To the Editor:
Diaphragm weakness in the intensive care unit (ICU) plays an important role in difficult weaning from mechanical ventilation. Diaphragm strength in mechanically ventilated (MV) critically ill patients has been assessed indirectly using phrenic nerve stimulation, which demonstrated that the pressure-generating capacity of the diaphragm was reduced in these patients (1–3). However, this technique cannot distinguish between impaired phrenic nerve function, abnormal neuromuscular transmission, and intrinsic abnormalities in the diaphragm muscle itself. Consequently, it is unknown whether intrinsic contractile weakness of diaphragm muscle fibers occurs in MV critically ill patients. If so, targeted treatment strategies that enhance contractility may improve the success of weaning. Such treatment strategies may include the administration of a novel class of small-molecule drugs, named fast skeletal troponin activators, which improve the contractile strength of skeletal muscle fibers (4). In this study, we obtained diaphragm biopsy specimens from critically ill patients (n = 10; MV for 28–603 h) undergoing laparotomy or thoracotomy, and compared them with control patients undergoing elective lung surgery (n = 10; MV 1–2 h, see Table E1 in the online supplement). The size and the contractile performance of isolated diaphragm muscle fibers were determined. In addition, we tested the ability of the fast skeletal troponin activator, CK-2066260, to improve contractile strength.
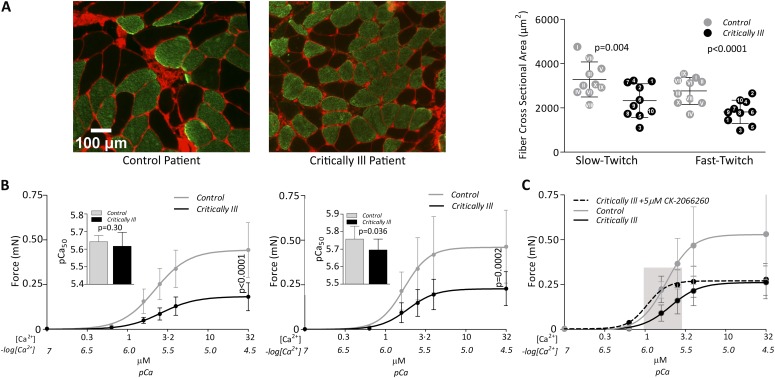
Diaphragm fiber cross-sectional area (CSA) was determined by means of immunohistochemical analyses with myosin heavy chain antibodies performed on cryosections of the biopsy specimens (5, 6). Figure 1A demonstrates atrophy of slow- and fast-twitch diaphragm fibers in critically ill patients (CSA slow-twitch fibers: control patients, 3,284 ± 793 μm2 vs. critically ill patients, 2,328 ± 763 μm2, P = 0.004; fast-twitch fibers: control patients, 2,766 ± 606 μm2 vs. critically ill patients, 1,819 ± 527 μm2, P < 0.0001).
Figure 1.
(A) Severe diaphragm muscle fiber atrophy in mechanically ventilated (MV) critically ill patients. Typical examples of serial diaphragm cross-sections stained with antibodies against slow-twitch myosin heavy chain (green). Wheat germ agglutinin (WGA) staining (red) was used to demarcate the muscle fibers. Scale bar = 100 μm (left panel). Quantification of, in total, 4,686 fibers revealed that, compared with control patients, fiber cross-sectional area (CSA) is 29% smaller in slow-twitch (control patients vs. critically ill patients: 3,284 ± 793 μm2 vs. 2,328 ± 763 μm2; P = 0.004) and 34% smaller in fast-twitch (control patients vs. intensive care unit [ICU] patients: 2,766 ± 606 μm2 vs. 1,819 ± 527 μm2; P < 0.0001) diaphragm muscle fibers of critically ill patients (right panel). Each dot with corresponding patient number indicates the mean CSA per patient (shaded dot, control patient; closed dot, critically ill patient). Horizontal bars indicate group mean; error bars indicate ±SD. (B) Severe diaphragm muscle fiber weakness in MV critically ill patients. Curves indicate the absolute force-calcium relation of diaphragm fibers of control patients and MV critically ill patients. Compared with control patients, the maximal absolute force—determined at maximally activating calcium concentrations (pCa 4.5)—was reduced by 56% in slow-twitch (control patients vs. ICU patients: 0.44 ± 0.16 mN vs. 0.19 ± 0.07 mN; P < 0.0001 [left panel]) and by 52% in fast-twitch (control patients vs. ICU patients: 0.49 ± 0.21 mN vs. 0.24 ± 0.09 mN; P = 0.0002 [right panel]) diaphragm fibers from critically ill patients. Indents show calcium sensitivity (pCa50). In slow-twitch fibers, pCa50 is not affected (control patients vs. critically ill patients: 5.64 ± 0.03 vs. 5.61 ± 0.08; P = 0.30 [left panel]), whereas in fast-twitch fibers pCa50 was significantly lower in critically ill patients (control patients vs. critically ill patients: 5.76 ± 0.07 vs. 5.70 ± 0.06; P = 0.036 [right panel]). Bullets and column bars indicate group mean; error bars indicate ±SD. (C) Diaphragm muscle fiber force is restored by a fast skeletal troponin activator. Curves show force response of fast-twitch fibers of a subset of patients to incremental calcium concentrations when exposed to vehicle (dimethyl sulfoxide [DMSO]) (gray solid line, control patients; black solid line, critically ill patients). Fibers from critically ill patients show a marked leftward shift of the force-calcium curve when treated with 5 μM CK-2066260 (dotted black line), such that, at physiological calcium concentration (indicated by gray bar), force is restored to levels observed in untreated fibers from control patients (force at pCa 5.8: 0.22 ± 0.05 mN [control patients with DMSO] vs. 0.22 ± 0.07 mN [critically ill patients with CK-2066260]; P = 0.954). Note that the magnitude of increase in pCa50 was comparable in both groups. Bullets indicate group mean; error bars indicate ±SD.
We measured the contractile performance of permeabilized single diaphragm fibers isolated from the biopsy specimens. Fibers were mounted between a force transducer and a length motor, and exposed to activating calcium solutions. Maximal contractile strength was markedly lower in critically ill patients (absolute force slow-twitch fibers: control patients, 0.44 ± 0.16 mN vs. critically ill patients, 0.19 ± 0·07 mN, P < 0.0001; fast-twitch fibers: control patients, 0.49 ± 0.21 mN vs. critically ill patients, 0.24 ± 0.09 mN, P = 0.0002; Figure 1B). After normalization of force to the CSA of these fibers (i.e., specific force), a deficit remained in diaphragm fibers of critically ill patients (see Figure E1). This suggests that, in these critically ill patients, there is not only a loss of contractile proteins, but also dysfunction of the remaining ones.
In addition, we measured the sensitivity of force to calcium. The negative logarithm of the calcium concentration needed to obtain 50% of maximal force (pCa50) was unaffected in slow-twitch fibers (control patients, 5.64 ± 0.03 vs. critically ill patients, 5.61 ± 0.08, P = 0.30), whereas, in fast-twitch fibers, the pCa50 was significantly lower in critically ill patients (control patients, 5.76 ± 0.07 vs. critically ill patients, 5.70 ± 0.06, P = 0.036) (Figure 1B). Thus, fast-twitch diaphragm fibers from critically ill patients not only have reduced maximal force, but also require more calcium to generate force.
We exposed diaphragm fibers of a representative subset of control patients (nos. I, IV, VI) and critically ill patients (nos. 1, 3, 4, 5) to the fast skeletal troponin activator, CK-2066260, which improves the sensitivity of the calcium sensor in the muscle sarcomere. Compared with vehicle, 5 μM of CK-2066260 significantly increased the calcium sensitivity of diaphragm fibers both in control patients (pCa50: 5.75 ± 0.04 vs. 6.18 ± 0.1, respectively; P < 0.001) and in critically ill patients (5.70 ± 0.07 vs. 6.00 ± 0.13, respectively; P < 0.01) (Figure 1C). Importantly, at physiological calcium concentrations, CK-2066260 restored the contractile force of fast-twitch diaphragm fibers of critically ill patients back to levels observed in untreated fibers from control patients (force at pCa 5.8: untreated control patients, 0.22 ± 0.05 vs. treated critically ill patients, 0.22 ± 0.07 mN; P = 0.954). See the online supplement for details.
The current study is the first to show that atrophy and contractile weakness of diaphragm muscle fibers develop in a clinically relevant group of MV critically ill patients. Interestingly, the reduction in the contractile force of diaphragm fibers of these critically ill patients is comparable to the reduction in diaphragm strength estimated previously by phrenic nerve pacing (1, 2), indicating that the reduction in diaphragm strength in these patients largely results from muscle fiber weakness. To date, no drug is approved to improve respiratory muscle function in MV critically ill patients. We made a step toward such a strategy by testing the ability of the fast skeletal troponin activator, CK-2066260, to restore diaphragm fiber strength. We observed that, upon exposure to CK-2066260, fast-twitch diaphragm fibers from critically ill patients regained strength at calcium concentrations that reflect activation during daily live activities to levels found in untreated fibers from control patients (Figure 1C). Because approximately 50% of fibers and total fiber area in the human diaphragm consists of fast-twitch fibers (Figure E3), fast skeletal troponin activators might significantly improve in vivo diaphragm strength. The potential of fast troponin activators is further strengthened by the notion that these drugs do not affect cardiac function (4), which would be an undesirable side effect in critically ill patients. The analog of CK-2066260, tirasemtiv (formerly CK-2017357), is currently under study in patients with amyotrophic lateral sclerosis (clinical trial no. NCT01709149).
What causes weakness of diaphragm muscle fibers in critically ill patients? It seems plausible that the observed diaphragm weakness was acquired during ICU stay, as we used strict exclusion criteria to rule out that our study patients had pre-existing diaphragm weakness. Also, during their stay in the ICU, patients received nutrition according to an optimized nutrition algorithm (7). A commonly suggested concept is that mechanical ventilation per se rapidly induces weakness and atrophy of muscle fibers due to contractile inactivity of the diaphragm (8–12). The critically ill patients we studied received MV for 28–603 hours before biopsy, a time frame that was associated with significant reductions in the CSA of diaphragm fibers in braindead organ donors (8, 13). Thus, the diaphragm muscle fiber atrophy and weakness that we observed may, at least partly, be explained by mechanical ventilation per se. Other ICU-related phenomena that could contribute to diaphragm muscle weakness include underlying disease, such as sepsis (14, 15). Clearly, to elucidate the main factors that contribute to the observed diaphragm muscle fiber weakness requires studies with larger cohorts of various patient groups.
Footnotes
Supported by National Institutes of Health grant HL121500 (C.A.C.O.); the drug compound was supplied by Cytokinetics Inc.
Authors Contributions: P.E.H.: study coordination, literature search, figures, data collection and analysis, data interpretation, writing; A.B.: study design, patient inclusion, data interpretation, writing; M.C.d.W.: patient inclusion, study coordination, writing; F.S.d.M.: data analysis, data interpretation, writing; J.W.V.: patient inclusion, writing; P.S.: obtaining biopsies, writing; R.A.B.: study design, writing; W.L.: data collection and analysis, writing; H.W.H.v.H.: data collection and analysis, writing; L.M.A.H.: data interpretation, writing; C.D.: obtaining biopsies, patient inclusion, writing; D.L.v.d.P.: obtaining biopsies, patient inclusion, writing; A.R.J.G.: study design, writing; J.R.J.: supply of compound, writing; F.I.M.: supply of compound, writing; G.J.M.S.: data interpretation, writing; K.J.H.: obtaining biopsies, patient inclusion, data interpretation, writing; M.A.P.: study design, obtaining biopsies, patient inclusion, writing; C.A.C.O.: study coordination, literature search, study design, data interpretation, writing.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this letters at www.atsjournals.org.
References
- 1.Jaber S, Petrof BJ, Jung B, Chanques G, Berthet J-P, Rabuel C, Bouyabrine H, Courouble P, Koechlin-Ramonatxo C, Sebbane M, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183:364–371. doi: 10.1164/rccm.201004-0670OC. [DOI] [PubMed] [Google Scholar]
- 2.Hermans G, Agten A, Testelmans D, Decramer M, Gayan-Ramirez G. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit Care. 2010;14:R127. doi: 10.1186/cc9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson AC, Hughes PD, Louise Harris M, Hart N, Ware RJ, Wendon J, Green M, Moxham J. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med. 2001;29:1325–1331. doi: 10.1097/00003246-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Russell AJ, Hartman JJ, Hinken AC, Muci AR, Kawas R, Driscoll L, Godinez G, Lee KH, Marquez D, Browne WF, IV, et al. Activation of fast skeletal muscle troponin as a potential therapeutic approach for treating neuromuscular diseases. Nat Med. 2012;18:452–455. doi: 10.1038/nm.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welvaart WN, Paul MA, Stienen GJ, van Hees HW, Loer SA, Bouwman R, Niessen H, de Man FS, Witt CC, Granzier H, et al. Selective diaphragm muscle weakness after contractile inactivity during thoracic surgery. Ann Surg. 2011;254:1044–1049. doi: 10.1097/SLA.0b013e318232e75b. [DOI] [PubMed] [Google Scholar]
- 6.Welvaart WN, Paul MA, van Hees HWH, Stienen GJM, Niessen JWM, de Man FS, Sieck GC, Vonk-Noordegraaf A, Ottenheijm CA. Diaphragm muscle fiber function and structure in humans with hemidiaphragm paralysis. Am J Physiol Lung Cell Mol Physiol. 2011;301:L228–L235. doi: 10.1152/ajplung.00040.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weijs PJM, Stapel SN, de Groot SDW, Driessen RH, de Jong E, Girbes ARJ, Strack van Schijndel RJM, Beishuizen A. Optimal protein and energy nutrition decreases mortality in mechanically ventilated, critically ill patients: a prospective observational cohort study. JPEN J Parenter Enteral Nutr. 2012;36:60–68. doi: 10.1177/0148607111415109. [DOI] [PubMed] [Google Scholar]
- 8.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 9.Vassilakopoulos T, Petrof BJ. Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;169:336–341. doi: 10.1164/rccm.200304-489CP. [DOI] [PubMed] [Google Scholar]
- 10.Ochala J, Renaud G, Llano Diez M, Banduseela VC, Aare S, Ahlbeck K, Radell PJ, Eriksson LI, Larsson L. Diaphragm muscle weakness in an experimental porcine intensive care unit model. PLoS ONE. 2011;6:e20558. doi: 10.1371/journal.pone.0020558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powers SK, Shanely RA, Coombes JS, Koesterer TJ, McKenzie M, Van Gammeren D, Cicale M, Dodd SL. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol (1985) 2002;92:1851–1858. doi: 10.1152/japplphysiol.00881.2001. [DOI] [PubMed] [Google Scholar]
- 12.Sassoon CSH, Caiozzo VJ, Manka A, Sieck GC. Altered diaphragm contractile properties with controlled mechanical ventilation. J Appl Physiol (1985) 2002;92:2585–2595. doi: 10.1152/japplphysiol.01213.2001. [DOI] [PubMed] [Google Scholar]
- 13.Hussain SNA, Mofarrahi M, Sigala I, Kim HC, Vassilakopoulos T, Maltais F, Bellenis I, Chaturvedi R, Gottfried SB, Metrakos P, et al. Mechanical ventilation–induced diaphragm disuse in humans triggers autophagy. Am J Respir Crit Care Med. 2010;182:1377–1386. doi: 10.1164/rccm.201002-0234OC. [DOI] [PubMed] [Google Scholar]
- 14.Shindoh C, Dimarco A, Nethery D, Supinski G. Effect of PEG-superoxide dismutase on the diaphragmatic response to endotoxin. Am Rev Respir Dis. 1992;145:1350–1354. doi: 10.1164/ajrccm/145.6.1350. [DOI] [PubMed] [Google Scholar]
- 15.Supinski GS, Wang W, Callahan LA. Caspase and calpain activation both contribute to sepsis-induced diaphragmatic weakness. J Appl Physiol (1985) 2009;107:1389–1396. doi: 10.1152/japplphysiol.00341.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]