Lung cancer develops in a complex and dynamic microenvironment that includes stromal cells, endothelial cells, inflammatory cells, and lymphocytes that interact with each other and with malignant cells. Depending on the cell types that are present, the microenvironment may promote tumor growth, invasion, and metastasis. However, the microenvironment may also promote successful antitumor immune responses that retard tumor growth or even lead to tumor rejection. For example, patients with non–small cell lung carcinoma can be divided into two groups based on the histological appearance of tertiary lymphoid structures (TLS) adjacent to tumors in the lung (1, 2). Those patients with TLS have a strikingly better overall prognosis and long-term survival than those without (1). Early studies show that the density of T cells and dendritic cells in the tumor-associated TLS correlates with better clinical outcomes (1). Now a follow-up study by Germain and colleagues (pp. 832–844) in this issue of the Journal (3) shows that the size and density of B-cell follicles, the size and number of germinal centers, and the number of antibody-secreting plasma cells in tumor-associated TLS also correlate with a better clinical outcome. Importantly, B cells responding in tumor-associated TLS appear to be participating in the antitumor immune response, as B cells cultured from TLS-containing biopsies produce tumor antigen–specific antibodies (3). Thus, there seems to be a functional link between the development of tumor-associated TLS and a protective response against the tumors.
How might TLS promote antitumor immunity? TLS in the lung are often termed bronchus-associated lymphoid tissue (BALT) and are typically observed after pulmonary infection or inflammation (4). These tissues have a structure similar to that of conventional lymphoid organs, consisting of large B-cell follicles surrounded by T-cell areas that also contain dendritic cells (5). BALT functions like a secondary lymphoid organ and can independently initiate local B- and T-cell responses (6) and serves as a reservoir of memory B and T cells (7). Importantly, mice with BALT are strikingly more resistant than mice without BALT to pulmonary infection with a variety of infectious agents (6, 8). In fact, mice that have BALT will easily survive a dose of influenza or the corona virus associated with severe acute respiratory syndrome that rapidly kills mice that lack BALT (6, 8). One way that BALT protects against infectious diseases in the respiratory tract is by accelerating the immune response. For example, T cell–dependent antibody responses to influenza and the corona virus associated with severe acute respiratory syndrome are generated earlier in mice with BALT than in mice without BALT (6, 8). Although the current study shows that BALT is associated with antitumor antibody responses, it is not yet clear whether the ability to make tumor-specific antibodies correlates with the protective effect (long-term follow-up studies are ongoing) or even whether the tumor-specific B cell response is an important immune effector mechanism against lung cancer.
Nevertheless, given the ability of BALT to accelerate antibody responses to infectious agents, it seems likely that the beneficial effect of BALT on the clinical outcome of lung cancer is due to the stimulation of antitumor immunity of some type. Unfortunately, a correlation between BALT formation and a better clinical outcome does not provide much information in the way of mechanism. For example, one possibility is that some patients had preexisting BALT in their lungs prior to the onset of lung cancer. Although the formation of BALT is often initiated by pulmonary infection or inflammation, once BALT is formed, it can respond to unrelated infections or antigens (9, 10). Thus, BALT formation may have been triggered by a prior infection and fortuitously promoted an antitumor immune response by making the immune system more sensitive to tumor antigens, accelerating or amplifying a nascent antitumor immune response, or even altering the accumulation and placement of T cells in the lung as noted in immune responses to Mycobacterium tuberculosis (11, 12). Given the current data (3), it seems that B cells will likely play an important role in antitumor immunity, perhaps by capturing and presenting tumor antigens to T cells or by generating tumor antigen–specific antibodies that target tumor antigens to dendritic cells that express receptors for antibody constant regions (Figure 1). Conversely, the development of BALT may be triggered by the lung tumors themselves. For example, some lung tumors may be more immunogenic than others and the immunogenic tumors may promote BALT formation as a consequence of antitumor immunity, including the activity of B cells. Thus, BALT may be either a cause or an effect of antitumor immune responses, and future studies will need to distinguish between these possibilities.
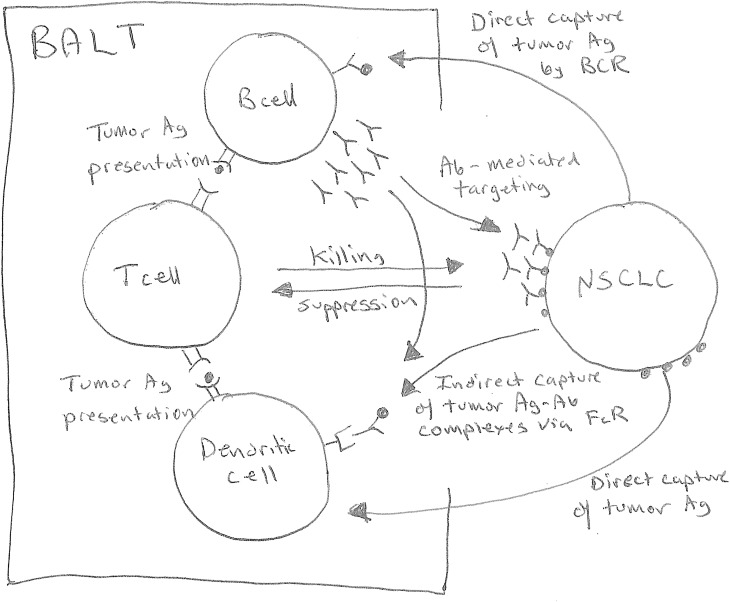
Figure 1.
Role of bronchus-associated lymphoid tissue (BALT) in immunity to lung cancer. B cells in BALT may facilitate antitumor immune responses by directly acquiring tumor antigens via tumor antigen–specific BCR and presenting processed tumor antigens to T cells. B cells may also produce tumor antigen–specific antibodies that bind tumor antigens and generate immune complexes that are taken up via FcR on dendritic cells, which then present processed tumor antigens to T cells. Once activated, T cells may kill tumor cells directly or may recruit inflammatory cells that either kill tumor cells or impair their growth. Tumor antigen–specific antibodies may also directly target tumor cells for destruction via complement-mediated or natural killer cell–mediated destruction. The presence of BALT adjacent to the tumor site facilitates antigen capture as well as the interactions between various immune cells that lead to immune activation and overcome tumor-mediated immune suppression. BCR = B-cell receptor; FcR = receptor for antibody constant region; NSCLC = non–small cell lung carcinoma.
How could understanding B-cell immunology help us clinically? The current dogma suggests that tumor surveillance is performed by T cells, specifically CD8+ T cells. However, a successful immune response is controlled by a complex interplay between the primary tumor, T cells, and B cells, with very limited data concerning B-cell immunology in lung cancer. The data in the current report (3) allow us to pose a variety of questions that, when answered, will yield clinically useful answers. For example, could TLS predict efficacy of therapy? In the current studies, TLS and their density of follicular B cells and mature dendritic cells predict a better outcome in patients with early and advanced disease (3). Therefore, TLS may be a surrogate for treatment response. Second, could TLS guide therapy through stratification of patients for optimal outcome? The current study suggests that assessment of TLS, in addition to typical clinical parameters, may stratify patients into high- or low-risk populations to predict outcome, perhaps by identifying individuals at high risk for recurrence who might benefit from adjuvant therapy, or individuals who do not need further therapy. Next, could TLS lead us to new therapeutic paradigms? In animal models of breast cancer, a unique subset of transforming growth factor-β–producing regulatory B cells triggers the differentiation of metastasis-promoting Foxp3-expressing regulatory CD4+ T cells that inactivate antitumor natural killer cells and effector CD8+ T cells (13). Therefore, transforming growth factor-β–producing regulatory B cells (Bregs) promote metastasis and tumor progression—a role for B cells apparently opposite of that identified in the current study. Thus, novel therapeutic approaches should suppress Breg function and promote the therapeutic activities of B cells. These findings may also impact drug or immunotherapy development for lung cancer. If the prognostic value of TLS is confirmed, future prospective clinical trials may need to stratify outcome based on TLS. Finally, can we change a TLS-negative tumor to a TLS-positive tumor? TLR ligands can promote BALT formation in mice (14) and may be useful to therapeutically alter the immunosuppressive microenvironment induced by cancer cells and lead to B-cell accumulation and activation adjacent to tumors in the lung.
In summary, the current study provides intriguing insight into B cell–mediated antitumor immunology in lung cancer and suggests novel therapeutic approaches. Key next steps in this field will be to identify tumor antigens recognized by B cells and use this information to develop novel vaccines.
Footnotes
T.D.R. is supported by NIH grants HL069409, AI097357, AI097876, and AI100127.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 2.Sautès-Fridman C, Cherfils-Vicini J, Damotte D, Fisson S, Fridman WH, Cremer I, Dieu-Nosjean MC. Tumor microenvironment is multifaceted. Cancer Metastasis Rev. 2011;30:13–25. doi: 10.1007/s10555-011-9279-y. [DOI] [PubMed] [Google Scholar]
- 3.Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in lung cancer patients. Am J Respir Crit Care Med. 2014;189:832–844. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- 4.Randall TD. Bronchus-associated lymphoid tissue (BALT) structure and function. Adv Immunol. 2010;107:187–241. doi: 10.1016/B978-0-12-381300-8.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodland DL, Randall TD. Anatomical features of anti-viral immunity in the respiratory tract. Semin Immunol. 2004;16:163–170. doi: 10.1016/j.smim.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 7.Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD, Lefrançois L, Cauley LS, Harmsen AG, Lund FE, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–654. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Wiley JA, Richert LE, Swain SD, Harmsen A, Barnard DL, Randall TD, Jutila M, Douglas T, Broomell C, Young M, et al. Inducible bronchus-associated lymphoid tissue elicited by a protein cage nanoparticle enhances protection in mice against diverse respiratory viruses. PLoS ONE. 2009;4:e7142. doi: 10.1371/journal.pone.0007142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halle S, Dujardin HC, Bakocevic N, Fleige H, Danzer H, Willenzon S, Suezer Y, Hämmerling G, Garbi N, Sutter G, et al. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J Exp Med. 2009;206:2593–2601. doi: 10.1084/jem.20091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day TA, Koch M, Nouailles G, Jacobsen M, Kosmiadi GA, Miekley D, Kuhlmann S, Jörg S, Gamradt P, Mollenkopf HJ, et al. Secondary lymphoid organs are dispensable for the development of T-cell-mediated immunity during tuberculosis. Eur J Immunol. 2010;40:1663–1673. doi: 10.1002/eji.201040299. [DOI] [PubMed] [Google Scholar]
- 11.Gopal R, Rangel-Moreno J, Slight S, Lin Y, Nawar HF, Fallert Junecko BA, Reinhart TA, Kolls J, Randall TD, Connell TD, et al. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol. 2013;6:972–984. doi: 10.1038/mi.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, Selman M, Becerril-Villanueva E, Baquera-Heredia J, Pavon L, et al. CXCR5+ T helper cells mediate protective immunity against tuberculosis. J Clin Invest. 2013;123:712–726. doi: 10.1172/JCI65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. 2011;71:3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, Kolls JK, Khader SA, Randall TD. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12:639–646. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]