Abstract
Rationale: Better phenotypic descriptions are needed for chronic lung disease among surviving premature infants.
Objectives: The purpose of this study was to evaluate the potential usefulness of respiratory inductance plethysmography in characterizing respiratory system mechanics in preterm infants at 32 weeks postmenstrual age.
Methods: Respiratory inductance plethysmography was used to obtain the phase angle, Φ, to describe rib cage and abdominal dyssynchrony in 65 infants born between 23 and 28 weeks gestation, all of whom were studied at 32 weeks postmenstrual age. Up to 60 breaths were evaluated for each subject. Sources of intrasubject variability in Φ arising from our methods were explored using mechanical models and by evaluating interobserver agreement.
Measurements and Main Results: The mean Φ from infants ranged from 5.8–162.9°, with intrasubject coefficients of variation ranging from 11–123%. On the basis of the mechanical model studies, respiratory inductance plethysmography recording and analysis software added <2.3% to the intrasubject variability in Φ. Potential inconsistencies in breaths selected could have contributed 8.1%, on average, to the total variability. The recording sessions captured 22.8 ± 9.1 minutes of quiet sleep, and enough breaths were counted to adequately characterize the range of Φ in the session.
Conclusion: Φ is quite variable during even short recording sessions among preterm infants sleeping quietly. The intrasubject variability described herein arises from the instability of the rib cage and abdominal phase relationship, not from the recording and analytical methods used. Despite the variability, Φ measurements allowed the majority (80%) of infants to be reliably categorized as having relatively synchronous or dyssynchronous breathing. Respiratory inductance plethysmography is easy to use and should prove useful in quantifying respiratory mechanics in multicenter studies of preterm infants.
Keywords: inductance plethysmography, premature infant respiration, phase angle
Chronic lung disease (CLD) complicates up to 35–45% of preterm births occurring between 24 and 28 weeks postmenstrual age (PMA) (1). The primary criterion used to label an infant with CLD is the apparent need for supplemental oxygen at 36 weeks PMA (2). There are problems, however, with equating this “phenotype” with CLD. For example, infants with minimal airways or airspace disease may have unstable oxyhemoglobin saturation because of periodic breathing (3–5). Furthermore, it is not clear how often unstable respiratory system mechanics are responsible for the apparent need for supplemental O2.
One barrier to a “higher-resolution” understanding of the mechanisms of CLD and to improving its phenotypic description is the paucity of validated methods that describe respiratory system mechanics and yield continuous rather than categorical results. One noninvasive method, respiratory inductance plethysmography (RIP), provides a continuous quantitative result, the phase angle (Φ), that reflects respiratory system resistance and compliance (6). Φ derived from RIP is used to quantify the degree of rib cage and abdominal movement dyssynchrony. Φ is 180° when dyssynchrony is complete, 0° when the rib cage and abdomen move in complete synchrony, and between 1–179° with increasing dyssynchrony.
Studies in infants have shown the potential usefulness of RIP in characterizing respiratory system mechanics in newborns. In a previous study, Φ was found to be 9–12° in healthy term infants, 38–60° in healthy preterm infants at 35–36 weeks PMA, and 70° or more in older infants with bronchopulmonary dysplasia (7). We therefore are using RIP as part of a large multicenter study in an attempt to quantify respiratory mechanics noninvasively in premature infants.
Early results of the study at our center show that Φ derived from RIP is quite variable during brief recordings. It is our hypothesis that this variability in Φ in premature infants arises from the infants themselves and not from the methods used for recording and analysis.
Methods
Study Center
This evaluation was conducted at Washington University School of Medicine and St. Louis Children’s Hospital as a single-center study within a larger multicenter collaboration (Premature Respiratory Outcome Program [PROP]) (8, 9). The study protocol was reviewed and approved by the Washington University Human Research Protection Office and the PROP Observational Studies Monitoring Board.
Subjects
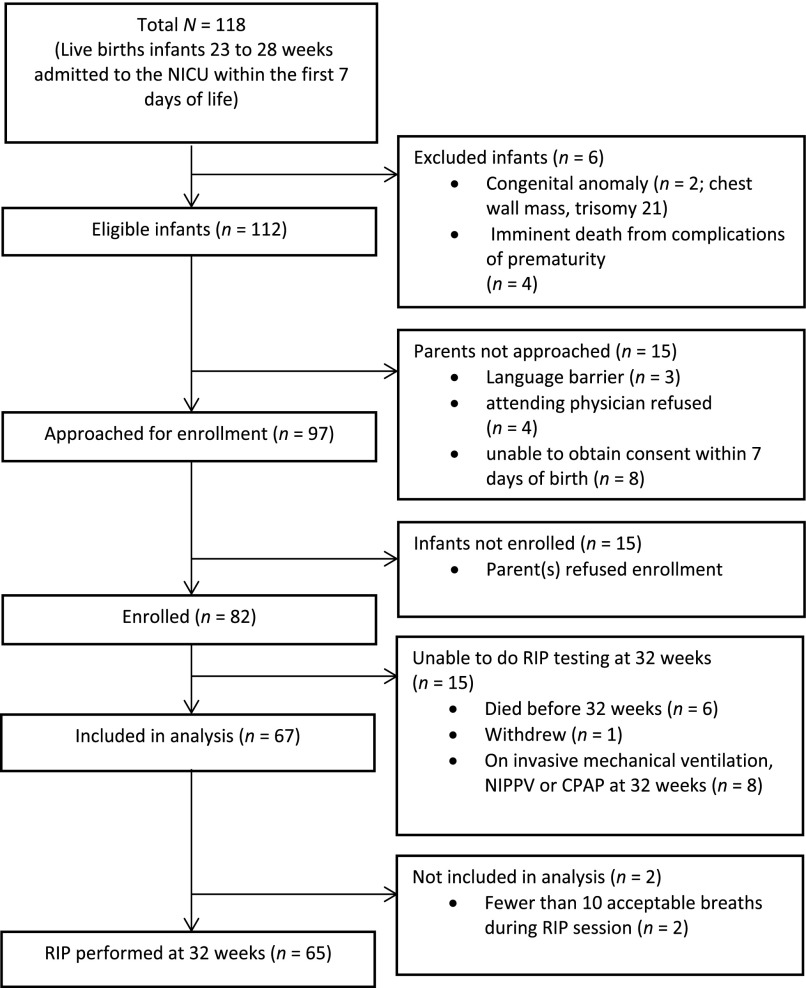
Premature infants born at 23–28 weeks gestation between August 12, 2010, and December 11, 2012, and hospitalized in the Newborn Intensive Care Unit at St. Louis Children’s Hospital were screened for enrollment at birth to be studied at 32 weeks PMA. PMA was the convention used within PROP for describing combined gestational and postnatal age. Exclusion criteria included known congenital anomalies or imminent death due to complications of prematurity. Families of all eligible infants admitted to the Newborn Intensive Care Unit were approached within 7 days of birth after acquiring permission from their attending neonatologists. Families were approached again at 32 weeks PMA prior to testing to confirm their informed consent. A sequential sample of 65 infants born between 23–28 weeks PMA was recruited for this study (Figure 1 and Table 1). The purpose of this study was to quantify intrasubject variability in Φ and how much of that variability arose from the methods used. The impact of supplemental oxygen or airflow rate on Φ was not addressed, and thus all infants were continued on the supplemental oxygen and flow rates as prescribed by their attending neonatologists. The fraction of inspired O2 ranged from 0.21–1.0, and flow rates were 0–5 L/min.
Figure 1.
Recruitment tree. NICU, Neonatal Intensive Care Unit.
Table 1.
Patient characteristics
| Infant Characteristics | Data |
|---|---|
| Gestational age (wk) | 26-6/7 wk (±9 d) |
| Age at time of testing (d) | 36 days (±9) |
| Birth weight | 940 g (±210) |
| Sex | Female (n = 39); male (n = 26) |
| Race | Black (n = 36); white (n = 28); Asian (n = 1) |
Respiratory Inductance Plethysmography in Infants at 32 Weeks PMA
The Φ obtained by RIP was the measurement of interest. Breaths occurring during behaviorally determined quiet sleep (QS) were selected. QS was defined by eyes closed, regular breathing, and no fluttering of eyelids or limbs (10, 11). The goal for each infant was to record at least 15 minutes of QS. All infants were spontaneously breathing, and none were supported by mechanical ventilation.
Measurements were obtained after a feeding with the infant supine in his or her incubator in the Newborn Intensive Care Unit. Two RIP bands, 38.0 cm long unstretched and 2.0 cm wide, were used, one at the nipple line and one at the umbilicus, to record rib cage and abdominal wall excursion. Scalar tracings from RIP were recorded in a cribside laptop computer using proprietary hardware and software (Biocapture; Cleveland Medical Devices, Cleveland, OH). The tracings were annotated every 3 minutes by an experienced sleep laboratory technician (C.M.C.) at cribside. The infant’s state was labeled as QS or rapid eye movement sleep/awake.
Selection and Analysis of Breaths
The duration of QS recorded for the 65 infants was 22.8 ± 9.1 minutes (range, 8–63 min). The investigators used the following standard protocol to choose breaths for analysis. (1) The duration in QS was divided into thirds, and the first 20 acceptable breaths within each time tercile were selected. (2) Acceptable breaths were defined by producing single closed or nearly closed Konno-Mead loops (12) when the thoracic and abdominal wall excursion tracings were plotted against one another (loops creating a figure-eight were not used). (3) If a time tercile did not include 20 acceptable breaths, fewer than 20 breaths from that tercile were used.
Our goal was to analyze a total of 60 acceptable breaths, 20 from the beginning of each time tercile. Of the 65 infants, 50 had 60 acceptable breaths. The 15 with fewer than 60 acceptable breaths had a median of 50 breaths (range, 19–58). Scalar tracings and corresponding rib cage versus abdominal Konno-Mead plots were inspected. Φ values were calculated using Φ = sin−1 (m/s) (13).
Variability of Phase Angle within Each Recording Session
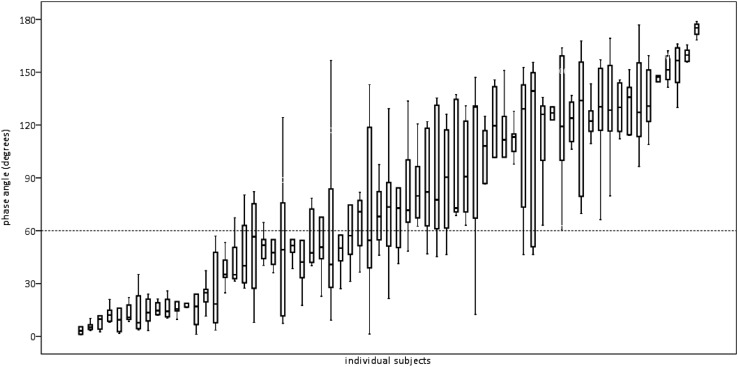
The between-time tercile variability in Φ for the selected breaths from each infant, usually 20 breaths from each time tercile, was compared using analysis of variance (ANOVA) or the Kruskal-Wallis test when appropriate. All statistical analysis was done using the IBM SPSS statistical software package (SPSS, Chicago, IL). The results for all breaths used from each infant were also described using means, SDs, medians, interquartile ranges, and coefficients of variation (Figure 2).
Figure 2.
Φ results for all breaths for each of 65 subjects. Plotted are the medians and the 25th and 75th percentiles with ranges. Of the 65 infants, 50 had 60 acceptable breaths and 15 had fewer than 60 acceptable breaths (median, 50 breaths; range, 19–58 breaths). Line at 60° is drawn to provide a magnitude context for these results by showing the mean value for Φ derived from a published report on infants with CLD and is not meant to indicate a cut point for normal versus abnormal (14). Indeed, the intent in assessing RIP is to validate a continuous variable.
Intraobserver Variability
It was unusual to identify 20 acceptable breaths at the beginning of each approximately 7-minute time tercile, making it necessary to select breaths from later in the tercile. Even in QS, Konno-Mead plots were often partially open, making breath selection potentially observer-dependent (14). To analyze the impact of interobserver differences in breaths chosen for Φ calculation, 25 of the 65 RIP tracings were inspected for acceptable breaths independently by two observers (L.N.U., J.S.K.). The two sets of Φ values from acceptable breaths chosen were then compared using Student’s t test or the Mann-Whitney U test. The more familiar techniques for assessing interobserver variability (e.g., Cohen’s κ test) were not appropriate, because there was no assurance that the same breaths were chosen by both observers. Furthermore, if both observers chose the same breaths, Φ values would have been identical.
Respiratory Inductance Plethysmography Using Mechanical Models
The purpose of using simple mechanical models was to evaluate variability arising from the thoracic and abdominal excursion recording acquisition hardware and software, as well as from the software used to evaluate the Konno-Mead plots and calculate the Φ value (VivoSense; Vivonoetics, San Diego, CA). Potential variability at two different “ventilatory” frequencies (Vf) was evaluated using simulations of synchronous and dyssynchronous breathing. Synchronous breathing was simulated using a 1-L anesthesia bag with two RIP bands placed contiguously on the approximate middle of the bag. The bag was initially inflated so that the bands could stay in place (the end-expiratory volume [EEV]) and so the bag could be “ventilated” at approximately 40 and 60 breaths per minute. The bag was gently inflated by hand using a syringe and emptied passively to EEV so that the bands did not change position. The volumes and pressures delivered by hand were not measured, as small breath-to-breath changes, within reasonable limits, should not cause discrepancies in measurements, such as Φ, based on timing rather than on volume or flow rate.
Dyssynchronous breathing was simulated using two 1-L anesthesia bags, each initially inflated so that a single RIP band stayed around the middle of the bag. The bags were further inflated dyssynchronously, but at the same frequencies, by separate mechanical ventilators at both 40 and 60 breaths per minute. We did not attempt to achieve complete dyssynchrony (i.e., Φ = 180°), but we sought to achieve obvious visible dyssynchrony. The settings were the same for both ventilators—frequency of 40 or 60, tidal volume of 60 ml, EEP of 5 cm H2O, and inspiratory time of 0.19 seconds.
How Many Breaths Should Be Used?
Whether infants with or without CLD could be misclassified by basing results for Φ on a small number of breaths is not known. The optimal number of breaths to be analyzed over the recording period was evaluated by comparing the mean Φ from 60 breaths, or all acceptable breaths if <60, to an estimate obtained when fewer breaths were included (15). Breaths from each tercile were used in all comparisons. Breaths were divided into 10 consecutive groups of 6 breaths. For the comparison of the mean of all breaths used with 10 breaths, the first breath from each group of 6 breaths was used. For the comparison with 20 breaths, the first 2 breaths from each group of 6 breaths were used. The latter method was continued for comparison with 30, 40, and 50 breaths.
Results
Subjects
The weight, age, and demographic information of the subjects are shown in Table 1. The sequence for subject selection is shown in Figure 1.
Variability of Phase Angle within Each Recording Session
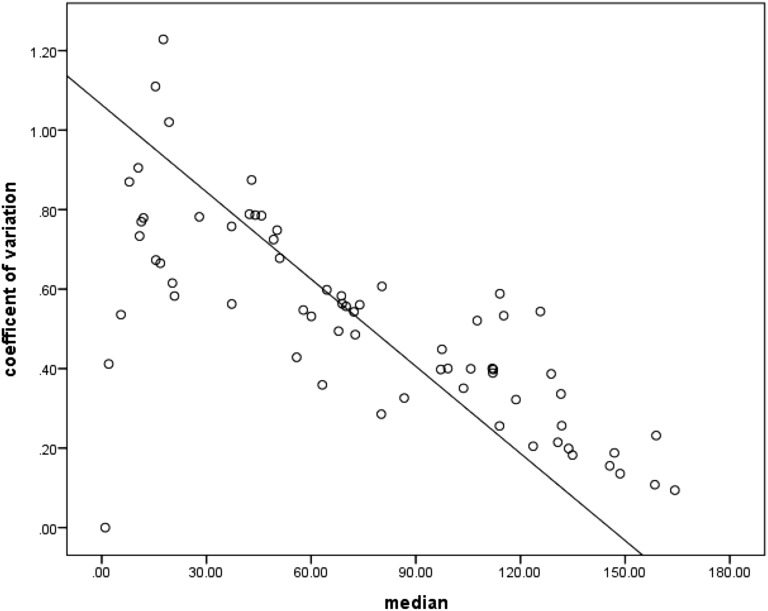
Figure 2 shows the Φ results for each infant. Coefficients of variation for Φ values for all 65 subjects ranged from 11–123%. Figure 3 explores the patterns of variability shown in Figure 2 in more detail.
Figure 3.
Coefficients of variation in Φ are plotted versus the median Φ for the breaths chosen. This figure illustrates that the variability shown in Figure 2, when corrected for Φ size, is actually larger at smaller Φ values and decreases with increases in the size of Φ. The smaller coefficient of variation for the larger Φ values likely reflects a “ceiling effect,” with no values >180° possible.
When consecutive acceptable breaths from each of the time terciles are compared, significant intrasession variability among the three sets of 20 breaths was found in 36 of the 65 subjects (P < 0.05 by ANOVA or Kruskal-Wallis test).
Mean Φ values and their coefficients of variation did not differ between infants not receiving high-flow cannula support and those receiving flow rates from 1–5 L/min (P = 0.559 and P = 0.375 by ANOVA, respectively).
Interobserver Variability
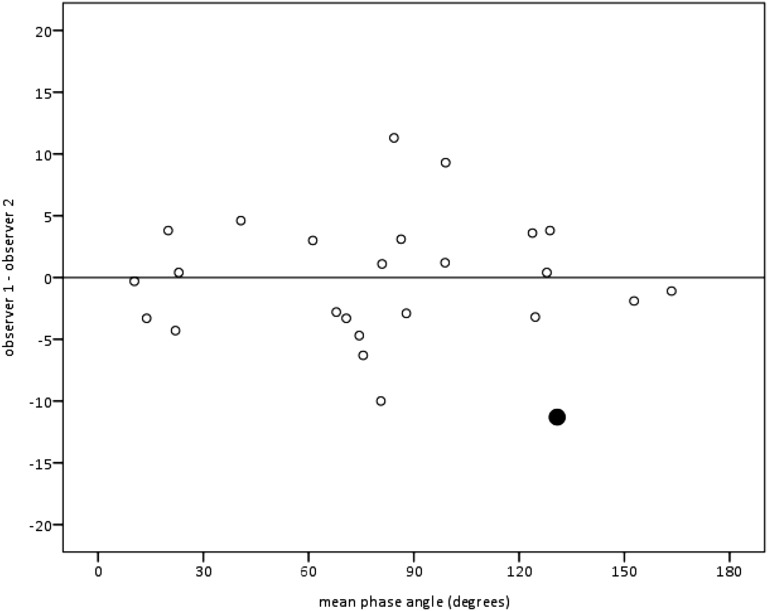
To assess interobserver variability, two investigators (L.N.U. and J.S.K.) independently scored recordings from 25 of the 65 subjects. There were no significant differences in Φ values between the two observers for 24 of the 25 subjects (P = 0.16–0.93 by Student’s t test or Mann-Whitney U test). For the one subject with a statistically significant difference (P = 0.023), the 95% CI for the difference between means was 21.0–5.6° (Figure 4). Both investigators found that rib cage and abdominal dyssynchrony was quite large, with mean Φ >> 100°.
Figure 4.
Interobserver differences for 25 infants illustrated by a Bland-Altman plot showing the differences in mean Φ obtained by two independent observers (L.N.U., J.S.K.) versus the average of their two means (mean observer 1 + mean observer 2, divided by 2). The result indicated by the large filled circle is for the 1 of 25 subjects compared when Φ results differed statistically between the two observers.
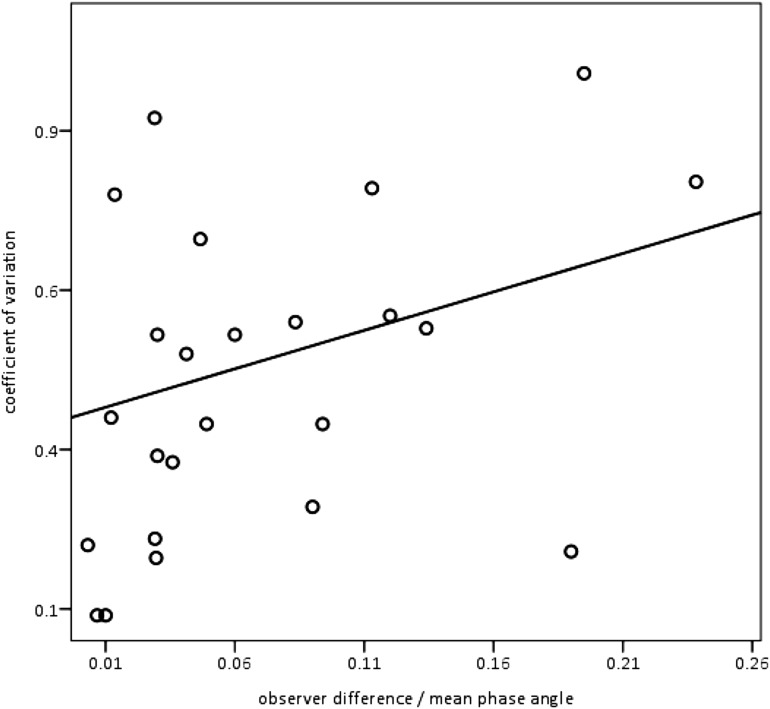
Figure 5 shows how much observer differences in breath selection might have contributed to the variability of the results. Differences in breaths chosen for analysis would have contributed, on average, only 8.1% to the intrasession variability. The results shown in Figure 5 are corrected for the magnitude of Φ (means ranged from 10.3–163.4°) because infants whose mean Φ values were relatively small (e.g., 20°) might still have had a relatively large coefficient of variation but not be wrongly labeled in a hypothetical categorization scheme based on Φ.
Figure 5.
Differences between observers in breath selection had a minimal impact on variability. For the 25 recordings scored by 2 observers, individual subjects’ coefficients of variation are plotted on the y-axis against interobserver differences divided by mean Φ r2 = 0.081. For clarity, dividing by the mean Φ was a necessary size correction because a small mean Φ with, for example, a SD of 10, would have a large coefficient of variation, whereas the same SD would be associated with a much smaller coefficient of variation if the mean Φ were, for example, >> 100°.
Phase Angle Variability when Using Mechanical Models
Figure 6) shows the Φ variability in mechanical models.
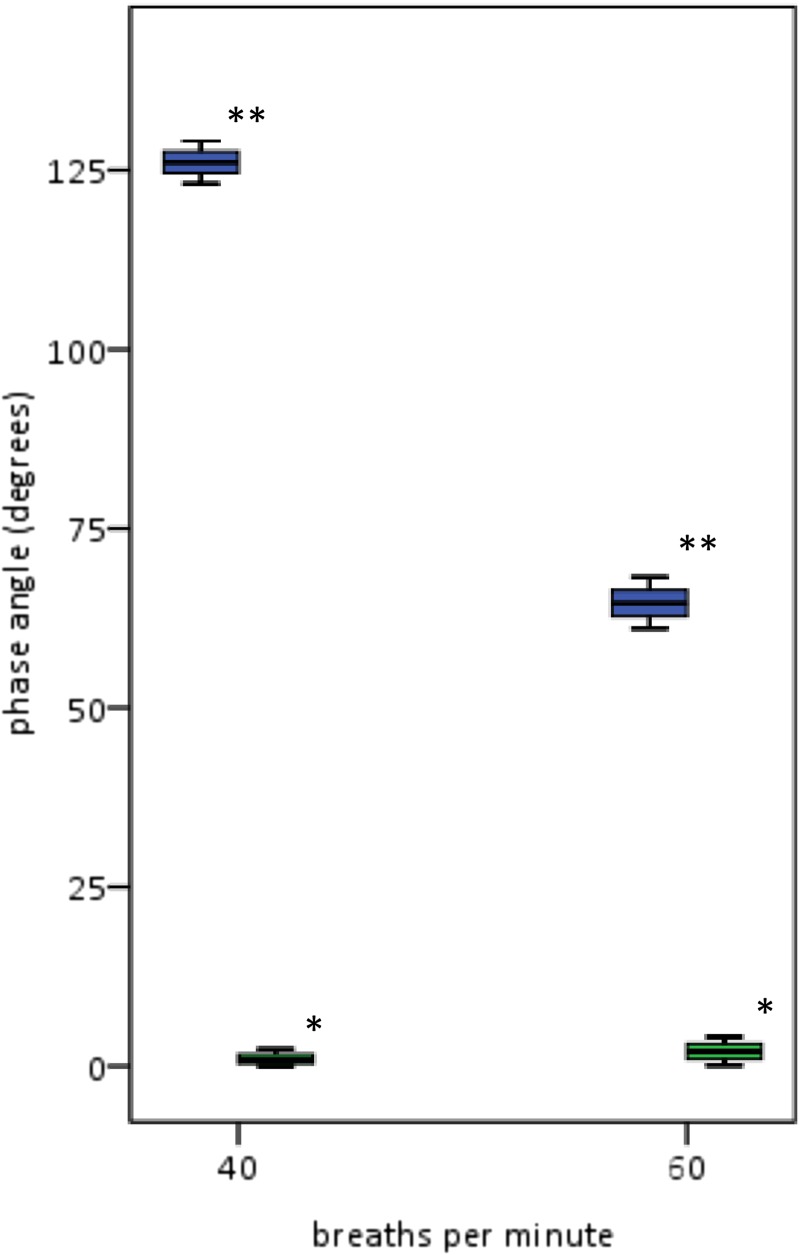
Figure 6.
Φ variability from synchronous and dyssynchronous mechanical models with Vf of approximately 40 and 60 breaths per minute. Shown are the 25th and 75th percentiles and the medians with ranges for Φ from the synchronous model (*) near 0°. For the dyssynchronous model (**), Φ coefficients of variation are 1.1% and 2.3% at Vf of 40 and 60 per minute, respectively. These results show that the recording and analysis equipment contributed minimally to the variability in Φ measurements.
Synchronous.
For the synchronous mechanical model, the expected Φ is 0°. At Vf of approximately 40 breaths per minute, mean Φ = 0.8 ± 0.1°. At Vf of approximately 60 breaths per minute, mean Φ = 1.9 ± 0.1°.
Dyssynchronous.
At Vf of 40 breaths per minute, mean Φ = 126.1 ± 1.4°. At Vf of 60 breaths per minute, mean Φ = 64.6 ± 1.5°. Coefficients of variation were 1.1% and 2.3%, respectively.
Number of Breaths Needed to Adequately Describe the Recording Session
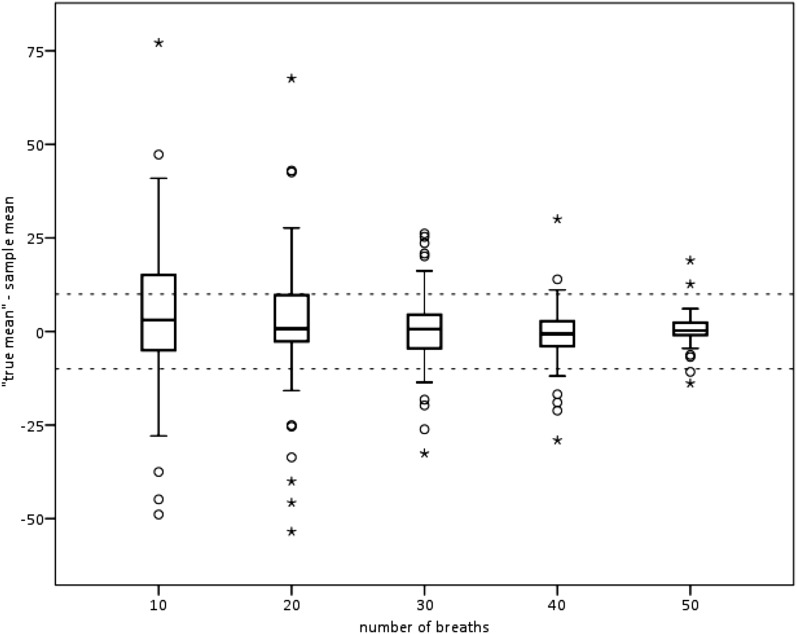
Our a priori assumption was that 60 breaths was enough to evaluate each subject, using 3 sets of 20 breaths. Fifty subjects had sixty breaths, and, for the majority, finding twenty usable breaths during QS within each time tercile was straightforward. More than 50 usable breaths were found in 55 (77%) of 65. Infants with <60 usable breaths had shorter QS times and were more likely to have variable EEVs leading to open Konno-Mead loops. The coefficients of variation for those 50 subjects with 60 usable breaths did not differ from those for the 15 subjects with fewer than 60 breaths (P = 0.98 by Student’s t test). Figure 7) shows the results from those 55 subjects with more than 50 usable breaths (54–60 breaths).
Figure 7.
How many breaths for evaluation are enough? Shown are the absolute differences between mean Φ calculated from 10, 20, 30, 40, and 50 breaths and the mean Φ for 60 breaths, or the highest number counted, for 55 subjects with more than 50 usable breaths. The dashed horizontal lines are at +10° and −10°. By 50 breaths, the mean Φ values were within 10° of the “true” mean for 90% of subjects and within 19° for all 55 subjects with more than 50 usable breaths. Shown are the 25th and 75th percentiles, the median, the 10th and 90th percentiles (T-bars), and the outlying results (circles and stars).
Discussion
RIP is a standard technique used in pediatric sleep laboratories. It has been used for over 20 years to describe chest wall movement in infants (13). The methods used for our investigation are simple, involving two stretchable bands placed around the thorax and abdomen of a supine infant sleeping quietly and a simple breath selection protocol. Many infants had marked variability in Φ values over a short period of time, but neither equipment imprecision nor observer bias contributed significantly to the variability observed. Rather, marked variability in rib cage and abdominal synchrony, of uncertain significance, is inherent to the premature infant. Nevertheless, the variability in Φ is manageable, and the variability documented will not preclude the use of Φ in quantitatively describing preterm infants by respiratory phenotype.
Our results seem to allay the important concern that different scorers might pick different breaths and get discrepant results, because an infant’s breathing pattern is erratic even in QS. Breaths selected for Φ calculation were chosen according to a standard protocol used by two observers working independently: behaviorally determined QS and total QS period divided into three time terciles (the first 20 breaths with closed Konno-Mead loops in each tercile). For 24 of 25 blinded, direct comparisons, the results did not differ statistically between two scorers. For the one example where Φ results differed between scorers, neither outcome would have caused the infant to be misclassified as having nearly “normal” Φ on the basis of Φ values for normal preterm infants described in previously published reports (7, 14). Figure 5 shows that interscorer differences in breaths selected added only 8.1% to the coefficients of variation.
We used mechanical models to show that the RIP acquisition hardware and software and the analysis software contributed little to apparent intrasubject variability. For a synchronously ventilated model, the Φ value was near 0 (1.91 ± 0.13° or less). For a dyssynchronous model, the coefficients of variation were 1.1% at 40 and 2.3% at 60 breaths per minute, respectively (Figure 6).
Among others, we want to recognize four potential limitations to this study. First, our emphasis was on the sources and amount of methodological variability, and we did not assess the impact of high flow through catheters on Φ and on stabilizing respiratory mechanics. Future studies will address the impact of nasal flow rates in a more systematic way. Second, our infants were near 32 weeks PMA. We did not study intrasubject variability in older infants—that is, near 36 weeks PMA, the age at which the diagnosis of CLD is typically assigned. However, we suspect that the results for those infants might be less variable and easier to analyze than the results for the infants included in our study. Third, although we inspected all scalar RIP tracings, we did not formally analyze whether both rib cage and abdominal tracings were nearly sinusoidal. Prisk and coworkers described the error that arises when assessing thoracoabdominal dyssynchrony if loops used for calculations are not derived from sinusoidal scalar tracings (16).
We can offer no quantitative assurances that the results would not have differed had we used breaths recorded over a longer time period, but we believe we have shown that the 23-minute recording period is adequately described with the number of breaths analyzed. Others have described the challenge of getting even 20 usable breaths among children (17). We were able to use more than 50 breaths in 55 of 65 subjects. Furthermore, Figure 7 suggests that counting more than 50 breaths in sessions of similar duration in the majority of infants would not change the results in an important way, e.g., mean Φ difference by >20°. The optimal number of breaths for fine distinctions was not explored, but it seems unlikely that RIP will be used for such comparisons (e.g., suggesting that differences in Φ as small as 10° or 20° indicate important physiological differences).
There is much potential appeal in having an investigational tool that is simple and noninvasive and reflects phenotypic abnormalities in upper-airway and intrathoracic airway resistance, as well as rib cage and lung elasticity, particularly when attempting to describe respiratory system health and to understand the action of beneficial interventions. When considering future uses of this technique, it seems likely that ill infants with unstable oxyhemoglobin saturation will have large and quite variable Φ that become smaller and less variable when the infants respond to, for example, high-flow nasal cannulas. It is also possible that the time course of response to “high flow” and the magnitude of the flow rate required to reduce Φ and its variability will suggest a site of action that will help us understand the physiological mechanisms of “high flow” benefit.
The coefficients of variation for Φ for all breaths were so large that the three time terciles had significantly different Φ values in 36 (65%) of 55 infants. Would the intrasubject variability obscure differences among infants that we want to detect? There are few publications that might provide quantitative guidance in this regard. Warren and coworkers showed that mean Φ among healthy preterm infants is near 60° (14). Figure 2 shows that the interquartile range of Φ in an individual infant spanned 60° in only 13 (20.0%) of 65 subjects.
We have used a simple technique and simple analytical algorithms to accurately described dyssynchronous breathing in 65 infants at 32 weeks PMA. Though the Φ values were variable over the recording period, we conclude that RIP can reliably add to the phenotypic description of CLD by identifying preterm infants with relatively normal and relatively abnormal respiratory system mechanics. The ultimate usefulness of methods such as these will be understood within the context of PROP and similar longitudinal studies of postneonatal clinical course and respiratory physiology.
Footnotes
Supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (U01 HL-101465) (A.H.).
Author Contributions: All authors contributed to the preparation of the manuscript. L.N.U.: contributed to the conception and design of the study; composed the first and subsequent drafts of the manuscript; acquisition, analysis, and interpretation of the data. A.H.: conception, design, and execution of the study; supervision of the acquisition, interpretation, and analysis of the data. T.W.F.: responsible for the concept, design, and execution of the study; supervised the acquisition, interpretation, and analysis of the data. O.M.R.: analysis of the data. C.M.C.: acquisition of the data. L.A.L.: recruitment of the subjects. J.A.H.: recruitment of the subjects. M.J.S.-S.: recruitment of the subjects. J.S.K.: concept and design of the study; acquisition, analysis, and interpretation of the data.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 3.Hoppenbrouwers T, Hodgman JE, Harper RM, Hofmann E, Sterman MB, McGinty DJ. Polygraphic studies of normal infants during the first six months of life: III. Incidence of apnea and periodic breathing. Pediatrics. 1977;60:418–425. [PubMed] [Google Scholar]
- 4.Poets CF, Southall DP. Patterns of oxygenation during periodic breathing in preterm infants. Early Hum Dev. 1991;26:1–12. doi: 10.1016/0378-3782(91)90038-5. [DOI] [PubMed] [Google Scholar]
- 5.Weintraub Z, Alvaro R, Kwiatkowski K, Cates D, Rigatto H. Effects of inhaled oxygen (up to 40%) on periodic breathing and apnea in preterm infants. J Appl Physiol (1985) 1992;72:116–120. doi: 10.1152/jappl.1992.72.1.116. [DOI] [PubMed] [Google Scholar]
- 6.Allen JL, Wolfson MR, McDowell K, Shaffer TH. Thoracoabdominal asynchrony in infants with airflow obstruction. Am Rev Respir Dis. 1990;141:337–342. doi: 10.1164/ajrccm/141.2.337. [DOI] [PubMed] [Google Scholar]
- 7.Allen JL, Greenspan JS, Deoras KS, Keklikian E, Wolfson MR, Shaffer TH. Interaction between chest wall motion and lung mechanics in normal infants and infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 1991;11:37–43. doi: 10.1002/ppul.1950110107. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya S, Go D, Krenitsky DL, Huyck HL, Solleti SK, Lunger VA, Metlay L, Srisuma S, Wert SE, Mariani TJ, et al. Genome-wide transcriptional profiling reveals connective tissue mast cell accumulation in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2012;186:349–358. doi: 10.1164/rccm.201203-0406OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kallapur SG, Presicce P, Senthamaraikannan P, Alvarez M, Tarantal AF, Miller LM, Jobe AH, Chougnet CA. Intra-amniotic IL-1β induces fetal inflammation in rhesus monkeys and alters the regulatory T cell/IL-17 balance. J Immunol. 2013;191:1102–1109. doi: 10.4049/jimmunol.1300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prechtl HFR. The behavioural states of the newborn infant (a review) Brain Res. 1974;76:185–212. doi: 10.1016/0006-8993(74)90454-5. [DOI] [PubMed] [Google Scholar]
- 11.Grigg-Damberger M, Gozal D, Marcus CL, Quan SF, Rosen CL, Chervin RD, Wise M, Picchietti DL, Sheldon SH, Iber C. The visual scoring of sleep and arousal in infants and children. J Clin Sleep Med. 2007;3:201–240. [PubMed] [Google Scholar]
- 12.Mayer OH, Clayton RG, Sr, Jawad AF, McDonough JM, Allen JL. Respiratory inductance plethysmography in healthy 3- to 5-year-old children. Chest. 2003;124:1812–1819. doi: 10.1378/chest.124.5.1812. [DOI] [PubMed] [Google Scholar]
- 13.Allen JL, Sivan Y.Measurements of chest wall functionInStocks J, Sly PD, Tepper RS, Morgan WJ, editorsInfant respiratory function testing New York: John Wiley and Sons, Inc; 1996344–349. [Google Scholar]
- 14.Warren RH, Horan SM, Robertson PK. Chest wall motion in preterm infants using respiratory inductive plethysmography. Eur Respir J. 1997;10:2295–2300. doi: 10.1183/09031936.97.10102295. [DOI] [PubMed] [Google Scholar]
- 15.Stocks J, Dezateux CA, Jackson EA, Hoo AF, Costeloe KL, Wade AM. Analysis of tidal breathing parameters in infancy: how variable is TPTEF:TE? Am J Respir Crit Care Med. 1994;150:1347–1354. doi: 10.1164/ajrccm.150.5.7952563. [DOI] [PubMed] [Google Scholar]
- 16.Prisk GK, Hammer J, Newth CJL. Techniques for measurement of thoracoabdominal asynchrony. Pediatr Pulmonol. 2002;34:462–472. doi: 10.1002/ppul.10204. [DOI] [PubMed] [Google Scholar]
- 17.Mayer OH, Jawad AF, McDonough J, Allen J. Lung function in 3-5-year-old children with cystic fibrosis. Pediatr Pulmonol. 2008;43:1214–1223. doi: 10.1002/ppul.20930. [DOI] [PubMed] [Google Scholar]