Abstract
Rationale: Individuals living with patients with tuberculosis (TB) are at elevated risk of infection and disease, with children at greatest risk. The World Health Organization recommends isoniazid preventive therapy (IPT) for HIV-positive contacts and those younger than 5 years. Despite these recommendations, household-level IPT programs are rarely implemented in high TB burden settings. Evidence is scarce about the age-specific efficacy of interventions, such as IPT and bacillus Calmette-Guérin (BCG) vaccination for preventing TB disease among exposed contacts.
Objectives: We estimate the age-specific efficacy of IPT and BCG for preventing TB disease using data from a large observational prospective cohort study of household contacts of patients with TB in Lima, Peru.
Methods: We identified all adults (>15 yr) with incident pulmonary TB (index cases) diagnosed at 106 public health centers in Lima from September 2009 to August 2012. Among 14,041 household contacts (of 3,446 index cases) assessed for infection and disease during the yearlong follow-up period, we identified 462 additional TB cases. We estimate risk ratios (RR) for pulmonary TB associated with BCG, IPT, and latent TB infection.
Measurements and Main Results: BCG confers protection against coprevalent and incident TB among HIV-negative children younger than 10 years (RR, 0.35; 95% confidence interval, 0.19–0.66). IPT confers protection against incident TB among HIV-negative contacts younger than 30 years (RR, 0.33; 95% confidence interval, 0.20–0.54). Risk of incident TB associated with latent TB infection is greatest for children younger than 5 years and decreases with age.
Conclusions: These findings support the use of IPT in older children and young-adult household contacts, in addition to children younger than 5 years.
Keywords: tuberculosis, isoniazid preventive therapy, bacillus Calmette-Guérin vaccination, household intervention
At a Glance Commentary
Scientific Knowledge on the Subject
The World Health Organization recommends administration of isoniazid preventive therapy (IPT) to HIV-positive contacts of patients with tuberculosis (TB) and those aged younger than 5 years. Despite these recommendations, programs in high TB burden settings rarely deliver IPT to household contacts.
What This Study Adds to the Field
Among household contacts of patients with TB in a high-burden setting, we show that IPT protects HIV-negative contacts aged younger than 30 years and that routine bacillus Calmette-Guérin vaccination protects contacts aged younger than 10 years.
Efforts to control tuberculosis (TB) in high-burden settings have traditionally used passive identification of symptomatic TB cases as the primary means for detecting infectious cases and interrupting the cycle of transmission. Although this approach has allowed TB programs to concentrate the resources required for diagnosis within existing health system infrastructure, it is increasingly recognized that eliminating TB will require more aggressive efforts to find and prevent new cases in the community, before patients self-present to diagnostic centers (1). Existing data do not generally support the hypothesis that untargeted efforts to actively seek out cases lead to better outcomes for those individuals in whom disease is detected by these means or to better TB control within communities (1, 2). To close the massive TB case detection gap globally, it is necessary to identify targeted strategies that can find and treat patients with TB as early as possible in their course of disease (3).
In both high and low TB burden settings, individuals who live with patients with TB are at elevated risk of infection and disease compared with individuals without such exposure (4, 5), with child contacts at particularly high risk (3, 6). This makes households an attractive place to use targeted interventions to reduce the risk of TB infection and disease among both children and adults. The World Health Organization currently recommends screening the household contacts of TB cases for disease (7) and the administration of isoniazid preventive therapy (IPT) to contacts younger than 5 years of age and HIV-positive contacts without TB disease (8).
These recommendations are reasonable given the heightened risk in these well-defined environments. Quantifying the age-specific effectiveness of IPT and routine bacillus Calmette-Guérin (BCG) vaccination for preventing TB among household contacts may suggest ways to improve these policies and increase the number of individuals who benefit from postexposure interventions. Although earlier placebo-controlled trials have demonstrated the efficacy of BCG against pediatric pulmonary TB disease (9), evidence from observational studies is important for understanding the efficacy of vaccination in the context of high-risk household exposure. Careful attention to how the efficacy of these interventions varies with age is particularly important in light of the expense and effort involved in reaching exposed contacts.
In this article, we analyze data from a large observational prospective cohort study of household contacts of diagnosed patients with TB in Lima, Peru. We use information collected during repeated home visits to estimate the efficacy of BCG vaccination and IPT, alone and in combination, for the prevention of disease among household contacts. In addition, we use statistical methods that allow us to account for unobserved sources of heterogeneity in risk arising from clustering of individuals (e.g., within neighborhoods) to estimate unbiased average intervention effects.
Methods
Data
Between September 2009 and August 2012, we identified all adults (>15 yr old) with incident pulmonary TB diagnosed at any of 106 participating public health centers located in our study catchment area of approximately 3.3 million inhabitants. Within 1 month of diagnosis of TB in these “index cases,” a study nurse visited the patient’s home and invited household contacts to participate in a baseline assessment of TB disease. Contacts with symptoms consistent with TB disease were referred to a study health center for smear microscopy and chest radiograph if indicated by the health center physician. Contacts found to have TB disease are termed secondary cases. Among contacts without TB disease, latent TB infection (LTBI) was assessed using the tuberculin skin test (TST); the skin test antigen used was Tuberculin RT23 (Statens Serum Institut, Copenhagen, Denmark). Consistent with existing Peruvian National TB Program guidelines, household contacts up to age 19 years old were offered 6 months of IPT, and some individuals older than 19 also elected to receive IPT. BCG vaccination status was assessed by questionnaire. The vaccination status of children was obtained from their parent or guardian. Households were visited again at 6 and 12 months after enrolment, and contacts free of LTBI and active TB at baseline were retested for LTBI and TB (the follow-up observations). Study protocols were reviewed and approved by research ethics committees at the Harvard School of Public Health and Peru’s National Institute of Health. Informed consent was obtained from all adult study participants and the parents or legal guardians of child participants. Figure 1 illustrates the study design.
Figure 1.
Study design. The figure illustrates the screening of a household with four contacts over the yearlong follow-up period. Each row illustrates a different individual, and an arrow connects successive observations. White circles with solid outline indicate no tuberculosis (TB) infection, white circles with dashed outline indicate latent TB infection (LTBI), and black circles indicate incident TB disease between screenings. The top case is coprevalent for TB disease with the household index case (not pictured) and not included in future screenings. The second row illustrates that individuals with a positive tuberculin skin test at any observation are not screened on successive observations, and the third row shows that individuals with a positive tuberculin skin test are still screened for active TB. The bottom row shows that individuals with neither LTBI nor TB disease at their previous observation are screened for LTBI at each successive observation.
Of the 3,446 enrolled index cases diagnosed with TB based on clinical presentation, 22% were eventually found to be sputum smear and culture negative (i.e., there was no microbiologic evidence of Mycobacterium tuberculosis after a thorough work-up). Although microbiologically unconfirmed TB cases are included in official notification statistics (10), these individuals are thought to be substantially less infectious than smear-positive TB cases (11). Consequently, in this study, all models are adjusted for the smear and culture status of the index case in each household. We denote smear-positive and culture-positive index cases as SCPIs, and smear-negative and culture-positive indexes as CPIs. Those index cases that are smear-negative and culture-negative are denoted as NIs. In our multivariate analysis of factors protective against onset of TB disease, we use the records of 12,875 household contacts with complete baseline observations. Individuals who did not complete 6- and 12-month follow-up interviews were included in the present analysis, because they were linked back to their original study record if they presented at a health center with TB disease at any time during the study period. Although BCG vaccination often leaves a characteristic scar, it is possible that vaccinated individuals may not scar (12). Consequently, we use the individual’s recall of vaccination rather than the presence of a BCG scar to estimate protective effects of BCG against disease.
From the 14,041 individuals in our original sample, 1,986 individuals were excluded from the multivariate analysis of incident TB because they were missing a baseline skin-test response, whereas 1,166 individuals were excluded from the analysis of coprevalent and incident TB because they were missing other covariates, such as BCG vaccination or household exposure status. There is a low prevalence of HIV in Peru (0.6% in 2006 [13]) and HIV was not commonly documented in this cohort. All index TB cases were tested for HIV infection, of which only 3% were found to be HIV coinfected. HIV testing was offered to all household contacts, and 59% of respondents agreed to be tested, and of these 27 were HIV-positive. An additional 27 household contacts also self-reported being HIV-positive; all 54 (0.38%) HIV-positive contacts are excluded from the present analysis. For a full analysis of the sensitivity of our results to these excluded cases, see the online supplement.
Models
We denote the size of the TST induration for individual i at time t to be and her infection status as . Consistent with most studies of contacts in low HIV prevalence populations, we used a 10-mm cutoff for defining LTBI (7). We classify individuals with greater than or equal to 10 to have LTBI () and those with less than 10 to be TST-negative . Individuals with 0 < < 10 are classified as being TST-reactive (R), and we denote this reactivity using a binary variable . Individuals who are nonreactive to the TST (i.e., with ) are denoted as NR.
TB Disease
To understand the effects of BCG vaccination and IPT on the risk of developing TB disease, we use a mixed-effects binomial regression model with a logarithmic link function to estimate risk ratios (RR) for covariates associated with diagnosis of TB disease at any point during the 1-year follow-up period. Because the small number of secondary cases of TB disease makes them relatively unlikely to cluster within households, we include random intercepts at only the health center level. We distinguish between coprevalent and incident cases (i.e., those contacts with TB disease at the time of the intake observation and those who developed disease during the yearlong follow-up). Because IPT was only offered after the baseline visit, there was no possibility for IPT to protect against disease in coprevalent cases. However, BCG vaccination may provide protection against both coprevalent and incident TB.
In addition to the protective effects of BCG and IPT, household environmental factors and socioeconomic status (SES) affect the risk of TB disease. To ensure that these factors do not confound our estimates of BCG and IPT efficacy, we include two additional variables in our analysis. The first is a measure of household overcrowding, defined as having three or more individuals per bedroom, which is likely to indicate both indoor environmental quality and SES. Because household income may be an unreliable indicator of SES, we use the quality of the household’s roof as another indirect measure of SES. Households with a mud or thatch roof are denoted as “unimproved roof” households, whereas those with a concrete or metal roof are denoted as “improved roof” households.
Systematic reviews have shown that children living within the homes of individuals with TB are at particularly high risk for development of TB disease (5, 6, 14). Consequently, in addition to understanding the effects of baseline TST, BCG, and IPT status on the probability of TB disease, we examined the moderating role of age on these effects. To do this, we estimate models including interaction terms with age for each of these predictors and selected a final model based on the Akaike information criterion and Bayesian information criterion, which allows us to compare the relative goodness of fit of these models while penalizing excess model structure. All mixed-effects models were fit with the lme4 package (15) for R 3.0.2.
Results
Descriptive Results
Among the HIV-negative household contacts there were 454 cases of TB (out of 462 total) during the yearlong follow-up period, of which 229 were coprevalent with the household index case (i.e., identified during the baseline investigation within the home), and the remaining 225 were incident cases over the course of the study period. Table 1 contains descriptive characteristics for the 13,975 HIV-negative individuals with complete demographic information. Figure 2 illustrates the age distributions of household index cases and all TB cases identified throughout the study period.
Table 1:
Descriptive Characteristics of Household Contacts by Age
| Age | N | Male | TB | Coprevalent | LTBI | Male TB | BCG | BCG & TB | IPT | IPT & TB |
|---|---|---|---|---|---|---|---|---|---|---|
| 0–4 yr | 1,791 (12.8%) | 899 (50.2%) | 73 (4.1%) | 41 (2.3%) | 230 (12.8%) | 35 (2.0%) | 1,728 (96.5%) | 67 (3.7%) | 915 (51.1%) | 9 (0.5%) |
| 5–9 yr | 1,464 (10.5%) | 750 (51.2%) | 35 (2.4%) | 23 (1.6%) | 298 (20.4%) | 19 (1.3%) | 1,405 (96.0%) | 31 (2.1%) | 767 (52.4%) | 8 (0.5%) |
| 10–14 yr | 1,340 (9.6%) | 665 (49.6%) | 45 (3.4%) | 19 (1.4%) | 363 (27.1%) | 18 (1.3%) | 1,280 (95.5%) | 43 (3.2%) | 629 (46.9%) | 9 (0.7%) |
| 15–19 yr | 1,401 (10.0%) | 637 (45.5%) | 76 (5.4%) | 30 (2.1%) | 384 (27.4%) | 44 (3.1%) | 1,293 (92.3%) | 67 (4.8%) | 466 (33.3%) | 7 (0.5%) |
| 20–29 yr | 2,468 (17.7%) | 1,115 (45.2%) | 99 (4.0%) | 54 (2.2%) | 929 (37.6%) | 63 (2.6%) | 2,337 (94.7%) | 95 (3.8%) | 88 (3.6%) | 1 (0.0%) |
| 30–39 yr | 1,734 (12.4%) | 738 (42.6%) | 48 (2.8%) | 26 (1.5%) | 764 (44.1%) | 22 (1.3%) | 1,624 (93.7%) | 44 (2.5%) | 64 (3.7%) | 0 (0.0%) |
| 40–49 yr | 1,558 (11.1%) | 522 (33.5%) | 26 (1.7%) | 12 (0.8%) | 787 (50.5%) | 11 (0.7%) | 1,498 (96.1%) | 26 (1.7%) | 60 (3.9%) | 0 (0.0%) |
| 50+ yr | 2,219 (15.9%) | 894 (40.3%) | 52 (2.3%) | 24 (1.1%) | 1,015 (45.7%) | 24 (1.1%) | 1,993 (89.8%) | 47 (2.1%) | 87 (3.9%) | 1 (0.0%) |
| Total | 13,975 (100.0%) | 6,220 (44.5%) | 454 (3.2%) | 229 (1.6%) | 4,770 (34.1%) | 236 (1.7%) | 13,158 (94.2%) | 420 (3.0%) | 3,076 (22.0%) | 35 (0.3%) |
Definition of abbreviations: BCG = bacillus Calmette-Guérin; IPT = isoniazid preventive therapy; LTBI = latent TB infection; TB = tuberculosis.
The table lists counts and percentages for each exposure or treatment variable. The column marked N contains the number of individuals in each age group and percentage of the total population is presented in italics. Percentages in the remaining columns reflect age-group–specific proportions. The column marked Male indicates the proportion male in each age group. Columns marked TB and Coprevalent indicate the total number of TB cases arising among household contacts and the subset who were coprevalent with the index case, respectively. The column marked Male TB contains the number TB cases among male household contacts at each age. LTBI indicates the number with latent TB infection (TST ≥ 10 mm) at baseline. BCG and IPT reflect the number and percent BCG-vaccinated or receiving IPT, whereas the columns marked BCG & TB and IPT & TB indicate the number of individuals receiving either BCG or IPT with TB disease at any point in the study (i.e., those who were either coprevalent with the index case or who later became an incident case).
Figure 2.
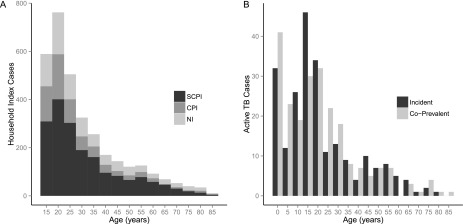
Age distributions of index cases and secondary tuberculosis (TB) cases in a household. (A) Distribution of ages for the 3,446 index TB cases in our analysis. Shading of the bars in the histogram indicates the proportion of index cases in each age group who were smear- and culture-positive for TB disease (SCPI), smear-negative and culture-positive (CPI), or smear-negative and culture-negative (NI). (B) Distribution of ages for the 454 HIV-negative cases of TB disease identified among 14,041 household contacts of 3,446 index TB cases. Bars are broken down to show the number of cases in each age group that were either coprevalent with the index case or developed TB after the baseline observation. Each bin in both histograms represents a 5-year age group.
TB Disease
Table 2 shows whole-sample and age-stratified crude RRs for coprevalent or incident TB disease as a function of receiving either BCG or IPT. At the whole-sample level, the effect of BCG vaccination against both coprevalent and incident TB disease is nonsignificant (RR, 0.76; 95% confidence interval [CI], 0.54–1.07). However, BCG-vaccinated individuals younger than 5 years (RR, 0.40; 95% CI, 0.18–0.88) and 5–9 years of age (RR, 0.33; 95% CI, 0.12–0.88) are significantly less likely to develop TB disease than their nonvaccinated peers. For IPT, a protective effect against incident TB disease is detectable for the entire sample (RR, 0.47; 95% CI, 0.32–0.70), and this effect is roughly consistent with the age-specific crude RRs for individuals younger than 20 years old. Individuals aged 20 and older are excluded from the stratified analysis of IPT efficacy because of the small number of individuals receiving IPT in older age groups.
Table 2:
Age-stratified Unadjusted Risk Ratios for Tuberculosis Disease by BCG and IPT Status
| BCG |
IPT |
|||
|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | |
| All ages | 0.76 | (0.54–1.07) | 0.47 | (0.32–0.70) |
| Age groups | ||||
| 0–4 yr | 0.40 | (0.18–0.88) | 0.26 | (0.11–0.58) |
| 5–9 yr | 0.33 | (0.12–0.88) | 0.88 | (0.29–2.70) |
| 10–14 yr | 1.01 | (0.25–4.00) | 0.49 | (0.22–1.11) |
| 15–19 yr | 0.62 | (0.32–1.20) | 0.29 | (0.13–0.68) |
| 20–29 yr | 1.32 | (0.50–3.50) | — | — |
| 30–39 yr | 0.74 | (0.27–2.00) | — | — |
| 40+ yr | 1.19 | (0.49–2.90) | — | — |
Definition of abbreviations: BCG = bacillus Calmette-Guérin; CI = confidence interval; IPT = isoniazid preventive therapy; RR = risk ratio.
RRs for IPT are not presented for older age groups because few individuals in these groups received IPT (see Table 1).
To assess whether the protective effect of BCG among individuals aged younger than 10 years remains significant after adjusting for covariates, we fit a mixed-effects logistic regression model to the full dataset, which included both coprevalent cases diagnosed with TB disease at the time of the first household visit and contacts who presented with TB disease during the yearlong follow-up period. This model is adjusted for age, IPT receipt, and exposure to a SCPI or CPI (Table 3). To assess age-specific effects of BCG vaccination, we include dummy variables for each 10-year age group, leaving 20-to-30 year olds as the reference group; this model includes interaction terms between BCG vaccination and membership in the 0- to 9- year and 10- to 19-year age groups. However, coefficients and 95% CIs for age-specific effects of BCG in Table 3 reflect the product of each age group interaction term and the term for BCG alone. This allows the BCG effect estimates to be interpreted relative to nonvaccinated individuals of the same age. Doing this, we find that BCG-vaccinated individuals younger than 10 years old are significantly less likely to develop TB disease over the yearlong follow-up than their nonvaccinated counterparts (RR, 0.35; 95% CI, 0.19–0.66), whereas this effect is nonsignificant for older individuals.
Table 3:
Risk factors for Coprevalent and Incident Tuberculosis at Baseline and over 1-Year Follow-up
| Variable | RR | 95% CI |
|---|---|---|
| Intercept | 0.02 | (0.01–0.05) |
| Demographics | ||
| Male | 1.30 | (1.08–1.57) |
| Socioeconomic status | ||
| Poor roof | 1.00 | (0.64–1.56) |
| Overcrowded | 1.20 | (0.99–1.47) |
| Age groups | ||
| 0–9 yr | 3.54 | (1.44–8.67) |
| 10–19 yr | 2.13 | (0.81–5.64) |
| 20–29 yr | REF | — |
| 30–39 yr | 0.72 | (0.50–1.04) |
| 40–49 yr | 0.46 | (0.29–0.74) |
| 50+ yr | 0.73 | (0.51–1.03) |
| Exposure | ||
| SCPI | 1.71 | (1.33–2.19) |
| CPI | 0.98 | (0.69–1.39) |
| NI | REF | — |
| Interventions | ||
| BCG (0–9 yr) | 0.35 | (0.19–0.66) |
| BCG (10–19 yr) | 0.72 | (0.35–1.51) |
| BCG (20+ yr) | 0.95 | (0.49–1.86) |
Definition of abbreviations: BCG = bacillus Calmette-Guérin; CI = confidence interval; CPI = smear-negative and culture-positive index cases; NI = smear-negative and culture-negative index cases; RR = risk ratio; SCPI = smear-positive and culture-positive index cases.
Table shows RR and 95% CIs for risk factors for coprevalent and incident tuberculosis disease. Statistically significant responses (P < 0.05) are highlighted in bold. Coefficients for BCG vaccination are presented relative to nonvaccinated individuals in each age group. Because coprevalent tuberculosis cases could not be targeted for isoniazid preventive therapy, we adjust for the effect of isoniazid preventive therapy among this group but do not present this coefficient.
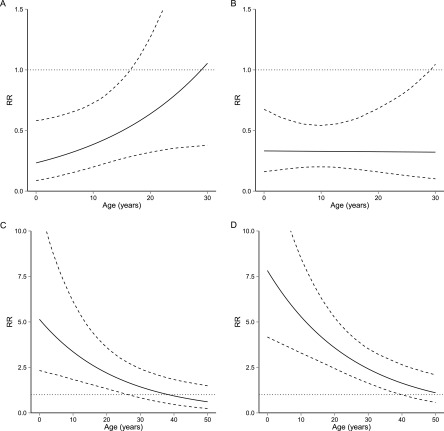
Because IPT and baseline TSTs were not administered to the 229 individuals with coprevalent TB disease, we fit a second mixed-effects logistic regression model to a dataset excluding coprevalent cases to assess the effects of IPT, baseline TST response, and BCG vaccination status on the probability of incident TB disease. In this model, we include interaction terms to estimate the age-specific effects of IPT, BCG, LTBI, and TST reactivity. Results from this model are presented in Table 4. To assess the range of ages over which these estimates are statistically significant, we present age-specific RRs and CIs from the model including all four age interactions (BCG, IPT, TST reactivity, LTBI) graphically in Figure 3. Figure 3A shows the protection against TB disease associated with BCG vaccination, and that this association becomes nonsignificant at age 17. Figure 3B shows that the effect of IPT on risk is constant across all ages that were eligible for preventive therapy. Figures 3C and 3D show that the RR of TB disease associated with TST reactivity and LTBI (as compared with TST nonreactivity) decline with age before becoming nonsignificant at ages 26 and 40, respectively.
Table 4:
Risk Factors for Incident Tuberculosis Disease during 1-Year Follow-up
| Variable | RR | 95% CI |
|---|---|---|
| Intercept | 0.01 | (0.00–0.04) |
| Demographics | ||
| Male | 1.18 | (0.87–1.60) |
| Age | 0.96 | (0.91–1.01) |
| Socioeconomic status | ||
| Poor roof | 1.25 | (0.59–2.63) |
| Overcrowded | 1.04 | (0.77–1.40) |
| Exposure | ||
| SCPI | 2.82 | (1.72–4.61) |
| CPI | 1.47 | (0.78–2.77) |
| TST | ||
| R | 5.15 | (2.32–11.40) |
| R × Age | 0.96 | (0.93–0.99) |
| LTBI | 7.83 | (4.22–14.51) |
| LTBI × Age | 0.96 | (0.94–0.98) |
| Interventions | ||
| BCG | 0.23 | (0.09–0.60) |
| BCG × Age | 1.05 | (1.00–1.11) |
| IPT | 0.33 | (0.16–0.68) |
| IPT × Age | 1.00 | (0.95–1.05) |
Definition of abbreviations: BCG = bacillus Calmette-Guérin; CI = confidence interval; CPI = smear-negative and culture-positive index cases; IPT = isoniazid preventive therapy; LTBI = latent TB infection; R = reactive; RR = risk ratio; SCPI = smear-positive and culture-positive index cases; TST = tuberculin skin test.
Statistically significant coefficients (P < 0.05) are highlighted in bold. LTBI is assessed using baseline skin test response. R state indicates TST > 0 mm and < 10 mm. LTBI indicates TST ≥ 10 mm.
Figure 3.
Interactive effect of age and baseline tuberculin skin test (TST) reactivity (R) or latent tuberculosis infection (LTBI) on probability of incident TB disease. The solid lines show point estimates for the risk ratio (RR) of TB disease at each age. (A and B) Protective effects of bacillus Calmette-Guérin vaccination (A) and isoniazid preventive therapy (B) as a function of age. (C and D) RR of TB disease associated with TST reactivity (C) or LTBI (D) as a function of age, relative to TST nonreactivity. Nonreactivity indicates TST induration of 0 mm, reactivity state indicates TST greater than 0 mm and less than 10 mm. LTBI indicates TST greater than or equal to 10 mm. The dashed lines show age-specific 95% confidence intervals for these quantities; the horizontal dotted lines are guides for assessing statistical significance.
We also estimate the combined effect of BCG and IPT on the risk of TB disease among individuals eligible for IPT (i.e., those who were not coprevalent with the index case). We first tested the hypothesis that BCG and IPT may have synergistic effects by including an interaction between BCG and IPT. Because this effect was nonsignificant, we used the product of the individual age-specific RR for BCG and IPT presented graphically in Figure 3 to estimate the age-specific effects of layering these interventions. To obtain confidence intervals for this combined effect, we used 10,000 draws from a multivariate normal distribution with parameters obtained from the fitted variance-covariance matrix of the fixed effects for the model in Table 4. We estimated that the combined use of IPT and BCG resulted in a statistically significant reduction in risk as compared with receiving neither BCG nor IPT for individuals aged younger than 1 year (RR, 0.07; 95% CI, 0.02–0.21), and that this protective effect declines before losing significance at 25 years of age (RR, 0.26; 95% CI, 0.07–0.92). For a graphical presentation of this combined effect, see Figure E2 in the online supplement.
We also use simulations from the fitted variance-covariance matrix to assess the range of ages over which the sum of these coefficients was significantly smaller than the individual ones [i.e., ]. We find that the combined effects of BCG and IPT were greater than BCG alone from birth until age 30, and greater than IPT alone from birth until age 18, both at the P less than 0.05 level.
Discussion
In our study setting, BCG vaccination and IPT are associated with reduced risk of TB disease in HIV-negative children and adults who live with patients with TB. We also find that BCG and IPT have independent protective effects, so that BCG-vaccinated individuals receiving IPT enjoy the full protection against disease afforded by each intervention. Our findings also illustrate how the discriminatory power of TST reactivity and LTBI as predictors of incident TB disease decline with age in this epidemiologic setting.
Although current guidelines stress the importance of screening household contacts of all ages for TB disease, the World Health Organization currently recommends that only household contacts without TB who are younger than 5 years of age or are HIV-positive receive IPT (1). The findings presented here, which demonstrate the efficacy of IPT for preventing TB disease among these older contacts, add weight to the argument for expanding age eligibility for IPT and provide a framework for assessing which contacts are most likely to benefit from IPT.
Although BCG vaccination has consistently been shown to prevent severe forms of TB disease, such as miliary TB and TB meningitis, in young children (16), estimates of the efficacy of BCG for preventing pulmonary TB vary from 0% to 80%, with efficacy lowest at tropical and subtropical latitudes with high TB burden (17). A placebo-controlled trial of BCG vaccination among American Indians and Alaska Natives suggested that BCG may confer long-term protection against all forms of TB, including pulmonary disease, for 40–50 years post-vaccination (18). However, the duration of protection among individuals with intense household exposure to TB has not been definitively established. Our findings show that, in a high-burden context, BCG vaccination conferred protection against incident TB disease among those with household exposure through the mid-teenage years. Although BCG vaccination is unlikely to be implemented as a postexposure intervention, understanding the implications of BCG for preventing TB disease among household contacts of patients with TB is important for assessing the efficacy of BCG vaccination programs. In addition, quantifying the degree to which IPT confers additional protection against disease among BCG-vaccinated individuals of different ages should help to assess the projected benefits associated with expanding these programs.
It is important to highlight potential limitations of our analysis. Although we have attempted to control for SES, household overcrowding, and sources of unobserved heterogeneity, the observational nature of our study means that we cannot definitively rule out other explanations for differences in TB disease risk among those receiving IPT and/or BCG. Another limitation comes from the possibility that the index case as defined in our cohort was not the first case that occurred in each household, which may result in misattribution of exposure of one individual to another. In addition, our data include only information about whether individuals were initiated on IPT, but not whether these individuals received a complete course of IPT. Consequently, if many individuals in the cohort did not complete the full course of IPT, our results may reflect an underestimate of the true efficacy of IPT.
Although our results only address the direct effects of IPT and BCG for preventing disease in individuals who live with patients with TB, these findings strongly suggest that expanding the reach of these interventions may also confer indirect protection by preventing secondary transmission at both the household and community levels. Future modeling studies should incorporate these findings to assess the extent to which such targeted household-level interventions can slow the pace of TB transmission in the community.
Footnotes
Supported by National Institutes of Health (NIH/NIAID grant U19 A1076217 to M.B.M., M.C.B., J.G., L.L., R.C., R.Y., C.C., Z.Z., and T.C.); the RAPIDD program of the Science and Technology Directorate, Department of Homeland Security (J.L.Z. and B.T.G.); the Fogarty International Center, National Institutes of Health (J.L.Z. and B.T.G.); the Bill and Melinda Gates Foundation (B.T.G.); and the Science and Technology Directorate, US Department of Homeland Security (Contract # HSHQDC-12-C-00058 to B.T.G.).
Author Contributions: J.L.Z., M.B.M., B.T.G., and T.C. designed the analysis, interpreted statistical results, and wrote the manuscript. J.L.Z. and T.C. analyzed the data and created figures. Z.Z. assisted with data management and analysis. M.B.M., M.C.B., J.G., L.L., R.C., R.Y., C.C., and T.C. contributed to design and implementation of the household contact study on which this analysis is based.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201310-1896OC on March 4, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health OrganizationGlobal tuberculosis report 2012. Geneva: World Health Organization; 2012 [Google Scholar]
- 2.Kranzer K, Afnan-Holmes H, Tomlin K, Golub JE, Shapiro AE, Schaap A, Corbett EL, Lönnroth K, Glynn JR. The benefits to communities and individuals of screening for active tuberculosis disease: a systematic review. Int J Tuberc Lung Dis. 2013;17:432–446. doi: 10.5588/ijtld.12.0743. [DOI] [PubMed] [Google Scholar]
- 3.Lönnroth K, Corbett E, Golub J, Godfrey-Faussett P, Uplekar M, Weil D, Raviglione M. Systematic screening for active tuberculosis: rationale, definitions and key considerations. Int J Tuberc Lung Dis. 2013;17:289–298. doi: 10.5588/ijtld.12.0797. [DOI] [PubMed] [Google Scholar]
- 4.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2012;41:140–156. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro AE, Variava E, Rakgokong MH, Moodley N, Luke B, Salimi S, Chaisson RE, Golub JE, Martinson NA. Community-based targeted case finding for tuberculosis and HIV in household contacts of patients with tuberculosis in South Africa. Am J Respir Crit Care Med. 2012;185:1110–1116. doi: 10.1164/rccm.201111-1941OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:359–368. doi: 10.1016/S1473-3099(08)70071-9. [DOI] [PubMed] [Google Scholar]
- 7.World Health OrganizationRecommendations for investigating contacts of person with infectious tuberculosis in low- and middle-income countries. Geneva: World Health Organization; 2012 [PubMed] [Google Scholar]
- 8.World Health Organization Stop TB Partnership Childhood TB Subgroup. Chapter 4: childhood contact screening and management. Int J Tuberc Lung Dis. 2007;11:12–15. [PubMed] [Google Scholar]
- 9.Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PEM, Rodrigues LC, Smith PG, Lipman M, Whiting PF, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58:470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 10.World Health OrganizationDefinitions and reporting framework for tuberculosis – 2013 revision. Geneva: World Health Organization; 2013 [Google Scholar]
- 11.Grzybowski S, Barnett GD, Styblo K. Contacts of cases of active pulmonary tuberculosis. Bull Int Union Tuberc. 1975;50:90–106. [PubMed] [Google Scholar]
- 12.Floyd S, Ponnighaus JM, Bliss L, Warndorff DK, Kasunga A, Mogha P, Fine PE. BCG scars in northern Malawi: sensitivity and repeatability of scar reading, and factors affecting scar size. Int J Tuberc Lung Dis. 2000;4:1133–1142. [PubMed] [Google Scholar]
- 13.Population Reference Bureau2006 World population data sheet. Washington, DC:2006
- 14.Shah S, Yuen CM, Heo M, Tolman AW, Becerra MC. Yield of contact investigations in households of drug-resistant tuberculosis patients: systematic review and meta-analysis. Clin Infect Dis. 2014;58:381–391. doi: 10.1093/cid/cit643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bates D, Maechler M.Ben Bolker, Walker S. lme4: Linear mixed-effects models using Eigen and S4 R package version 1.0-5. 2013 [accessed 2013 Aug 1]. Available from: http://cran.r-project.org/package=lme4
- 16.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 17.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 18.Aronson NE, Santosham M, Comstock GW, Howard RS, Moulton LH, Rhoades ER, Harrison LH. Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: a 60-year follow-up study. JAMA. 2004;291:2086–2091. doi: 10.1001/jama.291.17.2086. [DOI] [PubMed] [Google Scholar]