–766. doi: 10.1161/01.res.39.6.760. [DOI] [PubMed] [Google Scholar]
- 71.Vlahakes GJ, Turley K, Hoffman JI. The pathophysiology of failure in acute right ventricular hypertension: hemodynamic and biochemical correlations. Circulation. 1981;63:87–95. doi: 10.1161/01.cir.63.1.87. [DOI] [PubMed] [Google Scholar]
- 72.Hollenberg SM. Vasoactive drugs in circulatory shock. Am J Respir Crit Care Med. 2011;183:847–855. doi: 10.1164/rccm.201006-0972CI. [DOI] [PubMed] [Google Scholar]
- 73.Kerbaul F, Rondelet B, Motte S, Fesler P, Hubloue I, Ewalenko P, Naeije R, Brimioulle S. Effects of norepinephrine and dobutamine on pressure load-induced right ventricular failure. Crit Care Med. 2004;32:1035–1040. doi: 10.1097/01.ccm.0000120052.77953.07. [DOI] [PubMed] [Google Scholar]
- 74.Hirsch LJ, Rooney MW, Wat SS, Kleinmann B, Mathru M. Norepinephrine and phenylephrine effects on right ventricular function in experimental canine pulmonary embolism. Chest. 1991;100:796–801. doi: 10.1378/chest.100.3.796. [DOI] [PubMed] [Google Scholar]
- 75.Ducas J, Duval D, Dasilva H, Boiteau P, Prewitt RM. Treatment of canine pulmonary hypertension: effects of norepinephrine and isoproterenol on pulmonary vascular pressure–flow characteristics. Circulation. 1987;75:235–242. doi: 10.1161/01.cir.75.1.235. [DOI] [PubMed] [Google Scholar]
- 76.Schreuder WO, Schneider AJ, Groeneveld AB, Thijs LG. Effect of dopamine vs norepinephrine on hemodynamics in septic shock: emphasis on right ventricular performance. Chest. 1989;95:1282–1288. doi: 10.1378/chest.95.6.1282. [DOI] [PubMed] [Google Scholar]
- 77.Rich S, Gubin S, Hart K. The effects of phenylephrine on right ventricular performance in patients with pulmonary hypertension. Chest. 1990;98:1102–1106. doi: 10.1378/chest.98.5.1102. [DOI] [PubMed] [Google Scholar]
- 78.Cheung PY, Barrington KJ. The effects of dopamine and epinephrine on hemodynamics and oxygen metabolism in hypoxic anesthetized piglets. Crit Care. 2001;5:158–166. doi: 10.1186/cc1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Le Tulzo Y, Seguin P, Gacouin A, Camus C, Suprin E, Jouannic I, Thomas R. Effects of epinephrine on right ventricular function in patients with severe septic shock and right ventricular failure: a preliminary descriptive study. Intensive Care Med. 1997;23:664–670. doi: 10.1007/s001340050391. [DOI] [PubMed] [Google Scholar]
- 80.Evora PR, Pearson PJ, Schaff HV. Arginine vasopressin induces endothelium-dependent vasodilatation of the pulmonary artery: V1-receptor–mediated production of nitric oxide. Chest. 1993;103:1241–1245. doi: 10.1378/chest.103.4.1241. [DOI] [PubMed] [Google Scholar]
- 81.Leather HA, Segers P, Berends N, Vandermeersch E, Wouters PF. Effects of vasopressin on right ventricular function in an experimental model of acute pulmonary hypertension. Crit Care Med. 2002;30:2548–2552. doi: 10.1097/00003246-200211000-00024. [DOI] [PubMed] [Google Scholar]
- 82.Indrambarya T, Boyd JH, Wang Y, McConechy M, Walley KR. Low-dose vasopressin infusion results in increased mortality and cardiac dysfunction following ischemia–reperfusion injury in mice. Crit Care. 2009;13:R98. doi: 10.1186/cc7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lejeune P, Naeije R, Leeman M, Melot C, Deloof T, Delcroix M. Effects of dopamine and dobutamine on hyperoxic and hypoxic pulmonary vascular tone in dogs. Am Rev Respir Dis. 1987;136:29–35. doi: 10.1164/ajrccm/136.1.29. [DOI] [PubMed] [Google Scholar]
- 84.Holloway EL, Polumbo RA, Harrison DC. Acute circulatory effects of dopamine in patients with pulmonary hypertension. Br Heart J. 1975;37:482–485. doi: 10.1136/hrt.37.5.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leier CV, Heban PT, Huss P, Bush CA, Lewis RP. Comparative systemic and regional hemodynamic effects of dopamine and dobutamine in patients with cardiomyopathic heart failure. Circulation. 1978;58:466–475. doi: 10.1161/01.cir.58.3.466. [DOI] [PubMed] [Google Scholar]
- 86.Ferrario M, Poli A, Previtali M, Lanzarini L, Fetiveau R, Diotallevi P, Mussini A, Montemartini C. Hemodynamics of volume loading compared with dobutamine in severe right ventricular infarction. Am J Cardiol. 1994;74:329–333. doi: 10.1016/0002-9149(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 87.Acosta F, Sansano T, Palenciano CG, Falcon L, Domenech P, Robles R, Bueno FS, Ramirez P, Parrilla P. Effects of dobutamine on right ventricular function and pulmonary circulation in pulmonary hypertension during liver transplantation. Transplant Proc. 2005;37:3869–3870. doi: 10.1016/j.transproceed.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 88.Chen EP, Bittner HB, Davis RD, Jr, Van Trigt P., III Milrinone improves pulmonary hemodynamics and right ventricular function in chronic pulmonary hypertension. Ann Thorac Surg. 1997;63:814–821. doi: 10.1016/s0003-4975(97)00011-8. [DOI] [PubMed] [Google Scholar]
- 89.Deb B, Bradford K, Pearl RG. Additive effects of inhaled nitric oxide and intravenous milrinone in experimental pulmonary hypertension. Crit Care Med. 2000;28:795–799. doi: 10.1097/00003246-200003000-00031. [DOI] [PubMed] [Google Scholar]
- 90.Oztekin I, Yazici S, Oztekin DS, Goksel O, Issever H, Canik S. Effects of low-dose milrinone on weaning from cardiopulmonary bypass and after in patients with mitral stenosis and pulmonary hypertension. Yakugaku Zasshi. 2007;127:375–383. doi: 10.1248/yakushi.127.375. [DOI] [PubMed] [Google Scholar]
- 91.Kihara S, Kawai A, Fukuda T, Yamamoto N, Aomi S, Nishida H, Endo M, Koyanagi H. Effects of milrinone for right ventricular failure after left ventricular assist device implantation. Heart Vessels. 2002;16:69–71. doi: 10.1007/s380-002-8320-z. [DOI] [PubMed] [Google Scholar]
- 92.Eichhorn EJ, Konstam MA, Weiland DS, Roberts DJ, Martin TT, Stransky NB, Salem DN. Differential effects of milrinone and dobutamine on right ventricular preload, afterload and systolic performance in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1987;60:1329–1333. doi: 10.1016/0002-9149(87)90616-3. [DOI] [PubMed] [Google Scholar]
- 93.Singh R, Choudhury M, Saxena A, Kapoor PM, Juneja R, Kiran U. Inhaled nitroglycerin versus inhaled milrinone in children with congenital heart disease suffering from pulmonary artery hypertension. J Cardiothorac Vasc Anesth. 2010;24:797–801. doi: 10.1053/j.jvca.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 94.Sablotzki A, Starzmann W, Scheubel R, Grond S, Czeslick EG. Selective pulmonary vasodilation with inhaled aerosolized milrinone in heart transplant candidates. Can J Anaesth. 2005;52:1076–1082. doi: 10.1007/BF03021608. [DOI] [PubMed] [Google Scholar]
- 95.Wang H, Gong M, Zhou B, Dai A. Comparison of inhaled and intravenous milrinone in patients with pulmonary hypertension undergoing mitral valve surgery. Adv Ther. 2009;26:462–468. doi: 10.1007/s12325-009-0019-4. [DOI] [PubMed] [Google Scholar]
- 96.Haraldsson s A, Kieler-Jensen N, Ricksten SE.The additive pulmonary vasodilatory effects of inhaled prostacyclin and inhaled milrinone in postcardiac surgical patients with pulmonary hypertension Anesth Analg 2001931439–1445.Table of contents [DOI] [PubMed] [Google Scholar]
- 97.Parissis JT, Paraskevaidis I, Bistola V, Farmakis D, Panou F, Kourea K, Nikolaou M, Filippatos G, Kremastinos D. Effects of levosimendan on right ventricular function in patients with advanced heart failure. Am J Cardiol. 2006;98:1489–1492. doi: 10.1016/j.amjcard.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 98.Pitsiou G, Paspala A, Bagalas V, Boutou AK, Stanopoulos I. Inhaled iloprost plus levosimendan to decompensate right heart failure due to chronic thromboembolic pulmonary hypertension. Anaesth Intensive Care. 2013;41:554–556. [PubMed] [Google Scholar]
- 99.Barisin S, Djuzel V, Barisin A, Rudez I.Levosimendan reverses right-heart failure in a 51-year old patient after heart transplantation Wien Klin Wochenschr(In press) [DOI] [PubMed] [Google Scholar]
- 100.Maggio P, Hemmila M, Haft J, Bartlett R. Extracorporeal life support for massive pulmonary embolism. J Trauma. 2007;62:570–576. doi: 10.1097/TA.0b013e318031cd0c. [DOI] [PubMed] [Google Scholar]
- 101.Kolvekar SK, Peek GJ, Sosnowski AW, Firmin RK. Extracorporeal membrane oxygenator for pulmonary embolism. Ann Thorac Surg. 1997;64:883–884. [PubMed] [Google Scholar]
- 102.Deehring R, Kiss AB, Garrett A, Hillier AG. Extracorporeal membrane oxygenation as a bridge to surgical embolectomy in acute fulminant pulmonary embolism. Am J Emerg Med. 2006;24:879–880. doi: 10.1016/j.ajem.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 103.Olsson KM, Simon A, Strueber M, Hadem J, Wiesner O, Gottlieb J, Fuehner T, Fischer S, Warnecke G, Kühn C, et al. Extracorporeal membrane oxygenation in nonintubated patients as bridge to lung transplantation. Am J Transplant. 2010;10:2173–2178. doi: 10.1111/j.1600-6143.2010.03192.x. [DOI] [PubMed] [Google Scholar]
- 104.de Perrot M, Granton JT, McRae K, Cypel M, Pierre A, Waddell TK, Yasufuku K, Hutcheon M, Chaparro C, Singer L, et al. Impact of extracorporeal life support on outcome in patients with idiopathic pulmonary arterial hypertension awaiting lung transplantation. J Heart Lung Transplant. 2011;30:997–1002. doi: 10.1016/j.healun.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 105.Gregoric ID, Chandra D, Myers TJ, Scheinin SA, Loyalka P, Kar B. Extracorporeal membrane oxygenation as a bridge to emergency heart–lung transplantation in a patient with idiopathic pulmonary arterial hypertension. J Heart Lung Transplant. 2008;27:466–468. doi: 10.1016/j.healun.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 106.Berman M, Tsui S, Vuylsteke A, Snell A, Colah S, Latimer R, Hall R, Arrowsmith JE, Kneeshaw J, Klein AA, et al. Successful extracorporeal membrane oxygenation support after pulmonary thromboendarterectomy. Ann Thorac Surg. 2008;86:1261–1267. doi: 10.1016/j.athoracsur.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 107.Mydin M, Berman M, Klein A, Tsui S, Dunning J, Valchanov K, Vuylsteke A, Jenkins DP. Extracorporeal membrane oxygenation as a bridge to pulmonary endarterectomy. Ann Thorac Surg. 2011;92:e101–e103. doi: 10.1016/j.athoracsur.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 108.Srivastava MC, Ramani GV, Garcia JP, Griffith BP, Uber PA, Park MH. Veno-venous extracorporeal membrane oxygenation bridging to pharmacotherapy in pulmonary arterial hypertensive crisis. J Heart Lung Transplant. 2010;29:811–813. doi: 10.1016/j.healun.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 109.Rosenzweig EB, Brodie D, Abrams DC, Agerstrand CL, Bacchetta M. Extracorporeal membrane oxygenation as a novel bridging strategy for acute right heart failure in group 1 pulmonary arterial hypertension. ASAIO J. 2014;60:129–133. doi: 10.1097/MAT.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 110.Keogh AM, Mayer E, Benza RL, Corris P, Dartevelle PG, Frost AE, Kim NH, Lang IM, Pepke-Zaba J, Sandoval J. Interventional and surgical modalities of treatment in pulmonary hypertension. J Am Coll Cardiol. 2009;54(1) Suppl:S67–S77. doi: 10.1016/j.jacc.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 111.Strueber M, Hoeper MM, Fischer S, Cypel M, Warnecke G, Gottlieb J, Pierre A, Welte T, Haverich A, Simon AR, et al. Bridge to thoracic organ transplantation in patients with pulmonary arterial hypertension using a pumpless lung assist device. Am J Transplant. 2009;9:853–857. doi: 10.1111/j.1600-6143.2009.02549.x. [DOI] [PubMed] [Google Scholar]
- 112.Schmid C, Philipp A, Hilker M, Arlt M, Trabold B, Pfeiffer M, Schmid FX. Bridge to lung transplantation through a pulmonary artery to left atrial oxygenator circuit. Ann Thorac Surg. 2008;85:1202–1205. doi: 10.1016/j.athoracsur.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 113.Braunwald E.Pathophysiology of heart failure. In: Braunwald E, editor. Heart disease: a textbook of cardiovascular medicine. Philadelphia: Saunders; 1980. pp. 453–471 [Google Scholar]
- 114.Murray JF.The normal lung. Murray JF, editor. Philadelphia: W. B. Saunders Co.; 1986 [Google Scholar]
- 115.Cucci AR, Kline JA, Lahm T. Acute RV failure. In: Voelkel NF, Schranz D, editors. The RV in health and disease. New York: Humana Press; 2014.