Abstract
Pathological hallmarks indicative of Alzheimer’s disease (AD), which are the plaques of amyloid beta1–42 and neurofibrillary tangles, were found in brain of aged cynomolgus monkey. The aim of this study was to investigate if aged monkeys exhibiting spatial memory impairment and levels of biomarkers indicative of AD, had brain lesions similar to human patients suffering from senile dementia. Generating immunohistochemistry technique to biomarkers of amyloid beta1–42 and the phosphorylated tau 231, our study assessed the amyloidopathy, such as indicative to the senile plaques and cerebral amyloid angiopathy, and the tauopathy, to possible neurofibrillary tangles. Six aged monkeys were selected based on their spatial memory performance and profile of biomarkers of AD, divided equally to affected aged subject – with Memory-affected and low amyloid level, and aged with higher performance in memory and amyloid, as the age-matched subjects. Using immunohistochemistry, plaques of amyloid beta1–42 were observed in two out of three brains of aged subjects with memory impairment and biomarkers indicative of AD. The cerebral amyloid angiopathy was observed in both aged monkey groups, and unlike in the human, the amyloids were found to deposit in the small veins and capillaries. In one of the affected individuals, phosphorylated tau was positively stained intracellularly of the neurons, indicating a possibility of an early stage of the formation of tangles. These findings add to the body of evidence of the utility of the aged cynomolgus monkeys as a spontaneous model for Alzheimer-related disease.
Keywords: degenerative disease, immunohistochemistry, senile plaques, neurofibrillary tangles, cerebral amyloid angiopathy
Introduction
Tests for several behavioral tasks, developed within the human neuropsychological domain, have been successfully adapted for use with non-human primates (NHP), including delayed response tasks (DRT) (Amici et al., 2010) where delays of various lengths are imposed between the presentation of a stimulus and the desired response (Bartus and Dean, 2009; Lacreuse and Herndon, 2009; Rodriguez and Paule, 2009; Nagahara et al., 2010). This type of memory tests is appropriate in assessing Alzheimer’s disease (AD), and as well as other affected cognitive function such as executive function and divided attention (Johannsen et al., 1999; Giannakopoulos et al., 2007). As some of the earliest cerebral lesions in the AD progression are typically located in the hippocampus (Deiana et al., 2011), spatial memory tests are of particular interest.
Delayed response tasks performance in cynomolgus monkeys (Macaca fascicularis) has recently been described (Darusman et al., 2013a) demonstrating that old monkeys (more than 20 years of age) performed poorer than young (4–9 years) and middle aged (10–16 years) individuals. The DRT performance was correlated with the levels of the core biomarkers indicative of AD, especially the amyloid-beta1–42 (Aβ42) (Darusman et al., 2013b, 2014), where the DRT performance was positively correlated with concentrations of Aβ42. Structural magnetic resonance imaging (MRI) studies identified abnormalities, such as atrophy in hippocampus and morphological changes in the cortical areas in aged monkeys with poor memory and low Aβ42 levels (Darusman et al., 2014).
Earlier studies of brain sections from older cynomolgus monkey revealed pathological hallmarks indicative of AD (Nakamura et al., 1998), and based on the combined findings described above, the present study was carried out to investigate whether animals with poor DRT performance and circulating biomarkers levels indicative of senile dementia also exhibited brain lesions such as senile plaques (SP) of Aβ42 and tauopathy. In the human, the progression of cognitive decline and biomarkers levels reflect the AD pathological processes in the brain (Andreasen and Blennow, 2005; Perrin et al., 2009). Low levels of circulating Aβ42 and elevated total tau (t-tau) and phosphorylated tau (p-tau), are related with the development of SP and NFT, respectively (Brody et al., 2008; Blennow et al., 2010; Jack et al., 2010; Albert et al., 2011). In rhesus monkeys, the incidence, distribution, and chemical composition of the Aβ deposits in the brain of young and aged individuals have been described (Sani et al., 2003; Nishimura et al., 2012) with higher level of Aβ1–40 (Aβ40) than the Aβ42.
In the present study, we examined the presence of Aβ42 and the p-tau by immunohistochemistry analysis in brain sections of aged monkeys selected from a previous study (Darusman et al., 2013a,b), based on low total DRT and low levels of Aβ42 in cerebrospinal fluid (CSF) compared with aged monkeys with better memory performance and high CSF Aβ42 levels. The DRT were assessed by the short term memory test (STMT), long term memory test (LTMT), and memory load test (MLT).
Materials and Methods
Subjects
From previous studies, we selected aged subjects above 20 years old that met the requirements of low levels of Aβ42(<5000 pg/ml) and low DRT performance (<40%) for the memory-affected group (Darusman et al., 2013a,b). Aged subjects characterized by high-circulating Aβ42 and high DRT performance were selected for the age-matched control group. Subject age was determined from birth certificates for the animals born in captivity and from dental scaling (Swindler, 2002) and estimated year of born for animals born in the wild.
The subjects in this study (Table 1) were limited to old individuals that were destined for euthanasia due to progressive weight loss, paleness of the mucous membranes, reduced appetite, and/or general weakness. These criteria yielded three memory-affected monkeys (two females and one male), and three age-matched monkeys (one female and two males). Two young monkeys (one female and one male) were included to control for potential confounds associated with age.
Table 1.
The characteristics of the subjects.
| Tattoo/sex/age group | Total DRT (%) | Biomarker (pg/ml) |
Reference | Agea | ||
|---|---|---|---|---|---|---|
| Aβ42 | t-tau | pT231 | ||||
| C2538/female/young | 68.09 | 655.10 | 301.60 | 2.36 | Darusman et al. (2013a) | 9 |
| C0744/male/young | 60.50 | 620.00 | 568.60 | 6.27 | Darusman et al. (2013a) | 7 |
| 10063/female/memory-affected | 40.58 | 16.01 | 92.62 | 4.81 | Darusman et al. (2013b) | 30 |
| T3311/male/memory-affected | 40.00 | 368.36 | 319.46 | 4.19 | Darusman et al. (2013a) | 30b |
| I1112/female/memory-affected | 34.34 | 164.96 | 219.85 | 5.45 | Darusman et al. (2013a) | 29 |
| 10749/female/age-matched | 61.67 | 416.36 | 370.57 | 4.11 | Darusman et al. (2013b) | 30 |
| 9661/male/age-matched | 60.16 | 480.76 | 277.71 | 7.55 | Darusman et al. (2013b) | 27 |
| T3283/male/age-matched | 66.42 | 524.65 | 60.64 | 4.37 | Darusman et al. (2013b) | 30b |
Data are collected from the references Darusman et al. (2013a,b). DRT performance is presented as the collected percent of trials where the subject could correctly retrieve a hidden item following a predetermined delay. Biomarker levels were measured in serum and CSF.
aUp to the end of year of 2013.
bEstimated from the year of birth by a population survey in 1983.
All subjects were housed at the AAALAC-accredited Primate Research Center, Bogor Agricultural University (PRC, IPB) in pairs or social groups of various sizes. During the testing period, the subjects were individually housed indoors in adjacent cages, which permitted some tactile contact through perforated acrylic panels. The subjects’ housing conditions and test procedures were approved by the PRC IPB Animal Care and Use Committee. The subjects’ characteristics with their previous DRT performance and biomarker levels are presented in Table 1.
Histopathology
The animals were euthanized with pentobarbital and phenytoin injections (Euthasol™, Virbac, Fort Worth, TX, USA) followed by intracardial sodium chloride 0.9% perfusions and exsanguination; the intracardial perfusion was continued with paraformaldehyde (4%). Brains were collected and immersed in 10% paraformaldehyde solution for 24 h. Subsequently, the brains were fixated in buffered formalin 10% (pH 7). Fixated brains were sectioned in the coronal plane allowing for analyses of five distinct regions: the frontal, occipital, parietal and temporal lobes, and the hippocampus.
All sections were embedded in paraffin wax and cut into 5 μm sections. Hematoxylin-eosin (HE) staining was applied for routine examination of the brain, especially for observing degeneration signs (apoptotic neuron signs such as chromatolysis, gliosis, vacuolization) because we were not applying immunohistochemistry studies of these degeneration signs. Congo-red staining was also applied to observe amyloid in general. For immunohistochemistry of Aβ42 and p-tau, sections were treated with sodium citrate buffer at 95°C for 30 min, and to minimize non-specific binding, sections were treated with 3% hydrogen peroxide in methanol for 30 min at room temperature, and blocked using 1% fetal bovine serum and 10% skimmed milk in PBS for 30 min at room temperature. An AD detection kit (Millipore™, Temecula, CA, USA) was used to identify Aβ42 and p-tau threonine 231 (pT231). The kit contained polyclonal antibodies against human Aβ42 (catalog number AB5078P) and pT231 (catalog number MAB3420SP).
The sections were incubated with the specific antibodies (diluted 1:500 in PBS) overnight at 4°C. After washing with PBS, the sections were incubated with a biotinylated secondary antibody for 30 min, and subsequently with Streptavidin-Horse Radish Peroxidase (HRP) for 30 min at room temperature (DAKO-LSAB+ system HRP kit; Glostrup, Denmark). Diaminobenzidine (DAB) was applied at room temperature until an appropriate intensity was obtained (2–5 min). Sections were rinsed in distilled water, counterstained with HE to visualize tissue morphology and then mounted with Entellan® (product number 1079610100, Merck KGaA, Darmstadt, Germany). Negative controls (without secondary antibody), antibody control (treated with normal rabbit serum), and photomicrograph control, were included. All slides (three slides for each brain region, times each staining procedure were analyzed for each subject; i.e., 3 × 5 × 7 = 105) were scored independently by two trained pathologists that were blinded to the identity of the individuals. Scores are summarized in Table 2.
Table 2.
Scoring criteria of the histopathology of the brain.
| Score | Degeneration | Amyloid disorders |
Tauopathy | |
|---|---|---|---|---|
| CAA | Senile plaques | |||
| 1 | Condensed chromatin inside nuclei (chromatolysis) | Deposit of amyloid in vascular wall of arterioles | Diffused plaques – appearing as small numbers of nodules in the brain parenchyma | p-tau detected inside the cytoplasm of the neuron (intracellular stage) |
| 2 | Aggregation of satellite cell/glial cell (gliosis) | Deposit of amyloid in vascular wall of venules | Compacted plaques – appearing in larger amounts and sizes in the brain parenchyma | p-tau detected in the cytoplasm and/or axon of the neuron (fibrillar stage) |
| 3 | Formation of vacuole sites as indicative of active phagocytosis of the degenerated neuron (vacuolization) | Deposit of amyloid in vascular wall of capillaries | Compacted plaques in the brain parenchyma associated with degeneration of the surrounding nerve | p-tau detected as tangled formations outside of the neuron (extracellular stage) |
The degeneration score follows the progression of the apoptotic nerve cell (Kettemann et al., 2011). The degree of chromatolysis and the number of satellite cells or glial cells (Gliosis) was also incorporated in the degeneration score (Chen et al., 2013). Two aspects of amyloidopathy were scored: cerebral amyloid angiopathy (CAA) and SP (Heuer et al., 2012), where CAA scores were based on the localization of Aβ42 (Attems and Jellinger, 2004) and plaques were scored by morphology (lowest score was valued when the plaques appeared as small numbers and diffused, continued with compacted formation and compacted plaques with sign of degeneration surrounding as the highest score). The tauopathy was scored based on which stage the tangles formation was perceived to be in Augustinack et al. (2002).
Results
Degeneration
The histopathology findings (scores and the lobes where the lesion appeared) are presented in Table 3. Signs of brain degeneration were found in the aged monkeys, both the memory-affected and aged-matched subjects. Degeneration signs such as gliosis and vacuolization were present in the brains of all aged, but not in young animals. Early signs of degeneration were found in young animals, observed as a chromatolysis, while the later stages – the gliosis and vacuolization – were only observed in aged animals, both the memory-affected and age-matched subjects. These degeneration signs were observed in the frontal lobe of all the aged subjects (n = 6), in the parietal lobe of most (n = 5), and in the temporal and occipital lobes of some (n = 2). A degeneration of the hippocampus was found only in the memory-affected aged monkeys observed as chromatolysis.
Table 3.
Histopathology findings and summary of the scores.
| Tattoo | Group | Average score from two observers (lobesa) |
|||
|---|---|---|---|---|---|
| Degeneration | CAA | Senile plaques | Tauopathy | ||
| C2538 | Young | 1(P) | 0 | 0 | 0 |
| C0744 | Young | 1(P) | 0 | 0 | 0 |
| 10063 | Memory-affected | 2(F), 2(P), 1(O), 1(Hip) | 3(F), 2(T) | 0 | 0 |
| T3311 | Memory-affected | 3(F), 2(T), 1(Hip) | 3(F), 2(P), 1(O) | 2(F), 1(P), 1(Hip) | 0 |
| I1112 | Memory-affected | 3(F), 2(T), 1(O), 1(P), 1 (Hip) | 2(F), 2(T), 1(O), 1(P), 1 (Hip) | 2(F), 1(T), 1(O), 1(Hip) | 1 (T), 1 (O) |
| 10749 | Age-matched | 3(F), 2(P) | 2 (F), 2(T), 1(Hip) | 0 | 0 |
| 9661 | Age-matched | 3 (F), 2 (P) | 2 (F), 1(T), 1(P) | 0 | 0 |
| T3283 | Age-matched | 2(F), 2 (P), 1(O) | 2(F), 2 (T), 1 (O) | 0 | 0 |
aF, frontal lobe; T, temporal lobe; P, parietal lobe; O, Occipital lobe; Hip, hippocampus.
CAA
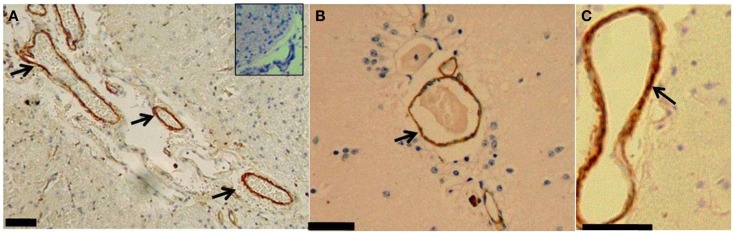
The CAA was found in the small vessels of the brain in all of the aged monkeys. Brown coloration from DAB appeared in the vascular wall of the brain capillaries, preferentially in small veins (Figure 1). The CAA was observed in the blood vessels of the frontal lobe (n = 6), temporal lobes (n = 5), occipital and parietal lobes (each n = 3), and hippocampus (n = 2). The CAA was observed in the capillaries of the frontal lobe in the Memory-affected monkeys (n = 2).
Figure 1.
(A) Brain section from the temporal lobe of a memory-affected animal (subject T3311) treated with a polyclonal serum against human Aβ42 and stained with DAB. The inset picture is a photomicrograph control treated with nonsense rabbit serum. (B) The occipital lobe of an Aged-matched subject (subject 10749); and (C) the hippocampus (subject I1112); arrows indicate deposition of the Aβ42 inside the walls of small veins. Scale bars: 60 μm.
Senile plaques
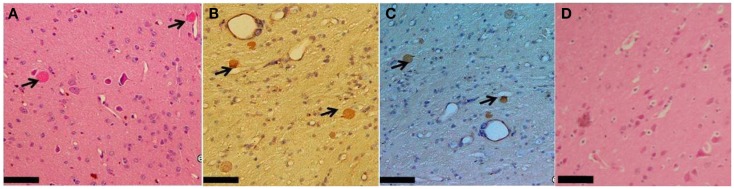
Plaques of Aβ42 in the parenchyma were observed in the brain from two monkeys of the memory-affected group (Figure 2); subjects I1112 and T3311. The plaques were principally found in the frontal, temporal and parietal lobes, and the hippocampus. The compacted shape of the plaques was observed only in frontal lobes, while the diffused shapes were found in the other aforementioned lobes.
Figure 2.
Brain section of the frontal lobe from a Memory-affected animal (subject I1112) demonstrating Aβ42 plaques in the parenchyma, (A) stained with HE, (B) Congo red and (C) with anti-Aβ42 antiserum/DAB, and (D) HE from Aged-matched subject (subject 10749). Arrows indicate Aβ42 plaques. Scale bars: 60 μm.
Tauopathy
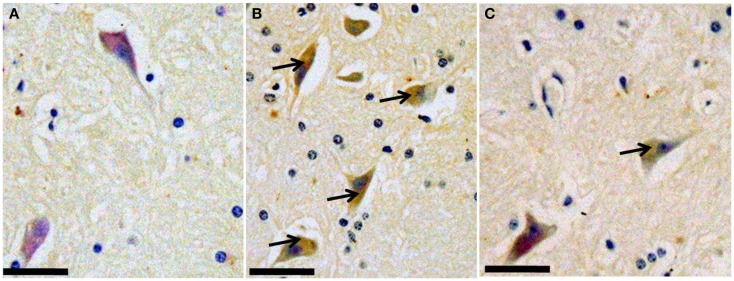
The pT231 was found in the temporal and occipital lobe from one subject belonging to the memory-affected group (I1112). The pT231-positive structures appeared only in the cytoplasm of the neuron cell (Figure 3). The brownish colorization from the pT231 antibody was weaker compared with the other DAB staining of the SP and CAA.
Figure 3.
The occipital lobe of an age-matched subject (T3283) (A), the occipital lobe of a memory-affected subject (I1112) (B), and temporal lobe (C). Sections have been treated with a polyclonal serum against human p-tau pT231 and stained with DAB. Arrows indicate the presence of pT231 in the cytoplasm of nerve cells. Scale bars: 60 μm.
Discussion
In addition to degenerative lesions, such as the microgliosis and vacuolization, we found the CAA in both aged groups and not in the young individuals – defined by an accumulation of amyloid beta in the brain vascular wall: cerebral beta-amyloid angiopathy (Rensink et al., 2003; Revesz et al., 2003, 2009; Greenberg et al., 2004). Since both aged groups developed the CAA, it suggested that the CAA may occur spontaneously in cynomolgus monkeys as in other aged NHP species, such as Rhesus monkeys (Walker, 1997; Heuer et al., 2012).
Qualitatively, the CAA in the memory-affected and aged-matched groups shared same relative amounts and similar to location in lobes where the lesions were present, namely the frontal and parietal-temporal lobes, which are the brain lobes related to memory function and also affected in AD and frontotemporal disease (FTD) in the human (Naggara et al., 2006; Rodriguez and Paule, 2009; Heuer et al., 2012), along with the hippocampus area (Johannsen et al., 2000; Deiana et al., 2011). CAA is present in almost in all cases of AD (Okamoto et al., 2009, 2012).
In humans, CAA is most prominent in the arterioles and less frequent in veins and capillaries (Preston et al., 2003), while interestingly our findings showed that amyloid beta deposits in the small veins and capillaries of the cynomolgus monkey. More thorough study of the mechanism of Aβ deposition in the veins is required in order to confirm whether or not the Aβ internalizes into endothelial cells, from blood passing through capillary pores, and thereby becomes detectable inside the vessel wall of veins and capillaries. The suspected CAA inside the capillaries and small veins correlates with AD pathology (Attems and Jellinger, 2004) and cognitive impairment (Eurelings et al., 2010). Our findings thus support studies of CAA in other aged old world monkeys (Walker, 1997; Nakamura et al., 1998; Oikawa et al., 2010).
In rhesus monkey and cynomolgus monkey, CAA and the parenchymal plaques of amyloid beta, are correlated (Nakamura et al., 1998; Levine and Walker, 2006) and our findings may support this. Our findings suggest that memory impaired cynomolgus monkeys suffer from an amyloid-related disease manifested as SP in the brain. On the other hand, animals with spontaneous amyloid-like disorder such as the dog with canine cognitive dysfunction had a fundamentally different relationship between the cognitive disorder and the retention of amyloid load sensitive marker Pittsburgh compound B (Fast et al., 2013).
Among three Memory-affected subjects, we found two subjects (I1112 and T3311) with the parenchymal form of amyloid beta inside the frontal, temporal lobes, and the hippocampus. The plaques were present in the same lobes where CAA was found. In the rhesus monkey, the deposits of Aβ were observed principally in the cortical, paralimbic, and core limbic cortical zones corresponding to mild, moderate, and heavy burden, respectively (Sani et al., 2003). Along with Nishimura et al. (2012), this study of rhesus monkeys also emphasizes the higher proportion of Aβ40 compared with the Aβ42. The absence of Aβ40 analysis in our study seems limit the whole interpretation.
However, another study in the species showed that the Aβ42 often predominate the Aβ40 in the cortical level of both temporal and occipital lobes, although the relative level of Aβ40 remain higher in the occipital cortex (Rosen et al., 2011). The additional immunohistochemistry of Aβ40 could add more information about the proportion of the Aβ in the cynomolgus monkey. This would support a biomarker study by Yue et al. (2014), which describes that Aβ42 was significantly associated with aging but not with the Aβ40 in cynomolgus monkey.
Similar to other studies (Oikawa et al., 2010; Heuer et al., 2012), we also found signs of both nerve damage and tangle formation in the brain of aged monkeys although the location of the plaques and the tangles were not correspondently the same. An old female from the memory-affected group (I1112), which had the parenchymal form of amyloid beta stained positively for pT231 in the body of nerve cells in the temporal and occipital lobes. In AD patients, this is the early stage of NFT, indicated by the intracellular form of NFT (Augustinack et al., 2002). The identified p-tau in the neuron’s cytoplasm was not corresponded with the SP, showed by the differences of the lobes’ location between SP and p-tau. This finding agrees with a previous study in cynomolgus monkey (Oikawa et al., 2010) that the pathological differences of SP and tauopathy in the species is specific and may differed with human’s AD pathology.
Although the present study was small, it adds to the body of evidence (Oikawa et al., 2010; Heuer et al., 2012) that support the neuropathology of the aged cynomolgus monkey as a potential model for studies of spontaneous disease of Alzheimer-type, especially the amyloid-related brain pathology and also suggests the potential for further study of p-tau mediated pathology in the species (Walker, 1997; Levine and Walker, 2006; Oikawa et al., 2010; Heuer et al., 2012). Future studies of high relevance would include more detailed analyses of the Aβ40, Aβ42, and the pT231 presence in each of the brain lobes to confirm the expression of both biomarkers and to complement the histopathology results.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the Director of the Primate Research Center IPB (Dr. Joko Pamungkas) and staff (Dr. Irma Suparto, Dr. Nengah Budiarsa, and Dr. Diah Pawitri), and the pathologist at the Primate Research Center (Dr. Sylvia A. Prabandari). This study was supported by the Directorate of Higher Education, Ministry of Education, and Republic of Indonesia through a PhD fellowship to Dr. Huda Shalahudin Darusman.
References
- Albert M. S., De Kosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amici F., Aureli F., Call J. (2010). Monkeys and apes: are their cognitive skills really so different? Am. J. Phys. Anthropol. 2010, 188–197. 10.1002/ajpa.21305 [DOI] [PubMed] [Google Scholar]
- Andreasen N., Blennow K. (2005). CSF biomarkers for mild cognitive impairment and early Alzheimer’s disease. Clin. Neurol. Neurosur. 107, 165–173. 10.1016/j.clineuro.2004.10.011 [DOI] [PubMed] [Google Scholar]
- Attems J., Jellinger K. A. (2004). Only cerebral capillary amyloid angiopathy correlates with Alzheimer pathology – a pilot study. Acta Neuropathol. 107, 83–90. 10.1007/s00401-004-0866-7 [DOI] [PubMed] [Google Scholar]
- Augustinack J. C., Schenider A., Mandelkow E. M., Hyman B. T. (2002). Specific tau phosphorylation sited correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 103, 26–35. 10.1007/s004010100423 [DOI] [PubMed] [Google Scholar]
- Bartus R. T., Dean R. L., III (2009). Pharmaceutical treatment for cognitive deficits in Alzheimer’s disease and other neurodegenerative conditions: exploring new territory using traditional tools and established maps. Psychopharmacology 202, 15–36. 10.1007/s00213-008-1365-7 [DOI] [PubMed] [Google Scholar]
- Blennow K., Hampel H., Weiner M., Zetterberg H. (2010). Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 6, 131–144 10.1038/nrneurol.2010.4 [DOI] [PubMed] [Google Scholar]
- Brody D. L., Magnoni S., Schwetye K. E., Spinner M. L., Esparza T. J., Stocchetti N., et al. (2008). Amyloid-β dynamics correlate with neurological status in the injured human brain. Science 321, 1221–1224. 10.1126/science.1161591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Errangi B., Li L., Glasser M. F., Westlye L. T., Fjell A. M., et al. (2013). Brain aging in humans, chimpanzees (Pan troglodytes), and rhesus macaques (Macaca mulatta): magnetic resonance imaging studies of macro- and micro-structural changes. Neurobiol. Aging 10, 2248–2260. 10.1016/j.neurobiolaging.2013.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darusman H. S., Call J., Sajuthi D., Schapiro S. J., Gjedde A., Kaliokoski O., et al. (2013a). Delayed response task performance as a function of age in cynomolgus monkeys (Macaca fascicularis). Primates 55, 259–267 10.1007/s10329-013-0397-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darusman H. S., Sajuthi D., Kalliokoski O., Jacobsen K. R., Call J., Schapiro S. J., et al. (2013b). Correlations between serum levels of beta amyloid, cerebrospinal levels of tau and phospho tau, and delayed response tasks in young and aged cynomolgus monkeys (Macaca fascicularis). J. Med. Primatol. 42, 137–146. 10.1111/jmp.12044 [DOI] [PubMed] [Google Scholar]
- Darusman H. S., Pandelaki J., Mulyadi R., Sajuthi D., Putri I. A., Kalliokoski O. H., et al. (2014). Poor memory performance in aged cynomolgus monkeys with hippocampal atrophy, depletion of amyloid beta 1-42 and accumulation of tau proteins in cerebrospinal fluid. In vivo 28, 173–184. [PubMed] [Google Scholar]
- Deiana S., Platt B., Riedel G. (2011). The cholinergic system and spatial learning. Behav. Brain Res. 221, 389–411. 10.1016/j.bbr.2010.11.036 [DOI] [PubMed] [Google Scholar]
- Eurelings L. S., Richard E., Carrano A., Eikelenboom P., van Gool W. A., Rozemuller A. J. (2010). Dysphoric capillary cerebral amyloid angiopathy mimicking Creutzfeldt-Jakob disease. J. Neurol. Sci. 295, 131–134. 10.1016/j.jns.2010.04.020 [DOI] [PubMed] [Google Scholar]
- Fast R., Rodell A., Gjedde A., Mouridsen K., Alstrup A. K., Bjarkam C. R., et al. (2013). PiB fails to map amyloid deposits in cerebral cortex of aged dogs with canine cognitive dysfunction. Front. Aging Neurosci. 5:1–11. 10.3389/fnagi.2013.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos P., Gold G., Kövari E., von Gunten A., Imhof A., Bouras C., et al. (2007). Assessing the cognitive impact of Alzheimer disease pathology and vascular burden in the aging brain: the Geneva experiences. Acta Neuropathol. 113, 1–12. 10.1007/s00401-006-0144-y [DOI] [PubMed] [Google Scholar]
- Greenberg S. M., Gurol M. E., Rosand J., Smith E. E. (2004). Amyloid angiopathy-related vascular cognitive impairment. Stroke 35, 2616–2619. 10.1161/01.STR.0000143224.36527.44 [DOI] [PubMed] [Google Scholar]
- Heuer E., Rosen R. F., Cintron A., Walker L. C. (2012). Nonhuman primate models of Alzheimer-like cerebral proteopathy. Curr. Pharm. 18, 1159–1169. 10.2174/138161212799315885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C. R., Jr., Knopman D. S., Jagust W. J., Shaw L. M., Aisen P. S., Weiner M. W., et al. (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 9, 119–128. 10.1016/S1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen P., Jakobsen J., Bruhn P., Gjedde A. (1999). Cortical responses to sustained and divided attention in Alzheimer’s disease. Neuroimage 10, 269–281. 10.1006/nimg.1999.0475 [DOI] [PubMed] [Google Scholar]
- Johannsen P., Jakobsen J., Gjedde A. (2000). Statistical maps of cerebral blood flow deficits in Alzheimer’s disease. Eur. J. Neurol. 7, 385–392 10.1046/j.1468-1331.2000.00088.x [DOI] [PubMed] [Google Scholar]
- Kettemann H., Hanisch U. K., Noda M., Verkhratsky A. (2011). Physiology of microglia. Physiol. Rev. 91, 461–553 10.1152/physrev.00011.2010 [DOI] [PubMed] [Google Scholar]
- Lacreuse A., Herndon J. G. (2009). “Nonhuman primate models of cognitive aging,” in Animal Model of Human Cognitive Aging, eds Bizon J. L., Woods A. (Humana Press, Springer; ), 29–58. [Google Scholar]
- Levine H., Walker L. C. (2006). “Models of Alzheimer’s disease,” in Handbook of Models for Human Aging, ed. Michael Conn P. (New York: Academic press; ), 121–134. [Google Scholar]
- Nagahara A. H., Bernot T., Tuszynski M. H. (2010). Age related cognitive deficits in rhesus monkeys mirror human deficits on an automated test battery. Neurobiol. Aging 31, 1020–1031. 10.1016/j.neurobiolaging.2008.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naggara O., Oppenheim C., Rieu D., Raoux N., Rodrigo S., Dalla B. G., et al. (2006). Diffusion tensor imaging in early Alzheimer’s disease. Acta Neuropathol. 82, 239–259 10.1016/j.pscychresns.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Nakamura S., Nakayama H., Goto N., Ono F., Sakakibara I., Yoshikawa Y. (1998). Histopathological studies of senile plaques and cerebral amyloidosis in cynomolgus monkeys. J. Med. Primatol. 27, 244–252. 10.1111/j.1600-0684.1998.tb00244.x [DOI] [PubMed] [Google Scholar]
- Nishimura M., Nakamura S., Kimura N., Liu L., Suzuki T., Tooyama I. (2012). Age-related modulation of ϒ-secretase activity in non-human primate brains. J. Neurochem. 123, 18–21. 10.1111/j.1471-4159.2012.07884.x [DOI] [PubMed] [Google Scholar]
- Oikawa N., Kimura N., Yanagisawa K. (2010). Alzheimer-type tau pathology in advanced old nonhuman primate brains harboring substantial amyloid deposition. Brain Res. 1315, 137–149. 10.1016/j.brainres.2009.12.005 [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Ihara M., Fujita Y., Ito H., Takahashi R., Tomimoto H. (2009). Cortical microinfarcts in Alzheimer’s disease and subcortical vascular dementia. Neuroreport 20, 990–996 10.1097/WNR.0b013e32832d2e6a [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Yamamoto T., Kalaria R. N., Senzaki H., Maki T., Hase Y., et al. (2012). Cortical hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol. 123, 381–394. 10.1007/s00401-011-0925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin R., Fagan A. M., Holtzman D. M. (2009). Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature 461, 916–922. 10.1038/nature08538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston S. D., Steart P. V., Wilkinson A., Nicoll J. A., Weller R. O. (2003). Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol. Appl. Neurobiol. 29, 106–117. 10.1046/j.1365-2990.2003.00424.x [DOI] [PubMed] [Google Scholar]
- Rensink A. A., de Waal R. M., Kremer B., Verbeek M. M. (2003). Pathogenesis of cerebral amyloid angiopathy. Brain Res. Brain Res. Rev. 43, 207–223 10.1016/j.brainresrev.2003.08.001 [DOI] [PubMed] [Google Scholar]
- Revesz T., Ghiso J., Lashley T., Plant G., Rostagno A., Frangione B., et al. (2003). Cerebral amyloid angiopathies: a pathologic, biochemical, and genetic view. J. Neuropathol. Exp. Neurol. 62, 885–898. [DOI] [PubMed] [Google Scholar]
- Revesz T., Holton J. L., Lashley T., Plant G., Frangione B., Rostagno A., et al. (2009). Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol. 118, 115–130. 10.1007/s00401-009-0501-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. S., Paule M. G. (2009). “Working memory delayed response tasks in monkeys,” in Methods of Behavior Analysis in Neuroscience, 2nd Edn, ed. Buccafusco J. J. (Boca Raton, FL: CRC Press; ), 247–266. [PubMed] [Google Scholar]
- Rosen R. F., Walker L. C., Levine H. (2011). PIB binding in aged primate brain: enrichment of high affinity sites in human with Alzheimer’s disease. Neurobiol. Aging 32, 223–234. 10.1016/j.neurobiolaging.2009.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sani S., Traul D., Klink A., Niaraki N., Gonzalo-Ruiz A., Wu C. K., et al. (2003). Distribution, progression and chemical composition of cortical amyloid-beta deposits in aged rhesus monkeys: similarities to the human. Acta Neuropathol. 105, 145–156. [DOI] [PubMed] [Google Scholar]
- Swindler D. R. (2002). Primate Dentistry: an Introduction to the Teeth of Non-Human Primates. Cambridge: Cambridge University Press. [Google Scholar]
- Walker L. C. (1997). Animal models of cerebral β-amyloid angiopathy. Brain Res. Rev. 25, 70–84 10.1016/S0165-0173(97)00017-9 [DOI] [PubMed] [Google Scholar]
- Yue F., Lu C., Ai Y., Chan P., Zhang Z. (2014). Age-associated changes of cerebrospinal fluid amyloid-β and tau in cynomolgus monkeys. Neurobiol. Aging 35, 1656–1659. 10.1016/j.neurobiolaging.2014.01.139 [DOI] [PubMed] [Google Scholar]